Department of Biology, Faculty of Science, University of Hail, Saudi Arabia
Corresponding author Email: n-almansor@hotmail.com
Article Publishing History
Received: 17/04/2019
Accepted After Revision: 20/06/2019
The red palm weevil (RPW), Rhynchophorus ferrugineus (Olivier) is an obtrusive, ruinous pest of date palms, (Phoenix dactylifera) causing critical economic losses in the Middle East including the Kingdom of Saudi Arabia. However, relying on insecticides alone for controlling the RPW (R. ferrugineus) can have negative effects on human health and the environment. Natural enemies are considered as the fundamental part of the biological control, which is safe for controlling the RPW. Therefore, knowledge of the natural enemies against the RPW (R. ferrugineus) is an important to create techniques for the integrated pest management (IPM). The present study aimed to isolate and identify the bacterial species associated the RPW (R. ferrugineus) in Hail region during. Adults of the RPW were monthly collected from infested date palm farms in various sites in Hail region. Several bacterial species were isolated from the investigated RPW and the obtained sequences were edited in MEGA7 software and compared to available sequences in the Gen Bank database. The 16S rDNA sequencing showed that bacteria isolated from the investigated RPW were mostly Gram positive and belonged to Proteus mirabilis (33.3%), Klebsiella pneumonia (25%), Serratia marcescens (25%), Staphylococcus sciuri (8.3%) and Providencia rettgeri (8.3%). Overall, the results of this study can be utilized a baseline data for applying the biological control program of the RPW.
Rhynchophorus ferrugineus, Biological control, Integrated pest management, Natural enemies, Bacteria, Chemical control
Alanazi N. A. Isolation and Identification of Bacteria Associated with Red Palm Weevil, Rhynchophorus ferrugineus from Hail Region, Northern Saudi Arabia. Biosc.Biotech.Res.Comm. 2019;12(2).
Alanazi N. A. Isolation and Identification of Bacteria Associated with Red Palm Weevil, Rhynchophorus ferrugineus from Hail Region, Northern Saudi Arabia. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2KH2cfv
Copyright © Alanazi, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
The red palm weevil Rhynchophorus ferrugineus (Olivier) (RPW) (Coleoptera: Curculionidae) is considered as a dangerous insect pest of date palm trees and makes huge losses to date palm’s farmers. Indeed, the Food and Agriculture Organization (FAO) has recorded the RPW as a class -1 pest of date palms in the Middle East (Al-Shawaf et al., 2013).This, subsequently, has encouraged to utilized several strategies to control the R. ferrugineus including chemicals, biotechnological frameworks utilizing semi chemicals or the improvement of the Sterile Insect Technique, and biological control (Mazza et al., 2014). Although chemical control has been broadly connected for controlling the R. ferrugineus, there is creating stress over the negative impacts of insecticides application on human health and the environment (Mazza et al., 2014; Asiry, 2015; Nicolopoulou-Stamati et al., 2016). R. ferrugineus collected from different typologies of prothoracic spots have been found in Malta, Sicily and Pakistan (Bannu, Khyber Pakhtunkhwa) which shows a degree of diversity in RPW population around the globe (Ul Haq et al., 2018).
Biological control can be characterized as an ecosystem service which advances the concealment of pests by their common natural enemies including their parasites, parasitoids, predators and pathogens (DeBach and Rosen, 1974; Bale et al., 2008). Biological control offers ecological and monetary favorable circumstances as yield loss might be diminished without undesirable natural outcomes coming with application of insecticides (Bianchi et al., 2006). The improvement of a biological control segment for a productive IPM requires the distinguishing proof of the common adversaries of the RPW and its defensive mechanisms against its regular natural enemies.There have been a few endeavors to separate pathogens from the RPW (Gindin et al., 2006; Güerri-Agulló et al., 2008; Salama and Abd-Elgawad, 2002; Salama et al., 2004). These reviews prompted to the disclosure of a cytoplasmatic polyhedrosis infection, and a yeast isolated from the RPW’s haemolymph. Be that as it may, none of these can be classified as potential biocontrol agents, mostly because their application in normal conditions is restricted (Banerjee and Dangar, 1995; Salama et al., 2004). Cytoplasmic polyhedrosis virus infected RPW larva showed 80-100 % mortality with a viral dose of 80×106 (Mahmoud et al., 2018). However, the accomplishment of biological control agents is frequently lacking and any control of the RPW is by all accounts.
Pathogenic entobacteria generally belong to the families Bacillaceae, Pseudomonadaceae, Enterobacteriaceae, Streptococcaceae and Micrococaceae (Tanada and Kaya, 1993). Although numerous bacteria can infect insects, just members of two genera of the order Eubacteriales, Bacillus and Serratia, have been enrolled for the control of insects (Tanada and Kaya, 1993). For the genus Rhynchophorus, bacteria have only been isolated from the RPW: Dangar and Banerjee (1993) found some pathogenic entobacteria belonging to Bacillus sp., Serratia sp. and the coryneform group in larvae and adults in India, while Alfazairy et al. (2003) and Alfazariy (2004) isolated Bacillus thuringiensis Berliner and Bacillus sphaericus Meyer and Neide from larvae and adults in Egypt. Alfazariy (2004) reported successful control of the RPW in laboratory conditions by infection with B. thuringiensis subspecies kurstaki isolated from larvae in Egypt. Conversely, different authors demonstrated an alternate weakness of the RPW to B. thuringiensis (Manachini et al., 2008a,b, 2009). Pseudomonas aeruginosa (Schroeter) was isolated from infected larvae collected in Kerala, India (Banerjee and Dangar, 1995). Research facility examines that this bacterium was pathogenic for weevils when ingested through force-feeding or when insects were forced to wade through a suspension of bacterial cells. Mortality occurred eight days after inoculation and small larvae were more susceptible than larger larvae (Banerjee and Dangar, 1995), probably most likely because of absence of antimicrobial cuticular compounds (Mazza et al., 2011a).The current study was carried out to characterize bacterial flora associated with the RPW in Hail region. Also to identify and screen bio-control bacterial strains. As a result, this can be set as a baseline data on the screening of natural enemies of the RPW in this region.
Materials and Methods
Collection of red palm weevils (RPW) samples
Monthly, 120 adults of the red palm weevils (RPW) were collected from five different infested farms in Hail region, namely: Al Gayed, Jubbah, Helala, Horir and Gutha Sharagiya. The study was conducted during the period October 2017 to December 2018. The pheromone traps were used for the RPW’s collection. In each area, 40 adults of the RPW were separately placed in plastic boxes. The collected RPW were kept in a freezer at −20 °C at the laboratory at the Department of Biology, Faculty of Science at University of Hail until used for investigating the bacteria.
Identification of microorganism flora: Isolation of bacteria
For bacterial isolation, the collected RPW were surface-sterilized with 70% ethanol for 5 min (Poinar and Thomas, 1978) and washed 3 times in sterile distilled water. The bodies of the investigated RPW were homogenized in nutrient broth using a glass tissue grinder, and the homogenate was filtered. Then, 10, 25, and 50 µL of sample extracts was plated on nutrient agar and incubated at 30 °C for 2–3 days. The remaining mixtures were incubated at 30°C for 3–4 h to enrich the number of bacteria that have low concentration. From these mixtures, 10, 25, and 50 µL were also plated on nutrient agar and incubated at 30°C for 2–3 days. Finally, the incubated RPW suspensions were heated in a water bath at 80°C for 10 min to eliminate nonspore-forming bacteria (Ohba and Aizawa, 1986). After heat inactivation, 10, 25, and 50 µL of the heated suspensions were plated on nutrient agar and incubated at 30 °C for 2–3 days. The bacterial Isolates were distinguished according to their colony color and morphology. Pure cultures of bacterial colonies were prepared and stocked in 20% glycerol at –80°C in the Microbiology Laboratory, Department of Biology, and Faculty of Sciences at University of Hail. Bacterial cultures were identified according to their morphology, nutritional features, and biochemical and molecular characteristics.
Molecular identification of the bacterial isolates
Inoculation of isolates: The bacterial isolates were grown on 5 ml tubes contained 2 ml of Luria broth (LB). The tubes containing isolates were incubated horizontally at 37°C for overnight with shaking at 200 rpm in an incubator shaker (Lab-line Instruments, Inc.). To precipitate bacterial pellets for extraction of DNA, 2 ml of LB media was centrifuged at 5000 rpm for 5 min.
DNA Extraction using modified Dellaporta procedure
DNA was extracted using modified method of Dellaporta et al., (1983) as the following protocol: Twenty mg of fresh harvested mycelium or bacterial pellet were ground with pestles in a 1.5 ml tube with 500 μl of Dellaporta buffer (100 mM Tris pH 8. 50 mM ethylenediamine-tetraacetate EDTA, 500 mM NaCl, 10 mM beta mercaptoethanol) (BME).Thirty three μl of 20% sodium dodecyl sulfate (SDS, w/v) were added, and the mixture was vortexed and incubated for 10 min at 65°C.One hundred and sixty μl of 5 M potassium acetate KoAc (Sigma chemicals) were added and vortexed.The mixture was then centrifuged for 10 min at 10,000 rpm, and 450 μl of supernatant was transferred to a new tube. Four hundred and fifty μl phenol, chloroform and isoamyl-alcohol (PCI) were added with a ratio of 25:24:1 and vortex for 5 min and then centrifuged for 5 min at 10,000 rpm. 400 μl of the upper phase were then removed to a clean microcent. The supernatant was removed and the total nucleic acid was precipitated in the bottom of the tube.The pellet was washed with 70% ethanol and spun 5 min at 10,000 rpm. Then, the pellet was re-suspended in 100 μl of Double-distilled water (ddH2O).
Polymerase Chain Reaction (PCR)
Amplification of the 16S rRNA gene from bacterial isolates was carried out using the universal primers; 27F 5′-AGA GTT TGA TCM TGG CTC AG-3′ and 1492R 5′-TACGGYTACCTTGTTACGACTT(Weisburg et al., 1991), in a total 50 μl of PCR reaction. The main PCR steps were programmed as follows: denaturation at 94 °C for 45 s, annealing at 55 °C for 60 s, and extension 72 °C for 60 s. in 30 amplification cycles, followed by a final extension step at 72 °C for 10 min. PCR was conducted in the ESCO Swift Maxi Thermal Cycler with initial denaturation at 95°C for 2 min, followed by 35 cycles of 95°C for 30 sec, 52°C for 30 sec, and 72°C for 30 sec, and the final cycle is a polymerization cycle performed at 72°C for 10 min. PCR Products were purified using QIAquick® PCR Purification Kit (Cat. No. 28106) according to manufacturing procedures.Macrogen Inc., (Korea), sequenced the purified PCR products and sequencing of the purified isolates was performed in both directions using ITS5 and ITS4 primer pairs. Sequence alignments were edited by MEGA7 (Kumar et al., 2016).
Results and Discussion
Rhynchophorus palm weevils are large insects belonging to the family Dryophthoridae, subfamily Rhynchophorinae, and tribe Rhynchophorini (Bouchard et al., 2011). All Rhynchophorus species are polyphagous and have a comparable life history and some of them are significant pests due to the serious economic damage they cause, specifically to several species of the family Arecaceae. In excess of 50 characteristic adversaries have been accounted to attack Rhynchophorus species, among the considered organisms, bacteria is a critical to be considered for incorporation in the integrated pest management programs. In the present study, obtained sequences were edited in MEGA7 and compared to available sequences in GenBank database. The 16S rDNA sequencing showed that bacteria isolated from the investigated RPW were mostly Gram positive and belonged to Proteus mirabilis (33.3%), Klebsiella pneumonia (25%), Serratia marcescens (25%), Staphylococcus sciuri (8.3%) and Providencia rettgeri ( 8.3%) as shown in Fig. 1 and 2.
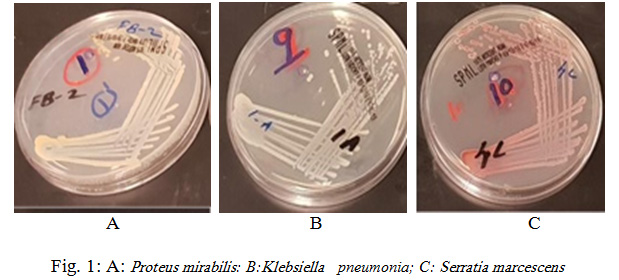 |
Figure 1: A: Proteus mirabilis: B: Klebsiella pneumonia; C: Serratia marcescens |
The dominated bacteria P. mirabilis was found to be on the bodies of the tested dead red palm weevil (RPW) and it was accounted 33.33% of all bacterial isolates from RPW body. Proteus species are part of the Enterobacteriaceae family of gram-negative bacilli. Proteus organisms are embroiled as genuine causes of diseases in humans along with Escherichia, Klebsiella, Enterobacter, and Serratia species. Proteus species are the most regularly found in the human intestinal tract as a component of ordinary human intestinal flora, alongside Escherichia coli and Klebsiella species, of which E. coli is the dominating inhabitant. Proteus is also found in various natural environments, including long-term care facilities and medical clinics (https://emedicine.medscape.com/article/226434-overview).
The most characterizing normal for Proteus microscopic organisms is a swarming marvel, a multicellular differentiation procedure of short rods to extended swarmer cells. It allows population of bacteria to move on strong surface. The virulence of Proteus rods has been related to several factors including fimbriae, flagella, catalysts (urease – hydrolyzing urea to CO2 and NH3, proteases corrupting antibodies, tissue framework proteins and proteins of the supplement system), iron acqusition frameworks and poisons: hemolysins, Proteus poison agglutinin (Pta), as well as an endotoxin – lipopolysaccharide (LPS)
Proteus mirabilis, a Gram-negative rod-shaped bacterium most noted for its swarming motility and urease activity, frequently causes catheter-related urinary tract infections (CAUTI) that are frequently polymicrobial (Chelsie et.al., 2017). P. mirabilis belongs to the class Gammaproteobacteria, and has long been perceived as a member of the order Enterobacteriales, family Enterobacteriaceae. However, one group recently created a reconstructed phylogenetic tree based on shared core proteins, ribosomal proteins, and four multilocus sequence analysis proteins, and has suggested that the order Enterobacteriales be renamed, putting Proteus within a new Morganellaceae family (Adeolu et. al., 2016).
P. mirabilis can be found in a wide assortment of environments, including soil, water sources, and sewage, yet it is transcendently a commensal of the gastrointestinal tracts of humans and animals (Armbruster and Mobley, 2012). While the bacterium is fit for causing an assortment of human diseases, including those of wounds, the eye, the gastrointestinal tract, and the urinary tract, it is most noted for infections of the siphoned urinary tract, known as catheter-associated urinary tract infections (CAUTI) (Warren et.al. 1982; Mobley and Warren 1987; Breitenbucher 1984; Jacobsen 2008; Armbruster et. al., 2016). These infections are common in long-term siphoned patients, for example, the individuals who dwell in nursing homes and chronic care facilities, and may be of particular danger to spinal cord injury patients. During infection, histological damage is brought about by cytotoxins including hemolysin and a assortment of proteases, some autotransported. The pathogenesis of infection including evaluation of individual genes or global screens for virulence or fitness factors has been evaluated in murine models of ascending UTI or CAUTI using both single-species and polymicrobial models. Global gene expression studies carried out in culture and in the murine model have revealed the remarkable metabolism of this bacterium (Chelsie et.al., 2017). Vaccines, utilizing MR/P fimbria and its adhesin, MrpH, have been appeared to be strong in the murine model.
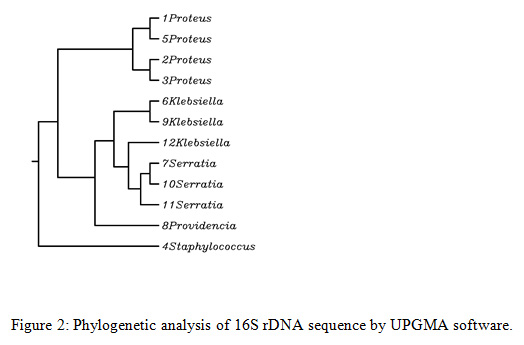 |
Figure 2: Phylogenetic analysis of 16S rDNA sequence by UPGMA software |
Klebsiella pneumoniae was also found in high numbers in the body of the tested RPW. It represented 25% of the total numbers of isolated bacteria. Klebsiella species are Gram-negative coliform bacteria that can cause mastitis, prompting noteworthy economic losses on dairy farms. K. oxytoca and K. pneumoniae are the species that are responsible for causing mastitis. Typical K. pneumoniae is an opportunistic pathogen, which for the most part influences those with debilitated immune systems and will in general reason for nosocomial infections. A subset of hypervirulent K. pneumoniae serotypes with elevated production of capsule polysaccharide can affect influence previously healthy persons and cause hazardous community acquired infections, such as pyogenic liver abscess, meningitis, necrotizing fasciitis, endo- phthalmitis and severe pneumonia. K. pneumoniae uses an assortment of virulence factors, particularly capsule polysaccharide, lipopolysaccharide, fimbriae, outer external membrane proteins and determinants for iron procurement and nitrogen source usage, for survival and immune avoidance during infection (Bei et. al., 2014).
pneumoniae, a member of the family Enterobacteriaceae, is a rod-shaped, Gram-negative, lactose-fermenting bacillus with an unmistakable case. Normal K. pneumoniae is an opportunistic pathogen that is widely found in the mouth, skin and intestines, as well as in hospital settings and medical devices. Opportunistic K. pneumoniae mostly influences those with compromised immune systems or who are weakened by other infections. Colonization of the GI tract by opportunistic K. pneumoniae generally occurs prior to the development of nosocomial infections, and K. pneumoniae colonization can be additionally found in the urinary tract, respiratory tract and blood (Podschun 1998). K. pneumoniae biofilms that structure on therapeutic gadgets (e.g., catheters and endotracheal tubes) provide a significant source of infection in catheterized patients (Schroll et. al., 2010). Nosocomial infections brought about by K. pneumoniae tends to be chronic due to the two following major reasons: K. pneumoniae biofilms formed in vivo protect the pathogen from attacks of the host immune responses and antibiotics (Jagnow and Clegg 2003 ); and nosocomial isolates of K. pneumoniae often display multidrug-resistance phenotypes that are commonly caused by the presence of extended-spectrum β-lactamases or carbapenemases, making it hard to choose appropriate antibiotics for treatment (Paterson et. al., 2004; Munoz-Price et. al. 2013).
Serratia marcescens was also found in high numbers in the body of tested RPW. It represented 25% of the total numbers of isolates. Analysis by the 16S rDNA sequences allotted the selected bacteria to the genus Serratia (family Enterobacteriaceae), with the most noteworthy similitude found for the species Marcescens. The genus Serratia includes, at least, 10 species (Grimont and Grimont 2006). Serratia is a bacterium found in the family Enterobacteriaceae that can cause opportunistic infections even though it is usually a weak pathogen. Analysis by the 16S rDNA sequences, classified our isolates into the species marcescens. The phylogenetic trees dependent on the 16S rDNA and the linked housekeeping gene sequences arranged our strains within the S. marcescens cluster (Figure -2 ).
This cluster was plainly particular from those of the other known red pigment-producing Serratia species (Grimont and Grimont 2006; de Araujo et al. 2010). S. marcescens has likewise been accounted for, at least for some red pigment-producing strains, to display an antimicrobial activity against some gram-positive and gram-negative bacteria (Ibrahim et al. 2014; Lapenda et al. 2015). I then verified if this was also the case for my isolates. S. marcescens is available as extracellular symbiont in various formative phases of the RPW. Additionally, the antimicrobial activity exhibited versus Bacillus spp., Paenibacillus spp., and Lysinibacillus spp., reported as insect pathogens and potential candidates for biocontrol agents, could attribute for S. marcescens a potential protective role (Maria Scrascia et. al. 2016).
Serratia marcescens is among the most widely recognized irresistible agents in infections related with Serratia. They cause infections with noteworthy mortality and morbidity in infants (Edmond et. al., 1999; Roy et. al., 1997; Ania et. al., 2008; Bayramoglu et. al., 2011). Moreover, S. marcescens is a significant irresistible agent that causes hospital‑acquired respiratory and urinary tract infections in neonatal‑adult intensive care unit and immunodeficient patients. The diffuse nearness of S. marcescens inside the infested palms highlighted the capacity of this bacterium to replicate and spread along the palm tissue.
The RPW is a singular insect with no or restricted contact between adult and developing individuals. Solitary insects, moreover to their own safeguards, can make utilize of symbionts to better protect themselves, offspring, or nutritional assets against pathogens, predateors, parasites, or parasitoids (Kellner 2002; Kaltenpoth et al. 2005; Brownlie and Johnson 2009). This protection can be interceded by various mechanisms, which include the production of antimicrobials. The cooperation among microorganisms and hosts have dependably been the object of escalated studies. Specifically, studies on the mutualistic relationships among bacteria and insects have dynamically uncovered the pertinent pretended by the formers on the life cycle of their hosts. The identification of red pigment-producing S. marcescens as extracellular symbiont of the RPW will add to the knowledge on a mutualistic connection among bacteria and the RPW (Maria Scrascia et. al. 2016). It has been reported that populations of certain weevils are sometimes definitely decreased by naturally occurring pathogens, for the most part under conditions for example, delayed high humidity or dense pest populations. Banerjee and Dangar (1995) isolated the bacterium Pseudomonas aeruginosa from naturally infected adults of R. ferrugineus. The bacterium is observed to be pathogenic to adults forced to feed on a suspension of bacterial cells and mortality happened 8 days after ingestion.
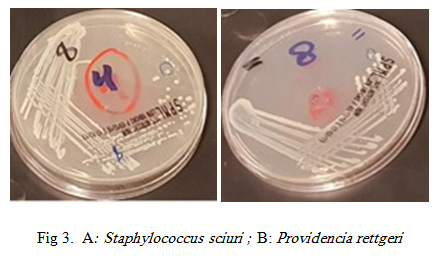 |
Figure 3A: Staphylococcus sciuri ; B: Providencia rettgeri |
Staphylococcus sciuri was identified in 8.3% of the bacterial isolates from the tested RPW body. S. sciuri belongs to the group of oxidase-positive, novobiocin-resistant coagulase negative staphylococci (CoNS) (Stepanovic et. al., 2006). This bacterium is widespread in nature and can be isolated from an assortment of pets, wild and domestic animals, insects, environment (soil, sand, water, air samples, etc.), and foods (Stepanovic et. al., 2006, Stepanovic et. al. 2005).
It has additionally been recuperated from the hospital environment (Dakic et. al., 2005) and in spite of the fact that S. sciuri is just occasionally isolated from humans, it has been associated with a number of serious infections such as septicemia, endocarditis, peritonitis, pelvic inflammatory disease, urinary tract infections and wound infections (Severin et. al., 2010; Stepanovic et. al., 2005). However, there is a little information regarding the pathogenicity of S. sciuri. Members of the S. sciuri group are widely distributed in nature, and they can be isolated from a variety of animals and the products of animal origin (Takeuchi et. al., Gianneechini et. al., 2002; Waage et. al., 1999) as well as from human (Stepanovic et. al., 2001; Couto et. al., 2000), but most of them are a pathogenic to animals. It has been reported that numerous bacteria pathogens to insects are dynamic makers of secondary metabolites harmful to insects or other organisms that can be utilized as novel particles for controlling both plant pathogens and pests (Bode, 2009).
Providencia represents a genus of urease producing, gram negative bacilli which although rare, are very omnipresent in the environment. Providencia species intently take after Proteus and Morganella species. They are often isolated from wounds, respiratory tract and urinary tract (P. alcalifaciens, P. rettgeri and P. stuartii), stool of humans (P. alcalifaciens), poultry, faeces from reptiles (P. rettgeri), throat, perineum, axilla and blood of humans (O’Hara et. al., 2000). A report from Nepal in 2014, a cluster of surgical infections with regards to the isolation of P. rettgeri, demonstrated the presence and significance of this organism in the Asia-Pacific region (Tada et al., 2014). P. rettgeri has been involved in the etiology of gastrointestinal sickness in 1986, traveler’s diarrhea in 2004, and ocular infection in 2006 (Muller 1986; Yoh et. al., 2005; Koreishi et al., 2006 ). P. rettgeri has additionally been involved as a causative agent of “purple bag syndrome”, where the enzymatic activity gives rise to a purple tinged urine (Peters et. al., 2011).
There are not many records about the occurrence of natural enemies of R. ferrugineus, which may be ascribed to the mysterious living space of the eggs, larvae and pupae, which protect them from such common adversaries. Ordinarily, the natural enemies do not play an important part in controlling of R. ferrugineus. Reginald (1973) suggested that natural enemies don’t have a significant impact in controlling world’s worst pest of palm trees, R. ferrugineus (RPW) and few studies have been conducted on natural enemies of Rhynchophorus (Murphy and Briscoe, 1999; Faleiro, 2006a,b). In this study, bacteria associated with the red palm weevils have been investigated by considering their pitfalls and potentialities in order to pinpoint management techniques to be considered in the development and reconciliation of biological control procedures.
Conclusion
The study demonstrated the many pathogenic bacteria were associated with the red palm weevil, Rhynchophorus ferrugineus Olivier adults, however, the pathogenicity of these bacteria could be attributed to the production of secondary metabolites harmful to the RPW. The extraction and recognizable proof of secondary metabolites delivered by the entomopathogenic bacteria isolated in this study as well as the in vivo activity of bacterial cells against R. ferrugineus require further investigation.
Acknowledgements
I am very grateful to the Deanship of Scientific Research (DSR) in the University of Hail who funded this research project (Number: BA-1511).
References
Adeolu M, Alnajar S, Naushad S, Gupta SR., ( 2016). Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. International Journal of Systematic and Evolutionary Microbiology.; 66:5575–5599. [PubMed: 27620848]
Alfazairy, A.A., Hendi, R., El-Minshawy, A.M., Karam, H.H., (2003). Entomopathogenic agents isolated from 19 coleopteran insect pests in Egypt. Egypt. J. Biol. Pest Control 13, 125.
Alfazariy, .A., (2004). Notes on the survival capacity of two naturally occurring entomopathogens on the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Egypt. J. Biol. Pest Control 14, 423.
Al-Shawaf, A. M., A. Al-Shagag, M. Al-Bagshi, S. Al-Saroj, S. Al-Bather, A. M. Al-Dandan, A. Ben Abdallah and J. R. Faleiro. (2013). A quarantine protocol against red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleptera: Curculiondae) in date palm. J. Plant Prot. Res. 53(4): 409-415.
Anía BJ. Serratia: Overview. eMedicine, WebMD 2008; (Retrieved 10.13. 08).
Armbruster CE, Mobley HLT(2012). Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Micro. 10:743–754.
Armbruster CE, Prenovost K, Mobley HLT, Mody L. How Often Do Clinically Diagnosed CatheterAssociated Urinary Tract Infections in Nursing Home Residents Meet Standardized Criteria? J Am Geriatr Soc. (2016); doi: 10.1111/jgs.14533.
Asiry, K. A. (2015). Species Richness and Abundance of Hymenopterous Parasitoids of the Family of Braconidae (Subfamily Aphidiinae) Within A barley Agro-ecosystem in Hail Region, Saudi Arabia. Egypt. Acad. J. Biolog. Sci., 8(1): 83-90.
Bale, J. S., van Lenteren, J. C. & Bigler, F. (2008). Biological control and sustainable food production. Philosophical Transactions of the Royal Society B-Biological Sciences 363(1492): 761-776.
Banerjee A. Dangar TK. (1995). Pseudomonas aeruginosa, a facultative pathogen of red palm weevil, Rhynchophorus ferrugineus. World J Microbiol Biotechnol.11:618–620. [PubMed]
Bayramoglu G, Buruk K, Dinc U, Mutlu M, Yilmaz G, (2011)Aslan Y Investigation of an outbreak of Serratia marcescens in a neonatal intensive care unit. J Microbiol Immunol Infect;44:111‑5
Bei Li, Yuling Zhao, Changting Liu, Zhenhong Chen and Dongsheng Zhou (2014). Molecular pathogenesis of Klebsiella pneumonia. Review. Future Microbiol. 9(9), 1071–1081.
Bianchi, F., Booij, C. J. H. & Tscharntke, T. (2006). Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proceedings of the Royal Society B-Biological Sciences 273(1595): 1715-1727.
Bode H.B., 2009. Enthomopathogenic bacteria as a source of secondary metabolites. – Curr. Op. Chem. Biol., 13: 224-230.
Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B.,(2011) Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children., Environmental Health Perspectives, 119:8, DOI: 10.1289/ehp.1003185.
Breitenbucher RB. Bacterial (1984) changes in the urine samples of patients with long-term indwelling catheters. Arch Intern Med. 144:1585–1588. [PubMed: 6331806]
Chelsie E. Armbruster, Harry L. T. Mobley, and Melanie M. Pearson (2017). Pathogenesis of Proteus mirabilis Infection. EcoSal Plus. 2018 February ; 8(1): . doi:10.1128/ecosalplus.ESP-0009-2017.
Couto I, Sanches IS, Sa-Leao R, de LH (2000) Molecular characterization of Staphylococcus sciuri strains isolated from humans. J Clin Microbiol 38: 1136–1143
Dakic I, Morrison D, Vukovic D, Savic B, Shittu A, Jezek P (2005) Isolation and molecular characterization of Staphylococcus sciuri in the hospital environment. J Clin Microbiol. 43: 2782–2785. https:// doi.org/10.1128/JCM.43.6.2782-2785.2005 PMID: 15956397
de Araujo, H. W., K. Fukushima, and G. M. Takaki. (2010). Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules 15:6931–6940.
DeBach, P. & Rosen, D. (1974). Biological control by natural enemies. New York: the Press Syndicate of the University of Cambridge.
Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP.(1999) Nosocomial bloodstream infections in United States hospitals: A three‑year analysis. Clin Infect Dis ;29:239‑44. 4.
Faleiro, J.R., (2006). A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci., 26: 135-154.
Gianneechini R, Concha C, Rivero R, Delucci I, Moreno LJ (2002) Occurrence of clinical and sub-clinical mastitis in dairy herds in the West Littoral Region in Uruguay. Acta Vet Scand 43: 221–230.
Gindin G., Levski S., Glazer I., Soroker V., (2006) Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus.- Phytoparasitica, 34: 370-379
Grimont, F., and P. A. D. Grimont. (2006) . The genus Serratia. Springer New York, New York. Hung EW, Darouiche RO, Trautner BW. Proteus bacteriuria is associated with significant morbidity in spinal cord injury. Spinal Cord. 2007; 45:616–620. [PubMed: 17179975]
Güerri-Agulló B., Asensio L., Barranco P., Gómez-Vidal S., Lopez-Llorca L. V., (2008) Use of Beauveria bassiana as a tool for biological control of Rhynchophorus ferrugineus, pp. 125. In: 41st Annual Meeting of Society for Invertebrate pathology and 9th international Conference on Bacillus thuringiensis, Warwick, UK, 3-7 August, 2008. (https://emedicine.medscape.com/article/226434-overview
Ibrahim, D., T. F. Nazari, J. Kassim, and S. H. Lim. (2014). Prodigiosin - an antibacterial red pigment produced by Serratia marcescens IBRL USM 84 associated with a marine sponge Xestospongia testudinaria. J. Appl. Pharm. Sci. 4:001–006
Jacobsen SM, Stickler DJ, Mobley HLT, Shirtliff ME. (2008) Complicated Catheter-Associated Urinary Tract Infections Due to Escherichia coli and Proteus mirabilis. Clin Microbiol 21:26–59. [PubMed: 18202436]
Jagnow J, Clegg S. (2003) Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology 149(Pt 9), 2397–2405.
Koreishi AF, Schechter BA, Karp CL. (2006) Ocular infections caused by Providencia rettgeri. Ophthalmology. 113(8):1463-66.
Lapenda, J. C., P. A. Silva, M. C. Vicalvi, K. X. Sena, and S. C. Nascimento. (2015). Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 31:399–406.
Manachini, B., Arizza, V., Parrinello, N., (2008a). Sistema immunitario del Punteruolo Rosso (Rhynchophorous ferrugineus). In: La ricerca scientifica sul punteruolo rosso e gli altri fitofagi delle palme in Sicilia, Regione Siciliana-Assessorato Agricoltura e Foreste Dipartimento Interventi Infrastrutturali, Servizi allo Sviluppo, Vol. I, pp. 133–136.
Mazza, G., Arizza, V., Baracchi, D., Barzanti, G.P., Benvenuti, C., Francardi, V., Frandi, A., Gherardi, F., Longo, S., Manachini, B., Perito, B., Rumine, P., Schillaci, D., Turillazzi, S., Cervo, R., (2011a). Antimicrobial activity of the red palm weevil Rhynchophorus ferrugineus. Bull. Insectol. 64, 33–41.
Mazza, G., Francardi V,m Simoni., Benvenuti C. Cervo R., Faleiro J.R., Llacer E., Longo S., Nannelli R., Tarasco E , Roversi PO.F., (2014) An overview on the natural enemies of Rhynchophorous palm weevils, with focus on R. ferrugineus. – Biol. Control, 77: 83-92.
Mahmoud, Y. A., Salama, H. S., Moawed, S. M., Ebadah, I. M. A., Sadek, H. E., & Khalifa, I. A. (2018). Virulence of a New Isolate of Cytoplasmic Polyhedrosis Virus against the Red Palm Weevil, Rhynchophorus ferrugineus (Oliv.) (Order: Coleoptera, Family: Curculionidae). Asian Journal of Agricultural and Horticultural Research, 2(2), 1-10.
Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L. (2016). Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front Public Health. 2016 Jul 18;4:148. doi: 10.3389/fpubh. 00148. PubMed PMID: 27486573; PubMed Central PMCID: PMC4947579.
Ohba M and Aizawa K (1986) Insect toxicity of Bacillus thuringiensis isolated from soils of Japan. J. Invertebr. Pathol. 47 12–20 Poinar, G.O. Jr, and Thomas, G.M., in: Diagnostic Manual for the identification of Insect Pathogens, p. 218. Plenum Press, New York (1978).
Kumar S., Stecher G., and Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33:1870-1874. (https://emedicine.medscape.com/article/226434-overview
Maria Scrascia, Carlo Pazzani, Franco Valentini, Marta Oliva, Valentina Russo, Pietro D’Addabbo1 & Francesco Porcelli (2016). Identification of pigmented Serratia marcescens symbiotically associated with Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae). MicrobiologyOpen; 5(5): 883–890.
Mobley HLT, Warren JW (1987). Urease-Positive Bacteriuria and Obstruction of Long-Term Urinary Catheters. Journal of Clinical Microbiology; 25:2216–2217. [PubMed: 3320089]
Muller HE (1986). Occurrence and pathogenic role of Morganella-Proteus-Providencia group bacteria in human feces. Journal Of Clinical Microbiology;23(2):404- 05.
Munoz-Price LS, Poirel L, Bonomo RA (2013) Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13(9), 785–796 .
Nicolle LE. Catheter-Related Urinary Tract Infection. Drugs & Aging. (2005); 22:627–639. [PubMed: 160607
O’Hara CM, Brenner FW, Miller JM (2000). Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clinical microbiology reviews. ;13(4):534-46.
Paterson DL, Ko WC, Von Gottberg A (2004). International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Intern. Med. 140(1), 26–32.
Peters P, Merlo J, Beech N, Giles C, Boon B, Parker B, (2011). The purple urine bag syndrome: a visually striking side effect of a highly alkaline urinary tract infection. Canadian Urological Association Journal. 5(4):233-34
Podschun R, Ullmann U. (1998). Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11(4), 589–603
Reginald, C., (1973). Principal Insect Pests. In: Coconuts. Tropical Agriculture Series, Reginald, C. (Eds.). Longmans, London.
Royo P, Boquete T, (1997) Epidemiology of Serratia marcescens between 1987 and 1995 at Vall d’Hebron Hospital. Enferm Infecc Microbiol Clin;15:519‑27.
Salama HS, Foda MS, ElBendary MA. AbdelRazek A, (2004). Infection of red palm weevil, Rhynchophorus ferrugineus, by sporeforming bacilli indigenous to its natural habitat in Egypt. J Pest Sci. 77:27–31.
Salama, H.S. and Abd-Elgawad, M. (2002) Activity of heterorhabditid nematodes at high temperature and in combination with cytoplasmic polyhedrosis virus. Pestic. Sci.75:78.
Tanada, Y., & Kaya, H. K. (1993). Insect Pathology. San Diego: Academic Press.
Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J, (1991). 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol.;17:697–703.
White, T. J., Bruns, T., Lee, S. J. W. T., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications, 18(1), 315-322.
Schroll C, Barken KB, Krogfelt KA, Struve C. (2010). Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 10, 179.
Severin JA, Lestari ES, Kuntaman K, Pastink M, Snijders SV, Lemmens-den Toom N, (2010). AMRIN Study Group. Nasal carriage of methicillin-resistant and methicillin-sensitive strains of Staphylococcus sciuri in the Indonesian population. Antimicrob Agents Chemother. 54: 5413–5417. https://doi. org/10.1128/AAC.00426-10 PMID: 20837756
Stepanovic S, Dakic I, Morrison D, Hauschild T, Jezek P, Petra´s P, (2005). Identification and characterization of clinical isolates of members of the Staphylococcus sciuri group. J Clin Microbiol. 43: 956– 958. https://doi.org/10.1128/JCM.43.2.956-958.2005 PMID: 15695717.
Stepanovic S, Hauschild T, Dakic I, Al-Doori Z, Svabic-Vlahovic M, Ranin L, (2006). Evaluation of phenotypic and molecular methods for detection of oxacillin resistance in members of the Staphylococcus sciuri group. J Clin Microbiol. 2006; 44: 934–937. https://doi.org/10.1128/JCM.44.3.934-937. PMID: 16517879
Murphy, S. T., and B. R. Briscoe. (1999). The red palm weevil as an alien invasive: biology and the prospects for biological control as a component of IPM. Biocontrol News and Information, 20(1):34n-46n.
Tada T, Miyoshi-Akiyama T, Dahal RK, Sah MK, Ohara H, Shimada K, (2014) . NDM-1 Metallo-beta-Lactamase and ArmA 16S rRNA methylase producing Providencia rettgeri clinical isolates in Nepal. BMC infectious diseases.;14:56.
Takeuchi S, Kobayashi Y, Morozumi T (1987) Proteolytic zymograms of Staphylococcus hyicus subsp. hyicus isolated from pigs, chickens and cows. Vet Microbiol 14: 47–52.
Ijaz Ul Haq and Sumaira Shams and Saima Khan and Asar Khan and Asif Hameed (2018). A novel report on morphological study of Red Palm Weevil (Rhynchophorus ferrugineus) from district Bannu KPK, Pakistan, Cogent Food & Agriculture,4:1 1425117-1425124.
Waage S, Mork T, Roros A, Aasland D, Hunshamar A, et al. (1999) Bacteria associated with clinical mastitis in dairy heifers. J Dairy Sci 82: 712–719
Yoh M, Matsuyama J, Ohnishi M, Takagi K, Miyagi H, Mori K, (2005) . Importance of Providencia species as a major cause of travellers’ diarrhoea. Journal Of Medical Microbiology. 54(Pt 11):1077-82.
Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC.(1982). A Prospective Microbiologic Study of Bacteriuria in Patients with Chronic Indwelling Urethral Catheters. Journal of Infectious Diseases. 146:719–723. [PubMed: 6815281]


