1Centre for Aquaculture, Col. Dr. Jeppiaar Research Park, Sathyabama Institute of Science and Technology, Jeppiar Nagar, Chennai – 600119,
Tamilnadu, India
2Department of Biotechnology, School of Bio and Chemical Engineering, Sathyabama University, Jeppiar Nagar, Chennai – 600119, Tamilnadu, India.
3CAS in Marine Biology, Faculty of Marine Sciences, Annamalai University, Parangipettai-608502, Tamil Nadu, India.
Corresponding author email: raji.shwehari@gmail.com
Article Publishing History
Received: 01/04/2020
Accepted After Revision: 27/05/2020
In the present study, stimulatory effect through dietary administration of probiotic bacteria, Bacillus firmus CAS 7 on growth, survival and skin colour of the tomato clown Amphiprion frenatus was investigated. A total of four different experiments with different concentrations of probiotic B. firmus with with basal diet (25, 50, 100 and 150 mg kg−1) and control (without probiotic) were planned and the fishes were fed for 120 days. The results obtained from the present study suggested that the fishes fed with the diets supplemented with probiotic at 100 and 150 mg kg−1 exhibited similar weight gain (21.06 ± 1.4 and 21.09 ± 1.5), specific growth (0.176), survival rate (100%) and feed conversion ratio (0.109), but higher than the control and other experiments. The carotenoid content in fish skin was comparatively higher in experiment III and IV (6.79 mg/g) than other experiments and control (4.48 mg/g). Although the stimulatory effect in terms of growth, survival and colour of fishes fed with diets containing 100 and 150 mg kg−1 of probiotic was higher than control and lower concentration, there was no significant difference between them and hence, it is recommended to use at a concentration of 100 mg kg−1 for the enhanced growth and color of the tomato clown Amphiprion frenatus which has significant importance in ornamental fish industry. Further study on immune stimulatory effect of this probiotic is under progress for its application in large scale to prevent any undesired effects.
Amphiprion frenatus, Probiotic, Bacillus firmus, Growth, Skin Color, Carotenoids.
Rajeswari M. V, Mitra A, Kanwar D, Aruni A. W, Balasubramanian T. Stimulatory Effect of Probiotic Bacterium Bacillus firmus CAS 7 on Growth, Survival and Colour of Tomato Clown Amphiprion frenatus (Brevoort, 1856). Biosc.Biotech.Res.Comm. 2020;13(2).
Rajeswari M. V, Mitra A, Kanwar D, Aruni A. W, Balasubramanian T. Stimulatory Effect of Probiotic Bacterium Bacillus firmus CAS 7 on Growth, Survival and Colour of Tomato Clown Amphiprion frenatus (Brevoort, 1856). Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2yO2T28
Copyright © Rajeswari et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The demand for marine ornamental fishes has gained a thrust among aquarium hobbyists due to their multitudinal colour and beauty. The annual worldwide market for ornamental marine reef fish has shown a steady increase over the past few years. Therefore, research on the commercial rearing of these fishes is an imminent necessity to save this fragile ecosystem. However, till date efforts in this direction have been extremely limited to very few coral fishes like damsels, neon gobies etc. and that too only in temperate conditions (Hunziker, 1990; Danillowicz & Brown, 1992; Ignatius, 2001).
Probiotics in aquaculture have been reported to provide beneficial effects and the use of probiotics is an important management tool in ornamental fish culture (Balcazar et al., 2006). In general, probiotic administration during early developmental stages is most effective, usually resulting in greater than an order of magnitude increases in survivorship (Gatesoupe, 2007). It has also been reported that in captive rearing, higher mortality occurs frequently (Benetti et al., 2008), and growth abnormalities lead to higher incidence of skeletal deformities (Fernandez et al., 2008). Probiotics as feed supplements benefit the host by improving the feed value, enzymatic contribution to digestion, inhibition of pathogenic microorganisms, antimutagenic and anticarcinogenic activity, growth promoting factors and increasing immune response (Harikrishnan et al., 2010).
Several reports suggested that the probiotics supplementation can reduce the cost of culture by improving the growth and feed utilization efficiency of fish (Mazurkiewicz et al., 2007). The genus, Bacillus as putative probiotics has been used extensively as aquaculture feed additives, because of its resistance to high temperature and high pressure (Rengpipat et al., 2000). Dietary supplementation of Bacillus spp. improved the growth performance, immunity and disease resistance of fish fish (Ai et al., 2011; Geng et al., 2012). In rainbow trout (Onchorhynchus mykiss), significant improvement of feed conversation ration (FCR), specific growth rate (SGR) and protein efficiency ratio (PER) was observed when the fish was fed with diets containing Bacillus spp (Bagheri et al., 2008; Merrifield et al., 2010).
Carotenoids, which are lipid soluble pigments, are responsible for skin colour of ornamental fishes, and can determine their commercial value (Paripatanamont et al., 1999). The ornamental fishes are unable to perform de novo synthesis of carotenoids (CD) like other animals and therefore rely on dietary supply to achieve their natural pigmentation (Goodwin, 1984). Under intensive farming conditions and aquarium rearing, ornamental fish are fed exclusively with compound feeds, which must therefore be supplemented with carotenoids (Wang et al., 2006). The spore-forming Bacillus species capable of synthesizing carotenoid pigments and the biochemical analysis on the carotenoids responsible for the yellow/orange pigmentation present in Bacillus sp. has been carried out and the identity of the carotenoids was elucidated (Perez-Fons, L., 2011).
Tropical marine species are beautiful as some marine species, such as members of Pomacentridae, are important in the world trade for ornamental fish (Wilkerson, 2001), and a popular subject of research (Arvedlund et al., 2000). Of which, clown fishes are considered to be most attractions of aquarists, and they are important in the aquarium trade in view of their bright colour, interesting display behavior and their ability adopt in captive conditions (Wilkerson, 1998). The tomato clownfish or, after its scientific name, Amphiprion frenatus (Brevoort, 1856), also known as black back anemonefish, fire clown, one band anemonefish, or red clown are under Order Perciformes (Jung, 2006). The tomato clown fish is considered to be major candidate species in ornamental fish industry and it plays a major role in world aquarium trade. Although the utility of probiotics has been recognized in aquaculture by several researchers worldwide, no attempts were made to improve its growth, survival and color through probiotic administration till date. In the present study, an attempt was made to investigate stimulatory effect of probiotic bacteria B. firmus CAS 7 on growth, survival and skin color of tomato clown, Amphiprion frenatus (Brevoort, 1856).
MATERIALS AND METHODS
Isolation and culture of probiotic bacteria B. firmus CAS 7
The probiotic bacteria, B. firmus CAS 7 was isolated from marine environment and identified by both conventional (morphology, physiology and biochemical) and molecular approaches (16S rRNA gene sequence). The 16S rRNA gene sequences of the probiotic strain CAS 7 obtained from the present study was deposited in NCBI with accession number HQ116811. Further, the probiotic strain B. firmus CAS 7 was cultured and prepared as described by (Sun et al., 2010). 500 mL of fresh nutrient broth was seeded with 1% inoculum (1.50 x 106CFU mL-1) and kept in a shaker incubator (200 rpm) at pH 7.5, temperature 28 oC, and salinity 30 PSU for 48 h. After incubation period, the cells were harvested by centrifugation at 5000 xg for 10 min, washed twice with phosphate-buffered saline (pH 7.5) and re-suspended in same PBS buffer. The growth of probiotic bacteria was estimated by measuring optical density at 600 nm from the aliquots withdrawn at every 6 h intervals.
Preparation of control and probiotic feed
The control basal diet was formulated using the ingredients such as fish meal, shrimp meal, soya bean meal, wheat flour, fish oil and vitamin mineral mix (Table1) (Sun, Y.Z., et al., 2010).
All the ingredients were dried overnight at 80º C in a hot air oven and powdered. The powdered ingredients were sieved through a fine-meshed screen (0.5 mm diameter) and mixed well. The dough was prepared by adding required amount of water with the ingredients, sterilized (autoclave at 121º C for 15 min) and incorporated with 3% (v/w) commercial vitamin mineral mix (EMIX PLUS, Mumbai, India) and pelletized using hand pelletizer to obtain 1 mm pellets. The pellets were initially sun dried and then oven dried at 60 ± 5ºC for 12 hours to get moisture content. Further, they were manually broken into smaller bits and stored at room temperature in an air tightened sterile polypropylene containers. The test feeds for the experiments were prepared by gently spraying the required amount of bacterial suspension on the control diet and mixing it part-by-part in a drum mixer to obtain a final concentration of 25, 50, 100 and 150 mg kg-1 in experiments I, II, III and IV respectively. The probiotic cell suspensions were added to the control diet after the dosage had been autoclaved and subsequently cooled, before pelletizing. The proximate composition (moisture, protein, ash, lipid and fibre) of all probiotic feeds and control feed were determined by the standard procedures of AOAC (1990). The probiotic strain-incorporated feeds were packed in sterile polypropylene containers and stored at 4ºC for viability studies.
Experimental setup
The juveniles of tomato clown Amphiprion frenatus (Brevoort, 1856) were obtained from MAV Breeders (Mandabam, Tamilnadu, India) and acclimatized for 4 weeks before the trial at Aquaculture breeding center, CAS in Marine Biology, Annamalai University, India. The feeding experiment was conducted in (20 L) rectangular fibreglass tanks, with temperature ranging from 26 – 30oC, salinity 28 -30 PSU, pH 7.4 – 7.8; and Dissolved oxygen 4.2 to 5.6 mg L-1. A total of 30 fish seeds were maintained in each tank throughout the experiment and each treatment was conducted in triplicate. A total of five trials were made during the study (control and four as experiments). The fishes were feed with prepared pellet feed alone in control and feed contains probiotic at a concentration 25, 50, 100 and 150 mg kg-1 in experiments I, II, III and IV respectively. The feeding rate was about 3% of biomass per day provided in equal rations at 8.00 AM, 1.00 PM, 6.00 PM for 120 days and the excess diet was collected and dried at 60 ºC, put in room temperature for 3 days to restore the natural moisture and then weighed. Daily feed was adjusted every 30 days by batch weighing of fish in each tank after a 24 h period of starvation. Experimental tanks were cleaned and water exchange was done once a week.
Growth indices
The growth parameters such as weight gain, specific growth rate (SGR), survival rate and feed conversion ratio (FCR) were assessed at 30, 60, 90 and 120 days. The weight gain (WG), specific growth rate (SGR) and feed conversion ratio (FCR) was evaluated based on standard formula as follows.
Weight gain = (Final weight – Initial weight)
Specific Growth Rate (SGR) = 100 (ln W2 – lnW1)/T
where, W1 and W2 are the initial and final weight, respectively, and T is the number of days in the feeding.
Feed conversion ratio FCR= total feed quantity given (g)/total weight gain (g).
Colour enhancement and carotenoid content estimation
The color enhancement was monitored by visual examination and estimation of carotenoid content in the skin of experimental fishes. The carotenoid content of the experimental fish skin was extracted according to the method of Torrissen and Naevdal (1984). The fishes were randomly sampled from each experiment per sampling period (30, 60, 90 and 120 days) and used for carotenoid content analysis in triplicate. Briefly, 2 mg of skin were collected from both sides between the abdominal and dorsal regions of the fish and then transferred to 10 mL of pre – weighed glass tubes after the fat layer had been removed from the skin and ground well with acetone containing anhydrous sodium sulphate and made up to 10 mL with acetone. The samples were stored for 3 days at 4oC in a refrigerator, and then extracted three times till no further colour could be obtained and centrifuged at 5000 xg for 5 min. The total carotenoid content of the samples was determined using spectrophotometer (Shimadzu, UV mini 1240) using extinction coefficients (E1%, 1 cm) of 2000 for astaxanthin (Hata & Hata, 1971) at 475 nm, and 2500 for carotenoids from alfalfa at 450 nm (Schiedt & Jensen, 1995).
RESULTS
Isolation and growth of probiotic bacteria B. firmus CAS 7
The bacterial strain was isolated from marine sediments of Parangipettai, Tamil Nadu, India and identified as B. firmus based on the morphology, physiological and 16S rRNA analysis (GenBank accession no. HQ116805) and was designated as B. firmus CAS 7. The growth of the bacteria was started from the stationary phase itself and it reached the maximum during logarithmic phase (24 – 30 h) (Fig.1). After incubation, cells were harvested from the culture broth by centrifugation (6000 xg), washed and resuspended with PBS buffer after the incubation and added to the basal diet at desired concentration and used for further study.
Growth analysis
In the present study, the fishes were fed with basal diet supplemented with probiotic at four different experiment and control (without probiotic) for 120 days and the growth parameters such as weight gain, SGR and FCR were determined and the results are presented in Table 3. The results suggested that the weight of experimental fishes was increased with the increasing days of culture in control and experiments. It was reported that there was significant weight gained in all experiments where fishes were fed with probiotic supplemented basal feed than control. The weight gain was found to be higher in experiment IV (21.09±1.5) followed by III (21.06±1.4), II (18.37±1.3), I (13.78±1.2) and control (10.81±1.4). Similarly, the fishes in experiment IV and III had significantly higher SGR (0.176) when compared to the fishes in experiment II (0.153), I (0.115) and control (0.090). Furthermore, feed conversion ratio (FCR) was similar in experiment IV and III (0.29) which comparatively lower than that of experiment II (0.33), I (0.44) and control (0.56). Likewise, survival rate in experiment IV, III, II, I and control was about 100, 100, 85,60 and 60% respectively. It seems that there was no mortality in experiment III and IV, whereas it was higher in experiment I, II and control setup. It seems that the probiotic supplemented in feed significantly stimulated the growth and survival of fishes in experiment III and IV. Although weight gain, SGR, FCR and survival rate was significantly higher in experiment III and IV than control and other experiments, no significant difference was reported when the feed supplemented with 100 and 150 mg kg-1 of probiotic.
Carotenoid content analysis
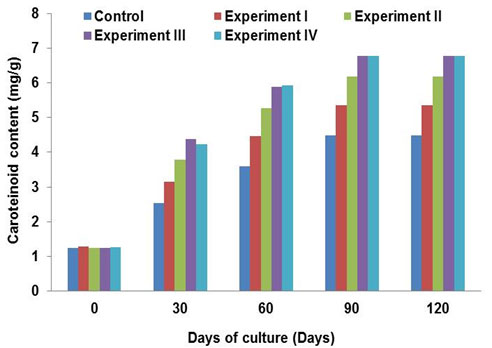
The results of the carotenoid content of skin suggested that the initial carotenoid content of the fish skin varied between 1.24 and 1.28 mg g-1 in experiments and control and it was increased gradually with increasing days of culture. Further, carotenoid content in experiments I, II, III, IV and control were about 5.3, 6.18, 6.79, 6.80 and 4.48 mg g-1 respectively (Fig. 2) at the end of experiment. It seems that the carotenoid content of fish skin in experiment III and IV where the feed was supplemented with 100 and 150 mg kg-1 of probiotic was comparatively higher than control and other experiments.
Table 1. Ingredients used for feed formulation and proximate composition of the prepared basal diet
| Ingredients | (g kg-1) | Proximate composition of the basal diet | (g kg-1) |
| Fish meal | 600 | Dry matter | 941 |
| Shrimp meal | 160 | Crude protein | 491 |
| Soybean meal | 20 | Crude lipids | 98 |
| Wheat flour | 140 | Ash | 96 |
| Fish oil | 40 | ||
| Soybean phospholipids | 20 | ||
| Vitamin mineral mix | 10 |
Vitamin mineral mix (EMIX PLUS, Mumbai, India) (Quantity per kg)
Vitamin A: 22 00 000 IU; Vitamin D3: 4 40 000 IU; Vitamin B2: 800 mg; Vitamin E: 300 mg; Vitamin K: 400 mg; Vitamin B6: 400 mg; Vitamin B12: 2.4 mg; Calcium Pantothenate: 1000 mg; Nicotinamide: 4 g; Choline Chloride: 60 g; Mn: 10 800 mg; I: 400 mg; Fe: 3000 mg; Zn: 2000 mg; Cu: 800 mg; Co: 180 mg; Ca: 200 g; P: 120 g; L L-lysine: 4 g; DL-Methionine: 4 g; Selenium: 20 ppm.
DISCUSSION
In the past decade of years, there are few reports on successful larval rearing of marine ornamental fishes such as Amphiprion clarkii, A. percula (Alava & Gomes, 1989; Malpass, 1996; Allen, 1998), Dascyllus albisella and D. aruanus (Danillowicz & Brown, 1992) in temperate waters. The benefits of probiotics in fish farming are improvements of growth performances, immunity and pathogen exclusions (Qi et al., 2009; Sun et al., 2010). Bacillus species significantly improved the growth in tilapia (Aly et al., 2008), Catla catla (Bandyopadhyay & Mohapatra, 2009), Labeo rohita (Ghosh et al., 2003), Macrobrachium rosenbergii (Keysami, M. A. et al., 2007) and Penaeus monodon (Rahiman, K. M. M. et al., 2010).
Table 2. Morphological, physiological and biochemical characteristics of the probiotic strain CAS 7
| Characteristics | Results |
| Shape | Rod |
| Gram stain | Positive |
| Spore formation | + |
| Motility | + |
| Glucose | + |
| Mannitol | + |
| Xylose | + |
| Starch Hydrolysis | + |
| Gelatin Hydrolysis | + |
| Fat Hydrolysis | + |
| Casein Hydrolysis | + |
| Catalase activity | – |
| Nitrate reduction | + |
| Indole | + |
| Citrate | + |
The probiotic bacterium, B. firmus CAS 7 isolated from marine environment was used as feed supplement to evaluate the stimulatory effect on growth, survival and skin colour of tomato clown Amphiprion frenatus (Brevoort, 1856). The growth studies suggested that the maximum cell growth was achieved at logarithmic phase (24th h). Similarly (Elayaraja, S. et al., 2011), studied the effect of B. cereus on the growth of polycheate and maximum cell growth as well as enzyme production at late logarithmic phase (36th h). Thus, maximum growth of this bacterium at shorter incubation period makes this a potential probiotic candidate species which could be used in the aquaculture industry.
Table 3. Growth and survival of tomato clown Amphiprion frenatus (Brevoort, 1856) fed with control (without probiotic) and experiments [basal diet supplemented with probiotic B. firmus CAS 7- 25 mg/kg (Experiment – I), 50 mg/kg (Experiment – II), 100 mg/kg (Experiment – III) and 150 mg/kg (Experiment – IV). The values are presented in mean ± SD, n = 3, FCR – feed conversion ratio; SGR – specific growth rate.
| Days of culture | Growth Parameters | Control | Experiment I
25 mg kg−1 |
Experiment II
50 mg kg−1 |
Experiment III
100 mg kg−1 |
Experiment
IV 150 mg kg−1 |
| 0-30 | Initial weight (g) | 7.33±0.38 | 7.61±0.31 | 7.57±0.29 | 7.63±0.32 | 7.89±0.41 |
| Final weight(g) | 10.27±1.6 | 11.89±1.1 | 13.49±1.2 | 15.53±1.4 | 15.94±1.1 | |
| Weight gain (g) | 2.94±1.6 | 4.28±1.3 | 5.92±0.29 | 7.9±0.30 | 8.05.24±1.2 | |
| SGR | 0.098 | 0.143 | 0.197 | 0.263 | 0.268 | |
| FCR | 0.061 | 0.089 | 0.123 | 0.164 | 0.167 | |
| Survival rate (%) | 60 | 60 | 85 | 90 | 90 | |
| 0-60 | Initial weight (g) | 7.33±0.38 | 7.61±0.31 | 7.57±0.29 | 7.63±0.32 | 7.89±0.41 |
| Final weight(g) | 12.83±1.5 | 15.13±1.3 | 16.92±1.6 | 20.57±1.1 | 21.56±1.3 | |
| Weight gain (g) | 5.5±1.5 | 7.52 | 9.35±1.2 | 12.94±1.4 | 13.67±1.4 | |
| SGR | 0.092 | 0.125 | 0.156 | 0.216 | 0.228 | |
| FCR | 0.057 | 0.078 | 0.097 | 0.134 | 0.142 | |
| Survival rate (%) | 60 | 60 | 85 | 90 | 90 | |
| 0-90 | Initial weight (g) | 7.33±0.38 | 7.61±0.31 | 7.57±0.29 | 7.63±0.32 | 7.89±0.41 |
| Final weight(g) | 15.24±0.8 | 17.78±1.3 | 19.32 ±1.5 | 24.02 ±1.3 | 24.56 ± 0.9 | |
| Weight gain (g) | 7.91±1.6 | 10.17 | 11.75 ±1.5 | 16.39±1.3 | 16.67±0.9 | |
| SGR | 0.008 | 0.113 | 0.131 | 0.182 | 0.185 | |
| FCR | 0.55 | 0.070 | 0.081 | 0.113 | 0.115 | |
| Survival rate (%) | 60 | 60 | 85 | 100 | 100 | |
| 0-120 | Initial weight (g) | 7.33±0.38 | 7.61±0.31 | 7.57±0.29 | 7.63±0.32 | 7.89±0.41 |
| Final weight(g) | 18.14±1.3 | 21.39±1.1 | 25.94±1.2 | 28.69±0.9 | 28.98±1.4 | |
| Weight gain (g) | 10.81±1.4 | 13.78±1.2 | 18.37±1.3 | 21.06±1.4 | 21.09±1.5 | |
| SGR | 0.090 | 0.115 | 0.153 | 0.176 | 0.176 | |
| FCR | 0.056 | 0.071 | 0.095 | 0.109 | 0.109 | |
| Survival rate (%) | 60 | 60 | 85 | 100 | 100 |
In the present study, the fishes were fed basal diet supplemented with probiotic B. firmus CAS 7 at 25, 50, 100 and 150 mg kg-1 and the growth performance such as weight gain, survival rate and color enhancement were evaluated for 120 days. The weight of fishes in experiment III (28.98±1.4) and IV (28.69 ± 0.9 g) was significantly higher than experiment II (7.57 ± 0.29 to 25.94 ± 1.2 g), I (7.61 ± 0.31 to 21.39 ± 1.1 g) and control (7.33 ± 0.38 to 10.81 ± 1.4 g). Moreover, the weight gain (21.09 ± 1.5 g) and survival rate (100%) were also significantly higher in experiment III and IV where fishes were fed with 100 and 150 mg kg-1 of probiotic mixed with basal diet respectively when compared other experiments and control. The specific growth rate (SGR) were significantly higher in fishes reared in experiment III and IV (0.176) followed by II (0.153), I (0.115) and control (0.090). Likewise, the FCR rate was in the range of 0.83 – 0.56, 0.57 -0.44, 0.41 – 0.33, 0.31-0.29 and 0.30 – 0.29 respectively in control, experiment I, II, III and IV. (Jafaryan et al., 2008), also reported that the probiotic (Bacillus sp.) supplemented diet significantly increased the weight, length and SGR of fish when compared to the control diet. (Giri, S.S. et al., 2013), suggested that the probiotic supplementation needs to be done for 60 days to obtain a significant improvement in SGR and FCR. Several studies suggested have that the probiotic Bacillus sp. supplementation has significantly increased the weight gain and SGR in Rachycentron canadum (Geng, X. et al., 2012), Labeo rohita (Giri, S.S. et al., 2013), Oreochromis niloticus (Aly et al., 2008), Epinephelus coioides (Sun, Y.Z. et al., 2010) and Larimichthys crocea (Ai et al., 2011). Thus, the reduction in FCR of fishes in experimental groups revealed dietary nutrients were utilized more efficiently when the diet was supplemented with probiotics.
Figure 1. Carotenoid content of skin of tomato clown Amphiprion frenatus (Brevoort, 1856) in control and experiments.
Carotenoids are commonly found in pigmented bacteria which are known to have a positive role in the intermediary metabolism of fish that could enhance nutrient utilization and may ultimately result in improved growth (Steiger, S. et al., 2012). The results of stimulatory effect on skin color study suggested that the carotenoid content in the skin of fishes fed with probiotic maintained coloration during periods of social interaction, suggesting that the probiotics may play important roles in maintaining fish skin coloration. After 30 days, the carotenoid content in the skin of fishes fed with probiotic supplemented feed exhibited increasing pigment levels and started to differ from that of control diet. The carotenoid content of fish skin was about 6.80 mg g-1 in experiment III and IV, whereas it was 4.48 mg g-1 in control. Thus, the results confirmed that the color enhancement in terms of total carotenoid content in the fish skin increased significantly with increasing probiotic bacteria concentration in the basal diet. Support to the above fact, (Hong, 2008), reported that the yellow/orange carotenoids formed by B. indicus HU36 significantly increased the pigmentation of the experimental fishes.
The result obtained from the present study suggested that the fishes fed with basal diet supplemented 100 and 150 mg kg-1 of probiotic B. firmus exhibited better stimulatory effect on growth, survival and skin colour of Amphiprion frenatus. Although the fishes fed with diets containing 100 and 150 mg kg−1 of probiotic exhibited higher effect than control and lower dosage, no significant difference reported between 100 and 150 mg kg−1 and hence, it is recommended to use at a concentration of 100 mg kg−1 for the enhanced growth and color of the tomato clown Amphiprion frenatus which has significant importance in ornamental fish industry.
ACKNOWLEDGEMENTS
The authors are greatly thankful to the authorities of Sathyabama University and Annamalai University for providing the facilities. The author, M.V. Rajeswari greatly acknowledged to University Grants Commission (UGC), Government of India for financial support through UGC-Postdoctoral fellowship for women.
REFERENCES
Ai, Q., Xu, H., Mai, K., Xu, W., Wang, J., & Zhang, W., (2011). Effects of dietary supplementation of Bacillus subtilis and fructooligo saccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture, 317, 155-161. http://dx.doi.org/10.1016/j.aquaculture.2011.04.036
Allen, B., (1998). Clowns in desert, Tropical fish hobbyist, 61-64.
Alva, V. R. & Gomes, L. A. O., (1989). Breeding marine aquarium animals: the anemone fish [a35], The NAGA ICLARM, pp.12-13.
Aly, S.M., Mohamed, M.F., & John, G., (2008). Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica (Oreochromis niloticus). Aquaculture Research, 39, 647- 656. https://dx.doi.org/10.1111/j.1365-2109.2008.01932.x
AOAC (1990). Official Methods of Analysis of the Association of Official Analytical Chemists, Vol. 1, 14th edn. 1102 p. Association of Official Analytical Chemists, Arlington,VA, USA. Aquatic Sciences, 8, 43-48.
Arvedlund, M., McCormick, M. I., & Ainswarth, T., (2000). Effect of photoperiod on growth of larval and juvenile of the anemonefish Amphiprion melanopus. Naga ICLARM Quarterly 23, 18-23.
Bagheri, T., Hedayati, S.A., Yavari, V., Alizade, M., & Farzanfar, A., (2008). Growth, survival and gut microbial load of rainbow trout (Onchorhynchus mykiss) fry given diet supplemented with probiotic during the two months of first feeding. Turkish Journal of Fisheries and Aquatic Sciences, 8, 43-48.
Balcazar, J. L., De Blas, I., Ruiz-Zarzuela, I., Cunningham, D., Vendrell,D., & M uzquiz,J. L., (2006). The role of probiotics in aquaculture. Veterinary Microbiology, 114, 173-186.
Bandyopadhyay, P., & Mohapatra, P.K.D., (2009). Effect of a probiotic bacterium Bacillus circulans PB7 in the formulated diets: on growth, nutritional quality and immunity of Catla catla (Ham.). Fish Physiololgy and Biochemstry, 35, 467- 478. https://dx.doi 10.1007/s10695-008-9272-8
Benetti, D.D., Sardenberg, B., Welch, A., Hoenig, R., Refik Orhun, M. & Zink, I., (2008). Intensive larval husbandry and fingerling production of cobia Rachycentron canadum. Aquaculture, 281, 22-27.
Danillowicz, B.S., & Brown, C.L., (1992). Rearing methods for two damsel species, DascyJlus albiseJla (Gill) and aruanus (L). Aquaculture, 106, 145-149.
Elayaraja, S., Annamalai, N., Murugesan, P., Mayavu, P. & Balasubramanian, T., (2011). Effect of amylase on growth, survival and proximate composition of the Polychaete, Perinereis cultrifera (Grube, 1840). Aquacuture Nutrition, 17, 627-633. http://dx.doi.10.1111/j.1365-2095.2011.00864.x
Fernandez, I., Hontoria, F., Ortiz-Delgado, J.B., Kotzamanis, Y., Estevez, A., Zambonino-Infante, J.L. & Gisbert, E., (2008). Larval performance and skeletal deformities in farmed gilthead sea bream (Sparus aurata) fed with graded levels of vitamin A enriched rotifers (Brachionus plicatilis). Aquaculture, 283, 102-115.
Gatesoupe, F. J., (2007). Live yeasts in the gut: Natural occurrence, dietary introduction and their effects on fish health and development. Aquaculture, 267, 20-30.
Geng, X., Dong, X.H., Tan, B.P., Yang, Q. H,, Chi, S.Y., Liu, H.Y., & Liu, X.Q., (2012). Effects of dietary probiotic on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Aquaculture Nutrition, 18, 46-55. https://dx.doi.org/10.1111/j.1365-2095.2011.00875.x
Ghosh, K., Sen, S.K., & Ray, A.K., (2003). Supplementation of an isolated fish gut bacterium, Bacillus circulans, in formulated diets for rohu, Labeo rohita, fingerlings. Israeli J. Aquaculture Nutrition, 55, 13-21. http://dx.doi.10.1111/j.1365-2095.2011.00875.x
Giri, S.S., Sukumaran, V., & Oviya, M., (2013). Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish Labeo rohita. Fish Shellfish Immunology, 34, 660-666. http://dx.doi.org/10.1016/j.fsi.2012.12.008
Giri, S.S., Sukumaran, V., Sen, S.S., & Jena, P.K., (2014). Effects of Dietary supplementation of potential probiotic Bacillus subtilis VSG1 singularly or in combination with Lactobacillus plantarum VSG3 or / and Pseudomonas aeruginosa VSG2 on the growth, immunity and disease resistance of Labeo rohita. Aquaculture Nutrition, 20, 163-171.
Goodwin, T.W., (1984). Biochemistry of the Carotenoids. vol II: Animals. 2nd edn. pp. 64–96. Chapman, Hall, London – NewYork.
Harikrishnan, R., Balasundaram, C., & Heo, M.S., (2010). Lactobacillus sakei BK19 enriched diet enhances the immunity status and disease resistance to streptococcosis infection in kelp grouper. Epinephelus bruneus. Fish Shellfish Immunology, 29, 1037-1043. http://dx.doi.10.1016/j.fsi.2010.08.017
Hata, M., & Hata, M., (1971). Carotenoid pigments in goldfish (Carassius auratus) II. Colour change and carotenoid pigment composition. International Journal of Biochemistry, 2, 182-184.
Hong, H. A., Huang, J. M., Khaneja, R., Hiep, L.V., Urdaci, M.C., & Cutting, S.M., (2008). The safety of Bacillus subtilis and Bacillus indicus as food probiotics, Journal of Applied Microbiology, 105, 510-520.
Hunziker, A., (1990). Belore it is too late. Tropical Fish Hobbyist, 39, 1-6.
Ignatius, B., Rathore, G., Jagadis, I., Kandasami, D., & Victor, A.C.C., (2001). Spawning and larval rearing technique for tropical clown fish amphiprion sebae under captive condition. Journal of Aquaculture in the Tropics, 16, 241-249.
Jafaryan, H., Asadi, R., & Bagheri, A. (2008). The promotion of growth parameters and feeding efficiency of Acipenser nudiventris larvae by using of probiotic Bacillus via bioencapsulation of Artemia urmiana. In: Resource management, natural, human and material resources for the sustainable development of aquaculture. Aquaculture Europe.
Jung, H. L., (2006). Spawning, development and larval rearing of false clownfish Amphiprion ocellaris under captive conditions. Master Thesis, Universiti Malaysia Terengganu.
Keysami, M.A., Saad, C.R., Sijam, K,, Daud, H.M., & Alimon, A.R., (2007). Effect of Bacillus subtilis on growth development and survival of larvae Macrobrachium rosenbergii (de Man) Aquaculture Nutrition, 13,131-136. http://dx.doi- 10.1111/j.1365-2095.2007.00463.x
Malpass, D. Jr., (1996). Raising Amphiprion percula, Tropical fish hobbyist, pp. 56-61.
Mazurkiewicz, J., Przybyl, A., Sip, A., & Grajek, W., (2007). Effect of Carnobacterium divergens and Enterococcus hirae as probiotic bacteria in feed for common carp, Cyprinus carpio. L. Arch. Polish Fish, 15, 93-102.
Merrifield, D.L., Dimitroglou, A., Bradley, G., Baker, R.T.M., & Davies, S.J., (2010). Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) I. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria. Aquaculture Nutrition, 16, 496-503. http://dx.doi.10.1111/j.1365-2095.2009.00689.x
Paripatanamont, T., Tangtrongpairoj, J., Sailasuta, A., & Chansue, N., (1999). Effect of astaxanthin on the pigmentation of goldfish Carassius auratus. Journal of World Aquaculture Society, 30,454-460.
Perez-Fons, L., Steiger, S., Khaneja, R., Bramley, P.M., Cutting, S.M., Sandmann, G., & Fraser, P.D., (2011). Identification and the developmental formation of carotenoid pigments in the yellow/orange Bacillus spore-formers, J Biochimica et Biophysica Acta 1811, 177-185. http://dx.doi.10.1016/j.bbalip.2010.12.009
Qi, Z.Z., Zhang, X.H., Boon, N. & Bossier, P., (2009). Probiotics in aquaculture of China-current state, problems and prospect. Aquaculture, 290, 15-21. http://dx.doi.10.1016/j.aquaculture.2009.02.012
Rahiman, K.M.M., Jesmi, Y., Thomas, A.P. & Hatha, A.A.M., (2010). Probiotic effect of Bacillus NL110 and Vibrio NE17 on the survival, growth performance and immune response of Macrobrachium rosenbergii (de Man). Aquaculture Research, 41, 120-134. http://dx.doi.10.1111/j.1365-2109.2009.02473.x.
Rengpipat, S., Rukpratanporn, S., Piyatiratitivorakul, S., & Menasa-veta, P., (2000). Immunity enhancement on black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture, 191, 271-288. http://www.elsiever.ni/locate/aquaonline/2000
Schiedt, K., & Jensen, S. L., (1995). Isolation and analysis. In: Britton, G., Liaaen-Jensen, S., Pfander, E. (Eds.), Carotenoids: Isolation and Analyses, vol. 1A. Birkha¨user, Basel, Switzerland, pp. 81-108.
Steiger, S., Perez-fons, L., Frase,r .PD. & Sandmann, G., (2012). Biosynthesis of a novel C30 carotenoid in Bacillus firmus isolates, Journal of Applied Microbiology, 113, 888-895. http://dx.doi.10.1111/j.1365-2672.2012.05377.x
Sun, Y.Z., Yang, H.L., Ma, R.L., & Lin, W.Y., (2010). Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunology, 29, 803-809. http://dx.doi.10.1016/j.fsi.2010.07.018
Torrisen, O.J., & Naedval, G., (1984). Pigmentation of Salmonids; Genetical variation in carotenoid deposition in Rainbow Trout. Aquaculture, 38, 59-66.
Wang, Y.B., Li, J.R., & Lin, J., (2008). Probiotics in aquaculture: challenges and outlook. Aquaculture, 281, 1-4. http://dx.doi.10.1016/j.aquaculture.2008.06.002
Wilkerson, J, D., (1998). Clown fishes [a29], A Guide to Their Captive Care, Breeding and Natural History. 1st edn. Microcosm, Shelburne: Vermont, pp. 240-260.
Wilkerson, J.D., (2001). Clown fishes: A guide to their captive care, breeding and natural history. Microsom Ltd., USA, 240p.