1Department of Biochemistry, Patna University, Bihar, India
2Department of Botany, Patna University, Bihar, India
3Department of Zoology, Patna University, Bihar, India
4Department of Biochemistry, Indira Gandhi Institute of Medical Science, Patna, Bihar, India
Corresponding author email: bb2mishra@gmail.com
Article Publishing History
Received: 10/12/2020
Accepted After Revision: 25/03/2021
Diabetes, an endocrine disorder, causes hyperglycemia along with oxidative stress that leads to diabetes-related complications. Diabetic nephropathy is one of the complications related to oxidative stress. Antioxidants play a pivotal role to protect body organs against damage caused by free radical species like Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). Nowadays, drugs that are used to treat diabetes mellitus cause serious side effects, leading to the gaining popularity of alternative herbal medicine. This study aims to investigate antioxidant potential concerning kidney tissue and hypoglycemic effect of Coriandrum sativum seeds in alloxan induced diabetic rats. The rats were divided into Normal, Diabetic and Coriander seed crude methanolic extract treated groups. Diabetes was induced by administering Alloxan at a dose of 100 mg per kg body weight. Diabetic rats were treated with crude methanolic Coriander seed extract at a dose of 500mg/kg bodyweight for 30 days using gavage. Blood serum was used for Glucose estimation whereas Kidney tissues were collected and stored in Tris Buffer for antioxidant assay. Glucose and antioxidant assays were carried out using previously reported methods with slight modifications. The results showed a significant (p<0.05) decrease in blood glucose level indicating its hypoglycemic effect. Besides, it caused a significant (p<0.05) increase in Antioxidant enzymes and compounds of kidney tissue such as Superoxide Dismutase (SOD), Glutathione-S-transferase (GST), Glutathione Peroxidase (GPx), Catalase (CAT), Glutathione Reductase (GSSG Red) and Reduced Glutathione (GSH) as compared to the diabetic group. Thus, it indicates that the crude methanolic extract of Coriandrum sativum seeds has the potential to combat hyperglycemia and oxidative stress-induced diabetic complications.
Diabetes, Hypoglycemic, Antioxidant, Coriandrum Sativum, Oxidative Stress
Mishra B. B, Padmadeo S. R, Thakur K. R, Jha D. K, VyomeshVibhaw, Pranay K, Kumar P. Hypoglycemic and Antioxidative Potential of Coriandrum Sativum Seed Extract in Alloxan Induced Diabetic Rats. Biosc.Biotech.Res.Comm. 2021;14(1).
Mishra B. B, Padmadeo S. R, Thakur K. R, Jha D. K, VyomeshVibhaw, Pranay K, Kumar P. Hypoglycemic and Antioxidative Potential of Coriandrum Sativum Seed Extract in Alloxan Induced Diabetic Rats. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/2YE3Bsn”>https://bit.ly/2YE3Bsn</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Diabetes, being, an Endocrine disorder having many complications (Adeyemi et al., 2010). xidative stress has been reported to play a key role in the initiation and progression of diabetes mellitus along with its complications. Diabetes-induced Hyperglycemia increases oxidative stress, which may be due to either increased free radical species production or a decrease in antioxidant defenses (Giacco and Brownlee, 2010). Studies indicate that oxidative damage caused due to oxidative stress is an important factor related to diabetic nephropathy (Zhang and Sun, 2015). Diabetic nephropathy, a chronic disease, caused due to diabetes mellitus, if left untreated, leads to end-stage renal failure (Chen et al., 2015; Ghaderian et al., 2015; Magee et al., 2017). Hyperglycemia and Oxidative stress together play an important role in the progression of Diabetic Nephropathy (Rehman and Akash, 2017). Chronic hyperglycemia is the prime factor of diabetes mellitus complications that modulates metabolism by elevating the production of reactive oxygen species (Rosca et al., 2005). Moreover, it has been reported that Diabetes mellitus is linked to elevation in oxidative stress (Ighodaro, 2018 Prabhakar et al., 2020).
Antioxidant enzymes such as Superoxide dismutase (SOD), Catalase (CAT), Glutathione reductase (GSSG Red), Glutathione Peroxidase (GPx), Glutathione Transferase (GST) and other antioxidant molecules such as Reduced Glutathione (GSH) scavenges the free radicals and provide protection to cells against oxidative damage (Bahadir et al., 2016). The antioxidant can decrease the oxidation rate of other molecules; various studies have indicated that antioxidants have the ability to suppress the complications of diabetes ( Ayala et al., 2014 Deepa et al., 2018). Previous works have proved the efficiency of plants in the regulation of oxidative stress related to diabetes mellitus (Taleb et al., 2009). Coriandrum sativum (Family Apiaceae) also known as Cilantro is one of the extensively used herbs as traditional medicine for gastrointestinal disorders etc. Several workers have reported that C.sativum has antioxidant (Wangensteen et al., 2004), antihyperglycemic (Eidi et al., 2009) and antihyperlipidemic (Chithra and Leelamma, 1999; Sreelatha and Inbavalli, 2012). In the present study, an attempt has been made to further assess the hypoglycemic and antioxidative effect of crude methanolic extract of Coriandrum sativum seeds with reference to the kidney in case of Diabetes mellitus.
MATERIAL AND METHODS
The Plant part was authenticated by Prof. S. R. Padmadeo, Former Head of Department of Botany, Patna University. The seeds of C.sativum were purchased from the local market during the harvesting season of the plant. Seeds of C. sativum were carefully washed with distilled water 3-6 times to remove dirt and other contaminating material. The plant materials were shade dried at ambient temperature and pressure until no moisture was left in it. The plant material was converted to fine powder using kitchen grinder followed by sieving with the help of muslin cloth to remove coarse particles. The powdered form of seeds of C.sativum respectively was stored in a well-labeled airtight container for further use. The methanolic crude extract of C.sativum was prepared using Soxhlet apparatus (Riviera, India). 100 grams of fine powder of plant material was weighed using a digital weighing machine (Wensar, India) and placed in the cellulose thimble using gloves. The thimble was carefully placed in the extraction chamber of the soxhlet apparatus while 500 ml of Methanol (100%) was placed in the boiling flask attached to the heating mantle. The Soxhlet apparatus was run for 48 hours at 60oC to ensure that all phytochemicals in the plant material have dissolved in methanol (Nafisa et al., 2007).
After 48 hrs cycle, the methanolic extract was collected from the Soxhlet apparatus and was further filtered using Whatman filter paper to get rid of any solid particle. The methanolic extract was concentrated by Rotavapour (Popular, India) at 60 o C and reduced pressure to one-twentieth volume (5 ml). it was further lyophilized to get thick greenish brown coloured residue in case of C. sativum which were stored in a well-labeled vial at 4oC. Alloxan monohydrate used in this study was a product of Sigma Chemical Company, St Louis, MO, U.S.A. Gluco-one glucometer was a product of Dr.Morepen, Delhi, India. UV-Vis Spectrophotometer (Systronics, India) was used to analyse enzymes and molecules. All other chemicals and assay kits used were products of Sigma-Aldrich Inc. and Merck, Germany, respectively. Healthy Wistar male albino rats (100–150 g) were kept under well-ventilated standard environmental conditions (temperature 25±2 °C, relative humidity 50±5 %) with a 12 h light / dark cycle. Animals were allowed to acclimatize for 7 days before the commencement of the experiment. The experiments were designed and conducted as per the current ethical norms and guidelines approved by the Ministry of Social justices and Environment, Government of India (Nafisa et al., 2007).
The rats were fed on Laboratory prepared pellet having the composition suggested by Subcommittee on Laboratory animal nutrition, National Research Council, USA and water ad libitum to ensure proper growth and nourishment. The extra supplement that was given was carrot, sprouted Bengal gram and green gram. Alloxan monohydrate 100 mg/kg body weight dissolved in 0.9% sterilized NaCl solution of pH 7.0 was administered in the tail vein of rats to induce diabetes mellitus. After 48 hours, their fasting blood glucose levels were monitored using a glucometer by collecting blood from the tail artery of animals. Those rats having fasting glucose levels in the range of 250 and 400 mg/dl were considered diabetic and used for the experiment (Nafisa et al., 2007).
The pure breed rats were kept in new polypropylene cages and were categorized into the following groups:-Group I – Normal/Control., Group II – Alloxan treated Diabetic rat, Group III – Crude Methanolic Coriander seed Extract treated ratMethanolic crude C. sativum seed extract at a dose of 500mg/kg. body weight was prepared from the stock solution according to the weight of the rats by dissolving in olive oil. Oral administration of the desired herbal extract was made through oral gavages for 10, 20 and 30 days. For the present research work, blood samples were collected by tail clipping for fasting glucose estimation and after an interval of 10, 20, and 30 days rats were sacrificed for organ collection and preservation. For the entire research work, a tissue sample of the kidney for the antioxidant assay of different parameters was kept in Tris-buffer at -20o C.
The kidney tissue was isolated, washed in 0.2 M Tris buffer solution, blotted dry and weighed. A 10% tissue homogenate was prepared in 0.2 M Tris buffer solution by a motor-driven Teflon pestle glass homogenizer. The tissue homogenate was centrifuged at 10,000 rpm for 20 min, to remove cell debris and then the supernatant was centrifuged at 15,000 rpm for 30 min. The supernatant obtained was used for various assays. The tissues collected at each interval were immediately processed and each tissue sample was analyzed separately (Rotruck et al., 1973).
Superoxide Dismutase (SOD) activity was measured by the method of Marklund and Marklund based on the inhibition of the autoxidation of pyrogallol (Marklund and Marklund, 1974). Catalase (CAT) activity was determined by measuring the rate of decomposition of H2O2 by the method of Claiborne, 1985. The Glutathione Peroxidase (GPx) activity was determined using H2O2 as a substrate according to the method of (Rotruck et al., 1973). Glutathione Peroxidase enzyme catalyzes the decomposition of H2O2 or other peroxides (-OH) with the simultaneous oxidation of GSH into GSSH (Rotruck et al., 1973).
The tissue GSH content was estimated by the method of Beutler based on the development of a stable yellow colored complex, with 5,5’-dithio, bis-2, nitrobenzoic acid (DTNB) or Ellman’s reagent (Beutler et al., 1967).The activity of GSH-R was measured by the oxidation of NADPH as described by Horn, 1963. The activity of GST was determined using 1-chloro 2,4-dinitrobenzene (CDNB) as substrate (Habig et al., 1974). Data were expressed as the Mean ± SEM. For statistical analysis of the data, group means were compared by one-way analysis of variance (ANOVA) followed by Tukey and Duncan post hoc test for multiple comparisons using Graph Pad Prism 8 software. p < 0.05 was considered to be statistically significant.
RESULTS AND DISCUSSION
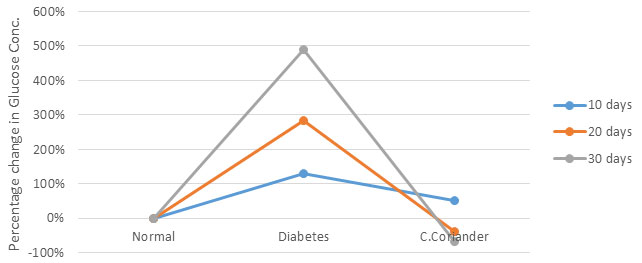
Diabetes is a disease, if not controlled in time can lead to a secondary pathological condition due to a rise in oxidative stress during the progress of the disease. In the present study, an attempt has been made to investigate the ameliorating impact of Coriander seed extract on diabetic nephropathy. Diabetes was induced with the help of alloxan, alloxan caused diabetes by the destruction of pancreatic Beta cells (Jelodar et al., 2007). Alloxan treated rats at a dose of 100 mg/kg body weight caused elevation in blood glucose up to 489% as compared to control leading to loss of weight and lethargic activity. Nevertheless, when the crude -coriander methanolic extract at a dose of 500 mg/kg body weight were administered to diabetic rat caused a significant decline in blood glucose level up to -74% (Fig.1). Kajal and Singh, (2019) reported similar findings through their work on petroleum ether extract of C.sativum seeds (Radenkovic et al., 2016; Kajal and Singh, 2019). The antioxidant effects of crude coriander were studied in terms of antioxidant enzymes like SOD, Catalase, GPx, GST and Glutathione Reductase along with antioxidant molecules like Reduced Glutathione (GSH) (Yasui and Baba, 2006; Kangralkar et al., 2010; Radenkovic et al., 2016).
The Superoxide Dismutase (SOD) belongs to the metalloenzyme group that forms defense against oxygen species (ROS) mediated injury by catalyzing the dismutation of superoxide anion free radical (O2-) into molecular oxygen and hydrogen peroxide (H2O2) thereby decreasing O2- concentration which harm cells (Yasui and Baba, 2006; Kangralkar et al., 2010; Radenkovic et al., 2016). In the diabetic rat group, SOD level considerably decreased (-84%), Nonetheless, Crude methanolic coriander seed extract treatment leads to a significant increase in enzyme activity (+100%) (p<0.005) as compared to the diabetic group suggesting a decrease in oxidation stress and ROS level (Landis and Tower, 2005) (Table 1). Catalase and Glutathione Peroxidase are other significant antioxidant enzymes that help to overcome stress by the elimination of H2O2 (Bagri et al., 2009).
There was a marked decline in catalase activity up to 98% in the diabetic group as compared to normal, which may be due to inactivation by superoxide radical and Glycation of Enzyme (Bagri et al., 2009). However, on treatment with plant extract for 30 days, catalase activity augmented 15.46 times as compared to the diabetic group showing a recovering trend (P<0.05) (Table 2), which may be due to the antioxidant potential of the plant. Similarly, GPx activity decreased substantially by 90% in the diabetic group. Reduced activity of GPx in the Diabetes group may be attributed to free radical induced inactivation and Glycation of the enzyme (Zhang and Tan, 2000; Rajasekaran et al., 2005).
Administration of crude methanolic C.sativum extract increased the activities of GPx by (+82%) on day 30 (p<0.05) with respect to the diabetic group (Table 3), which is in agreement with the result of (Sreelatha and Inbavalli, 2012). GST is multifactorial enzymes that play a key role in the detoxification of electrophilic metabolites (Hayes et al., 2005). GST activity in alloxan induced diabetes group illustrated a two-fold decrease with respect to normal on day 30. However, on treatment with methanolic crude coriander seed extract, there was an elevation in enzyme activity by 1.42 fold from day 10 to day 30 showing the beneficial effect of the extract (Table 4), which is similar to the reports of Rai et al., 2010. The decreased activity of GST noted in the diabetic group may be due to deactivation caused by ROS. This suggests that plant extract may assist in neutralizing ROS (Andalu and Vardacharlu, 2003; Sreelatha and Inbavalli, 2012). Glutathione reductase (GSSH red) another key antioxidant enzyme assists in regenerating reduced glutathione (GSH) from the oxidized form of Glutathione that is produced due to oxidation of GSH and therefore ratio of cellular GSH: GSSG is maintained (Dym and Eisenberg, 2001; Taleb et al., 2009; Sato et al., 2011).
Glutathione reductase activity followed the declining trend in the case of the diabetic rat group (-58%) but on treatment with crude seed extract of coriander, there was 43% increase in enzyme activity as compared to the diabetic group on day 30 (P<0.05) (Table 5), which is in consensus with the work of (Taleb et al., 2009). Reduced Glutathione, a tripeptide antioxidant that protects the cellular system from the deleterious effect and scavenging free radicals besides being acting as co-substrate for detoxification by glutathione peroxidases (Anantham et al., 2004; Nain et al., 2012). In the present study, the GSH level was reduced to -24% in the diabetic group as compared to Normal that suggests increased oxidative stress (Table 6). Treatment with crude coriander seed extract leads to 25% increase in GSH level in the diabetic rat (p<0.05) in contrary to the diabetic group without treatment, which is in congruence with the findings of (Ozsoy et al., 2006; Hussien, 2008; Nain et al., 2012).
Figure 1: Effect of methanolic extract of crude coriander seeds on blood glucose
Table 1. Effect of Methanolic extract of crude Coriander seeds on SOD (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | C.Coriander |
| 10 days | 172.1567± 0.494821 | 130.3967± 0.471926* | 4.243333± 0.024037*# |
| 20 days | 172.1567± 0.494821 | 97.06333± 0.087432* | 7.256667± 0.016667*# |
| 30 days | 172.1567± 0.494821 | 27.59667± 0.800861* | 55.30333± 0.104775*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 2. Effect of Methanolic extract of crude Coriander seeds on Catalase (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | C.Coriander |
| 10 | 413.7567± 57.7487 | 271.85± 1.66872 | 17.26± 0.355387# |
| 20 | 413.7567± 57.7487 | 51.48± 0.931522 | 26.34667± 0.7349# |
| 30 | 413.7567± 57.7487 | 8.703334± 0.275096 | 266.8567± 0.280496# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 3. Effect of Methanolic extract of crude Coriander seeds on GPx (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | C.Coriander |
| 10 days | 12.28667± 0.01453 | 4.26± 0.05* | 1.233333± 0.082529*# |
| 20 days | 12.28667± 0.01453 | 2.093333± 0.027285* | 1.94± 0.04*# |
| 30 days | 12.28667± 0.01453 | 1.23± 0.005773* | 2.243333± 0.024037*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 4. Effect of Methanolic extract of crude Coriander seeds on GST (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | C.Coriander |
| 10 days | 0.612667± 0.00491 | 0.481± 0.001155* | 0.331± 0.001155*# |
| 20 days | 0.612667± 0.00491 | 0.462667± 0.00318* | 0.749333± 0.001202*# |
| 30 days | 0.612667± 0.00491 | 0.311± 0.002309* | 1.297± 0.006506*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 5. Effect of Methanolic extract of crude Coriander seeds on Glutathione Reductase (U/ mg of protein) in Kidney Tissue.
| Days | Normal | Diabetes | C.Coriander |
| 10 days | 0.774333± 0.001453 | 0.693± 0.003055* | 0.202667± 0.000882*# |
| 20 days | 0.774333± 0.001453 | 0.462± 0.000577* | 0.322± 0.000577*# |
| 30 days | 0.774333± 0.001453 | 0.323333± 0.001856* | 0.463667± 0.001764*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 6. Effect of Methanolic extract of crude Coriander seeds on GSH (U/ ml of sample homogenate) in Kidney Tissue
| Days | Normal | Diabetes | C.Coriander |
| 10 days | 11.56967± 0.024333 | 10.21667± 0.006667* | 5.241± 0.024*# |
| 20 days | 11.56967± 0.024333 | 9.613± 0.024* | 5.966± 0.024*# |
| 30 days | 11.56967± 0.024333 | 8.767667± 0.041858* | 10.94167± 0.041858*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
CONCLUSION
Diabetes mellitus shows its severity through complications that are caused by oxidative stress generated by ROS due to hyperglycemia leading to diabetic nephropathy. The study reveals that crude methanolic Coriandrum sativum seeds are effective in lowering blood glucose level and has the potential to alleviate diabetes mellitus related oxidative stress from organs such as the kidney. Thus, it can be concluded that it has both hypoglycaemic and antioxidant potential. However, it needs further investigation to identify active components, as this study was performed in a small population group with limited resources.
ACKNOWLEDGEMENTS
The authors are thankful to the Department of Biochemistry, Patna University, Patna India for providing necessary equipments along with chemicals and to Mr. Ravinder for renderinmg helping in research.
Conflict of Interest: The authors declare that there are no conflicts of interest regarding publication or any other activity related to this article.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of the experiments were designed and conducted as per the current ethical norms and guidelines approved by the Ministry of Social justices and Environment, Government of India.
REFERENCES
Adeyemi, D. O., Komolafe, O. A., Adewole, O. S., Obuotor, E. M., Abiodun, A. A. and Adenowo, T. K. (2010). Histomorphological and morphometric studies of the pancreatic islet cells of diabetic rats treated with extracts of Annona muricata. Folia Morphologica. 69(2), 92–100.
Anantham, R., Latha, M., Ramkumar, K.M., Pari, L.,Bhaskar, C. and Narmatha, B.V. (2001). Modulatory effect of Gymnema montanum leaf extract on alloxan induced oxidative stress in wistar rats. Nutrition 20,280-285.
Andalu, B. and Vardacharlu, N.C. (2003). Antioxidant role of Mulberry (Mones indica L.cv. Ananthe) leaves in streptozotocin diabetic rats. Clin. Chem Acta 338, 3-10.
Ayala, A., Munoz, M. F. and Arguelles, S. (2014). Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity, 1–31.
Bagri. B.P., Mohd, A., Aeri, V., Bhowmik, S. and Sultana, S. (2009).Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells, lipid peroxidation and antioxidant enzymes in experimental diabetes. Food and chemical toxicology 47, 50-54.
Bahadir, M. V., Yildirim, Y., Baran, O. P., Polat, S., Akkoc, H. and Tunik, S. (2016). The potential beneficial effects of ethyl pyruvate on diabetic nephropathy: an experimental and ultrastructural study. Polish Journal of pathology: official journal of the Polish Society of Pathologists 67(3), 250–257.
Beutler, E., Duron, O. and Kelly, B.M. (1967). Improved method for the determination of blood glutathione. J Lab Clin Med. 61, 882-888.
Chen, J., Cui, W., Zhang, Q., Jia, Y., Sun, Y., Weng, L. and Yang, B. (2015). Low molecular weight fucoidan ameliorates diabetic nephropathy via inhibiting epithelial‐mesenchymal transition and fibrotic processes. American Journal of Translational Research 7(9), 1553–1563.
Chithra, V. and Leelamma, S. (1999). Coriandrum sativum changes the levels of lipid peroxides and activity of antioxidant enzymes in experimental animals. Indian J Biochem Biophys. 36(1), 59–61.
Claiborne, A., (1985). Catalase activity. In: Greenwald, R.A. (Ed.), Handbook of Methods for Oxygen Radical Research. CRC Press, 283–284.
Deepa, R., Subbulakshmi, P. and Krishnamoorthy, G. (2018). A review on role of antioxidants in diabetes. Asian Journal of Pharmaceutical and Clinical Research. 11, 48–53.
Dym, O. and Eisenberg, D. (2001). Sequence-structure analysis of FAD-containing proteins. Protein science: a publication of the Protein Society 10(9), 1712–1728.
Eidi, M., Eidi, A., Saeidi, A., Molanaei, S., Sadeghipour, A., Bahar, M. and Bahar, K. (2009). Effect of Coriander seed (Coriandrum sativum L.) ethanol extract on insulin release from pancreatic beta cells in Streptozotocin induced diabetic rats. Phytother Res. 23(3), 404–406.
Ghaderian, S. B., Hayati, F., Shayanpour, S., Beladi and Mousavi, S. S. (2015). Diabetes and end‐stage renal disease: A review article on new concepts. Journal of Renal Injury Prevention 4(2), 28–33.
Giacco, F. and Brownlee, M. (2010). Oxidative stress and diabetic complications. Circ Res. 107(9), 1058–1070.
Habig, W.H., Pabst, M.J. and Jakoby, W.B. (1974). Glutathione S transferase.The first enzymatic step in mercapturic acid formation. J Biol Chem 249, 71301-09.
Hayes, J.D., Flanagan, J.U. and Jowsey, I.R. (2005). Glutathione transferases. Annu Rev Pharmacol Toxicol 45, 51-88.
Horn, H.D., (1963).Glutathione reductase. In: Bergmeyer HU.ed. Methods in Enzymatic Analysis. New York: Academic Press, 875-879.
Hussien, M.A., (2008). Antidiabetic and antioxidant activity of Jasonia montana extract in streptozotocin induced diabetic rats. Saudi Pharmaceutical Journal 16, 214-221.
Ighodaro O. M. (2018). Molecular pathways associated with oxidative stress in diabetes mellitus. Biomedicine & pharmacotherapy, 108, 656–662.
Jelodar, G., Mohsen, M. and Shahram, S., (2007). Effect of Walnut leaf, Coriander and Pomegranate on blood glucose and histopathology of Pancreas of alloxan induced Diabetic rats. AJTCAM 4(3), 299-305.
Kajal, A. and Singh, R. (2019) Coriandrum sativum seeds extract mitigate progression of diabetic nephropathy in experimental rats via AGEs inhibition. PLOS ONE 14(3), 1-12.
Kangralkar, V.A., Patil, S.D. and Bandivadekar, R.M. (2010), Oxidative stress and diabetes: a review. Intl. J. Pharm Appl 1, 38-45.
Landis, G.N. and Tower, J. (2005). Superoxide dismutase evolution and life span regulation. Mech Ageing Dev 126(3), 365-379.
Magee, C., Grieve, D. J., Watson, C. J. and Brazil, D. P. (2017). Diabetic nephropathy: A tangled web to unweave. Cardiovascular Drugs and Therapy 31(5‐6), 579–592.
Marklund, S. and Marklund, G. (1974). Involvement of superoxide anion radical and a convenient assay of superoxide dismutase. Eur J Biochem 47, 469-474.
Nafisa, P.C., Chakradnar, V.L., Vandana, S.P. and Suresh, R.N. (2007). An experimental evaluation of the antidiabetic and antilipidaemic properties of a standardized Momordica charantia fruit extract. BMC Complement Alternat Med 7, 29–55.
Nain, P.,Saini, V.,Sharma, S. and Nain, J. (2012). Antidiabetic and antioxidant potential of Emblica officinalis Gaertn leaves extract in streptozotocin induced type-2 diabetes mellitus (T2DM) rats. Journal of Ethnopharmacology 142, 65-71.
Ozsoy-Sacan, O., Yanardag, R., Orak, H., Ozgey, Y., Yarat, A. and Tunali, T. (2006). Effects of parsley (Petroselinum crispum) extract versus glibornuride on the liver of streptozotocin-induced diabetic rats. Journal of Ethnopharmacology 104(1-2), 175–181.
Prabhakar, Y.K., Janardhan, Y.E., Sreenivasulu, D., Raju, K., Kumar, K.J. and Prabhusaran,N. (2020). Ameliorative effects of Mentha aquatica on diabetic and nephroprotective potential activities in STZ-induced renal injury. Comp Clin Pathol. 29,189-99.
Radenkovic, M., Stojanovic, M. and Prostran, M. (2016), Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharmacol Toxicol methods 78, 13-31.
Rai, P.K., Jaiswal, D., Rai, D.K.,Sharma, B. and Watal, G. (2010).Antioxidant potential of oral feeding of Cynodon dactylon extract on diabetes induced oxidative stress. Journal of Food Biochemistry 34, 78-92.
Rajasekaran, S., Sivagnanam, K. and Subramanian, S. (2005). Antioxidant effect of Aloe vera gel extract in streptozotocin induced diabetic rats. Pharmacol Rep. 57, 90-96.
Rehman, K. and Akash, M. S. H. (2017). Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked? Journal of Cellular Biochemistry 118(11), 3577–3585.
Rosca, M. G., Mustata, T. G., Kinter, M. T., Ozdemir, A. M., Kern, T. S., Szweda, L. I. and Weiss, M. F., (2005). Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. American Journal of Physiology‐Renal Physiology 289(2), 420–430.
Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G. and Hoekstra, W.G. (1973). Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073), 588-590.
Sato, I., Shimatani, K., Fujita, K., Abe, T., Shimizu, M., Fujii, T., Hoshino, T. and Takaya, N. (2011). Glutathione reductase/glutathione is responsible for cytotoxic elemental sulfur tolerance via polysulfide shuttle in fungi. The Journal of biological chemistry 286(23), 20283–20291.
Sreelatha, S. and Inbavalli, R. (2012). Antioxidant, Antihyperglycemic and Antihyperlipidemic effects of Coriandrum sativum leaf and stem in alloxan induced diabetic rats. Journal of food science 00, 1-5.
Taleb-senouci, D., Ghomari, H., Krouf, D., Bouderbala, S., Prost, J., Lacaille-Dubois, M.A. and Bouchenak, M. (2009). Antioxidant effect of Ajuva iva aqueous extract in streptozotocin induced diabetic rats. Phytomedicine 16, 623-631.
Wangensteen, H., Samuelsen, A.B. and Malterud, K.E. (2004). Antioxidant activity in extracts from coriander. Food Chem. 88(2), 293–297.
Yasui, K. and Baba, A. (2006). Therapeutic potential of Superoxide Dismutase (SOD) for resolution of inflammation. Inflamm Res 55(9), 359-363.
Zhang, H. and Sun, S.C. (2015). NF-κB in inflammation and renal diseases. Cell Biosci. 5, 63.
Zhang, X.F. and Tan, B.K. (2000). Antihyperglycemic and antioxidant properties of Andrographis paniculata in normal and diabetic rats. Clin. Exp. Pharmacol. Physiol. 27, 358-363.