Plant Molecular Biology Laboratory, Department of Botany, Raiganj University, Raiganj – 733134, Uttar Dinajpur, West Bengal, India
Corresponding author Email: sanjoysadhukhan@gmail.com
Article Publishing History
Received: 27/12/2018
Accepted After Revision: 21/02/2019
Jute, an important lingo-cellulosic rich natural bast fibre, is the second only after cotton in production. It is obtained from the plant belonging to Malvaceae. The advantages of the fi bre lies in its properties i.e. environment-friendly, good insulating, antistatic properties, low thermal conductivity and moderate moisture regain.High lignin content of jute fibre hinders its utilization in textile industry and diversified value‐added products. To make jute suitable for diverse use, especially in textiles, many efforts were made to modify its chemical composition and physicochemical characteristics. With the aim of developing jute fibre with reduced lignin content, the comparative histobiochemical and physicochemical parameters were studied between normal jute and a stable mutant jute line with decreased lignin content. An x‐ray‐induced mutant line (CMU 013) of jute (Corchorus capsularis) was histobiochemically and physico-chemically evaluated along with its normal parent (JRC 321). The anatomy and morphology of mutant jute differ from normal jute in regard to fibre formation and deposition of fibre forming materials. The primary and secondary vascular bundles are normal in both JRC 321 and CMU 013 but bast fi bre formation is less pronounced in mutant jute. The percentage of lignin content is reduced from 19.27% (JRC 321 jute) to 8.627% and cellulose content increased from 48.237% (JRC 321 jute) to 6.397%. X-ray diffraction analysis revealed that the mutant jute has less rigidity and is brittle in nature. Histobiochemical evidence of jute stem of JRC 321 demonstrates that the bast fibre contains decent amount of cementing material, i.e. lignin which is validated by the biochemical analysis of the fibre. Deposition of the lignin was found to be prerequisite for generation of well-developed fi bre as corroborated by SEM. The fragile nature of the fibre as seen in the SEM of CMU 013 jute was validated by the X-ray diffraction analysis and tensile strength. This mutant may serve as a model to understand the molecular mechanism that organizes the development of secondary wall thickening in jute bast fibre cells.
Jute Fibre, Lignin, Mutant, Scanning Electron Microscopy, Ftir Spectroscopy, Strength
Sadhukhan S. Histobiochemical and Physicochemical Characterization of Mutant Jute Corchorus Capsularis CMU 013 with Poorly Developed fibre. Biosc.Biotech.Res.Comm. 2019;12 (1).
Sadhukhan S. Histobiochemical and Physicochemical Characterization of Mutant Jute Corchorus Capsularis CMU 013 with Poorly Developed fibre. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2MgfsJU
Copyright © Sadhukhan, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Jute, a lignocellulosic natural bast fibre obtained from the plant Corchorus sp. is a dicotyledonous annual crop belonging to the family Malvaceae. The fibre originates from the secondary phloem tissue of the stem. It consists of long extraxylary cells which mechanically support the phloem (Guerriero et. al. 2017), also known as bast fibre. The jute fibre is one of the most versatile natural fi bres and it has considerable commercial significance for the generation of diversified value-added industrial products i.e. immense potentiality for industrial production of packaging, textiles, non-textile, construction, and agricultural sectors. Advantages of jute include environment-friendly, renewable resources (Gorshkova 2012), good insulating and antistatic properties, as well as having low thermal conductivity and moderate moisture regain. Of late, it has been well established that a score of value-added commercial products of great utility can be produced based on diverse utilization of jute fibre due to its high strength, high modulus, and high flexural rigidity. However, some problems have been encountered with the quality of jute fibre that figures as critical for their suitability for generation of its value-added textile industrial end products. Utilization of natural fibres including jute depends on its physical and chemical properties. An extensive comparative study has been made on different physical and chemical properties of different natural fibres(McGovern et al., 1983 and Young, 1993) and those properties have been found to be the basis of the historical utilization of the natural fibre. It has also been shown that strength, stiffness, and extensibility of jute fiber decrease with gradual removal of lignin. The deposition of lignin into cellulose and other cell wall materials might be one of the reasons behind the strength and stiffness of natural fiber like jute (Roy, 1953; Meredith 1956). Lignin is a secondary plant metabolite which hinders high-end industrial applications of several plant products including jute (Choudhary et al 2017).
Anatomical studies on jute fibre showed that the strength and fineness of the fi bre are correlated with number of cells per bundle, length-breadth ratio of fibre cells and cross-sectional area of the fibre cells. The crystallinity of cellulose has a large influence on the mechanical properties of natural fibres. Jute bast fibres are composed of xylan with high degree of lignifications whereas fl ax, ramie and hemp bast fibres, have a high abundance of crystalline cellulose (Guerriero et. al. 2017). Fibres like jute, hemp, fl ax, and ramie have their cellulosic chains oriented nearly parallel to the fi bre axis (in the range 7-12°), and they exhibit high strength in contrast to cotton fibre. Cotton fibres, with appreciably lower tensile strengths, are characterized by spiraled cellulose chains that are oriented approximately 30° to the fibre axis (Rials et al., 1997). Moisture content and
fibre purity also influence the degree of crystallinity in jute and mesta fibres (Ray, 1969). X-ray diffraction and Fourier Transformed Infra-Red (FTIR) Spectroscopy are powerful tools to evaluate the structure of jute (Bera et. al. 2002). The bast fibre lignin is significantly different from that of the typical ‘woody plant lignin’ (Day et. al. 2005). Development of bast fibre in jute (Corchorus spp.) is a complex process involving differentiation of secondary phloem fibres from the cambium accompanied by lignification of the fibre cell wall (Kundu et. al. 2012).
The formation of secondary phloem fibres starts long after the initiation of secondary xylem (Snegireva 2015).
In the present study, comparison between an induced mutant (CMU 013) of Corchorus capsularis having an altered form of bast fibre and a normal jute (JRC 321) plant was performed on the basis of the histobiochemical and physicochemical characters of jute fibres.
Materials and Methods
Transverse sections of jute stems of 45 days after sowing (DAS) normal and mutant were prepared, dehydrated and stained with lignin-specific stain phloroglucinol- HCl (Srivastava 1966). The stained sections were observed and photographed using Leica DM LS optical microscope.Freehand transverse sections were prepared of jute stems of 60 days old both normal and mutant were prepared and dehydrated. The surface morphology of dry jute fi bers of both cultivated and mutant varieties was evaluated by scanning electron microscopy using JEOL, JSM 5800, at the Central Research Facility, IIT Kharagpur. The images of scanning electron microscopy were taken at electron volt of 20 KeV.
Estimation of cellulose, hemicellulose, and lignin
For composition analysis, neutral detergent solution (NDS) and acid detergent solution (ADS) were used. Hemicellulose, cellulose, and lignin were estimated following the method (Sadasivam and Manickam 2008). For estimation of these components, the jute fibers were refluxed separately with NDS and ADS followed by washing the insoluble part with hot water and acetone in each case. The insoluble parts obtained after treatment with NDS and ADS are termed as Neutral Detergent Fiber (NDF) and Acid Detergent Fiber (ADF) respectively. The hemicellulose content of jute fibers was obtained asa difference of NDF and ADF. The cellulose content of jute fibers was obtained as the difference of ADF and residue after extraction of ADF with 72% sulphuric acid. The lignin content of jute fiber was obtained as the difference of residue after extraction of ADF with 72% sulphuric acid and ash obtained by igniting ADF. In typical procedure hemicellulose, lignin and cellulose of jute fiber were estimated as follows:
For determination of NDF about 1 g of jute fiber powder was taken in a round bottom fl ask to which 10 ml cold NDS, 2 ml decahydronaphthalene and 0.5 g sodium sulphate were added and refluxed for 1h. The solid residue was fi ltered in a sintered glass crucible (G-2) and washed with hot water followed by washing twice with acetone. The residue was collected, dried at 100°C for 8 h and stored after weighing in a desiccator. This residue was designated as NDF. For determination of ADF about 1g of jute fi ber powder was taken in a round bottom fl ask to which 100ml of ADS were added. The content of the fl ask was heated to boiling in a controlled way to avoid foaming for 5 to 10 min followed by refluxing for 1h. The residue was fi ltered through sintered glass crucible (G-2), washed with hot water and acetone. The washed residue was then dried at 100°C for overnight. The dried residue was designated as ADF and was stored in a desiccator.
For determination of Acid Detergent Lignin (ADL), weighed amount of ADF was taken in a 100ml beaker to which 25 to 50ml 72% sulphuric acid and 1g asbestos fibre was added. The content of the beaker was continuously stirred for 3h. Then the content was diluted with distilled water and the residue was filtered through a preweighed Whatman No. 1 filter paper and washed with water to remove last traces of acid. The residue of the filter paper was dried at 100°C for 2 h. The weight of the residue after drying on filter paper was taken. The filter paper with the residue was transferred on a preweighed silica crucible, which was heated in a muffle furnace at 550°C for 3 h. The weight of ash was calculated after weighing the cold crucible with the ash. For obtaining % ADL, a blank test was performed by taking 1 g asbestos fi bre following the same procedure as before. In this blank test, weights of two residues, one weight after drying on fi lter paper at 100°C for 2 h and the other weight after heating at 550°C for 3 h in a muffle furnace in an identical way: (Protocol adopted from, Sadasivam and Manickam 2008)
NDF = Hemicellulose + Cellulose + Lignin + Minerals
ADF = Cellulose + Lignin + Minerals
Hemicellulose = NDF – ADF
Cellulose = ADF – Residue after extraction with 72% H2SO4
Lignin = Residue after extraction with 72% H2SO4 – ash
The tensile strength of normal and mutant jute fibers was determined by subjecting the fibers to Hounsfield Universal tensile testing machine with the following parameters: load range =10.000N, extension range = 3.000mm, speed = 0.0500mm/min, temperature = 25C, relative humidity = 70%. Clean and dry strands collected from the middle portion of the fiber reeds were used for the measurement after proper gripping through paper boards. Before testing, the specimens were kept in desiccators for 2 days. A stress-strain curve of the cultivated and mutant jute fibers was plotted.IR study of both normal and mutant jute fibers was done using a Nexus 870 Nicolet FTIR spectrophotometer (Edwards et al., (1997), Samal et al., (1995), Rana et al., (1997), Pan et al., (1999). Jute fi bers were crushed with liquid nitrogen into a fine powder and dried in desiccators. An equal weight of both cultivated and mutant fibers was taken and made pellet with potassium bromide and subjected to IR spectrophotometer.
X-ray diffraction (XRD) analysis: XRD of dry jute fibres were carried out by Philips PW 1729 X-ray generator using Co target (y=0.179 nm) at a scanning speed of 2ɸ / min and the data recorded every 0.02ɸ (2ɸ) for the angular range of 2ɸ = 10-50ɸ (Varma et al., 1984).
Results
Anatomical study of jute stems
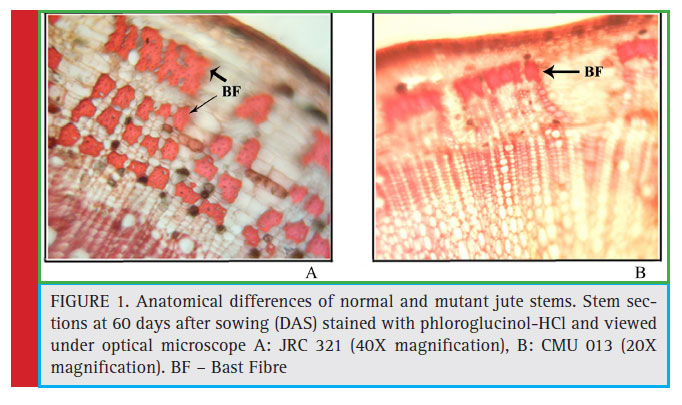
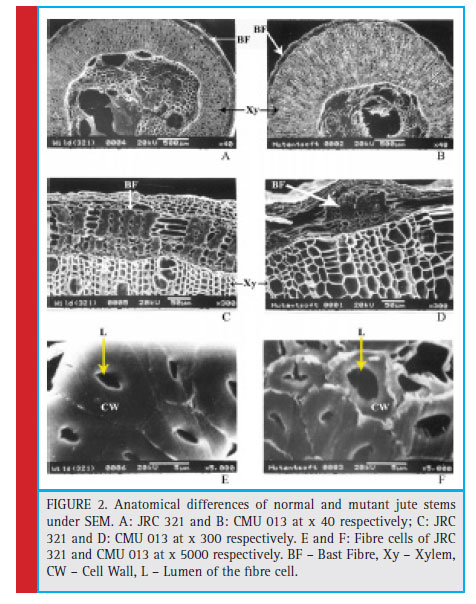
The morphological differences of the mutant (CMU-013) having discontinuous fibre directed to study the developmental pattern in fibre formation in its stem compared to cultivated variety JRC 321. Thus, jute stem of 60 days old from both the JRC 321 and CMU 013 was sectioned transversely and stained with phloroglucinol-HCl and analyzed microscopically. It was revealed that the bast fibre (secondary phloem fi bre) was poorly developed in CMU 013 (Figure 1) indicating a weaker deposition of the fibre forming components.The comparison was also studied by scanning electron microscopy (SEM) to measure the fiber-forming zone in both the CMU 013 and JRC 321. It was found that the thickness of the bast cell wall in JRC 321 was 4.5 μm whereas it was 2.2 μm in case of CMU 013. Moreover, the diameter (3.65 μm) of the lumen in jute fi bre of CMU 013 was found to be more than the JRC 321 (2.25 μm), which indicated further lack of deposition of the fi bre forming components in CMU 013 (Figure 2).
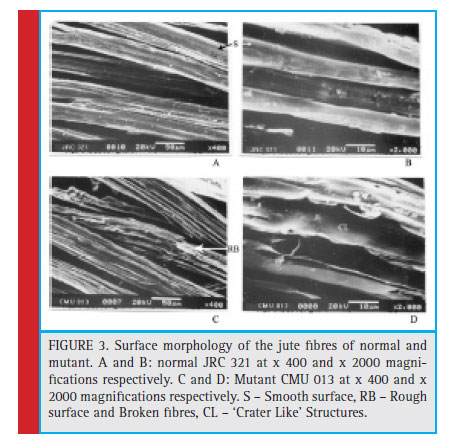
It was also observed from scanning electron micrograph that the surface morphology of the jute fibre isolated from CMU 013 was entirely different than that of JRC 321; the fiber of JRC 321 showed a smooth surface compared to that of the mutant CMU 013 having a rough surface and broken fibres. At a higher magnification, a crater-like structure was found to be present on the surface of the mutant jute fiber (Figure 3). Thus, it could be assumed that the fi bre formation in the mutant is underdeveloped due to improper functions of the fibre forming elements.
The chemical components of cultivated and mutant jute fibers were estimated by gravimetric method. It was found that cultivated type fibers have more lignin and less cellulose compared to that of the mutant. Mutant has 28.42 % more cellulose than the cultivated fiber and whereas the cultivated fiber has 55 % more lignin than the mutant fiber.
The jute fibers of both the cultivated variety (JRC 321) and the mutant (CMU 013) were assessed for its tensile strength. The tensile strength of the jute fibers was measured by a Universal Tensile Testing Machine (Hounsfield) with a load cell 10,000 N, extension range = 30 mm, speed = 0.0500 mm/min, temperature of 25 0C and 70 % relative humidity. Clean and dry strands collected from the middle portion of the fi ber reeds were used for the measurement. It was observed that the fibre of the cultivated variety showed to be stronger (tensile strength of 952.4 MPa) with an elongation percentage at break of 2.51 % than the mutant jute (tensile strength of 600.8 MPa) with an elongation percentage at break of 1.67 %. It was found that the jute fiber of JRC 321 covered a higher total area under the stress-strain curve compared to that of the mutant CMU 013 (Figure 4). This indicated that the jute fiber in the cultivated variety was stronger than that of the mutant. From a calculation of the area under the stress-strain plot it was found that the jute of the cultivated variety needs more energy than that of the mutant for breaking the fibre under a tensile load.
FTIR vibrational bands of the cultivated and mutant area are shown in Figure 5, and Table 2. In the mutant type of fibre shifting of H bonded –OH stretching frequency value to higher region indicates that intermolecular H-bonding decreases and whereas in case of cultivated type of fibre shifting of H bonded –OH stretching frequency value to lower region indicates that intermolecular H-bonding increases. Peaks at 2903.76 cm-1 and 2916.84 cm-1 correspond to C-H stretching in methyl and methylene groups (in cellulose and hemicellulose) in jute. Asymmetric C-O-C stretching bands at 1110.67 cm-1 and 1111.85 cm-1 indicates the presence of the chain of anhydroglucose ring, i.e., cellulose. The 1427.11 cm-1 and 1426.78 cm-1 may be due to the presence of lignin on both cultivated and mutant jute fibres.
The fibres of both the cultivated and mutant jute were subjected to analyze by X-ray diffraction study within the angular range of 100 – 500 to measure the percentage crystallinity of the fibre. It was revealed from figure 6 that the percentage crystallinity of the fibre in cultivated variety was found to be 20.53 % whereas that of the mutant fibre was 13.9 % indicating more rigidity and brittle nature of the jute fibre in the cultivated variety.
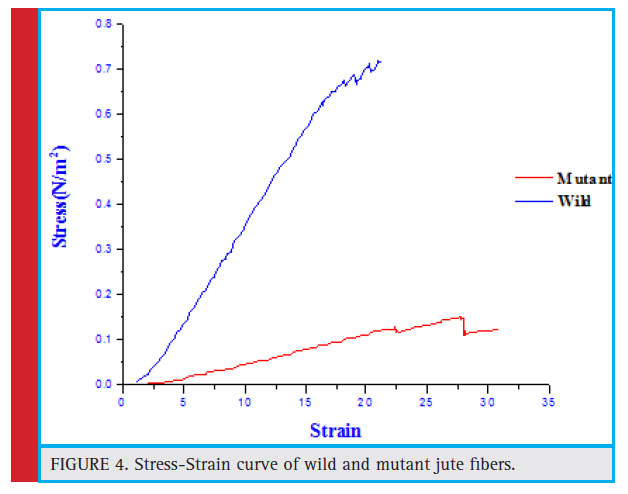 |
Figure 4: Stress-Strain curve of wild and mutant jute fibers. |
Discussion
The present study constitutes the first attempt to understand the physico-chemical differences between normal jute fibre and its mutant fibre. Jute (Corchorus capsularis) is the most important fibre producing crop in the world next to cotton in terms of yield. Anatomically, jute fibres, also known as bast fi bres, are sclerenchyma cells with secondary cell wall thickening and are rigid in structure (Kundu, 1944; Fahn, 1990; Evert 2006, Crang et. al. 2018). Proper utilization of this fibre is hampered due to its coarse nature. Any attempt to improve the quality of the phloem fibre of jute would be valued for potential diversification of jute usage (Palit, 1999, 2001). Continuous attempts through breeding processes, X-ray induced mutation for generation of improved jute variety and also in improved retting process (Kharkwal et. al. 2004; Das et. al. 2018), to get the improved quality of jute fibres are being made without any significant outcome. Here, an induced mutant of Corchorus capsularis having an altered bast fi bre was compared with a normal jute plant and identify some of the histobiochemical and physicochemical characters of jute fibres.
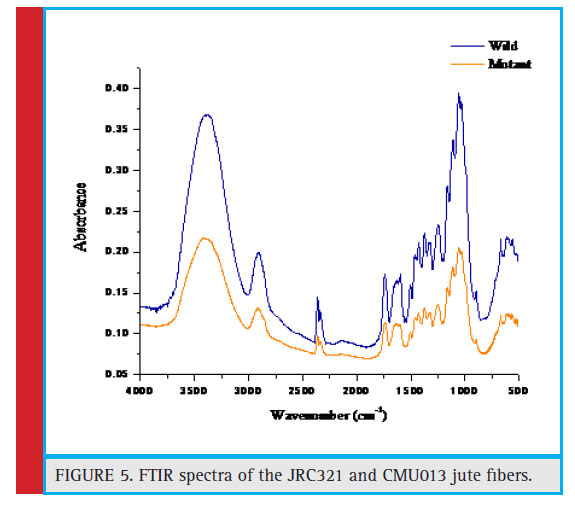 |
Figure 5: FTIR spectra of the JRC321 and CMU013 jute fibers |
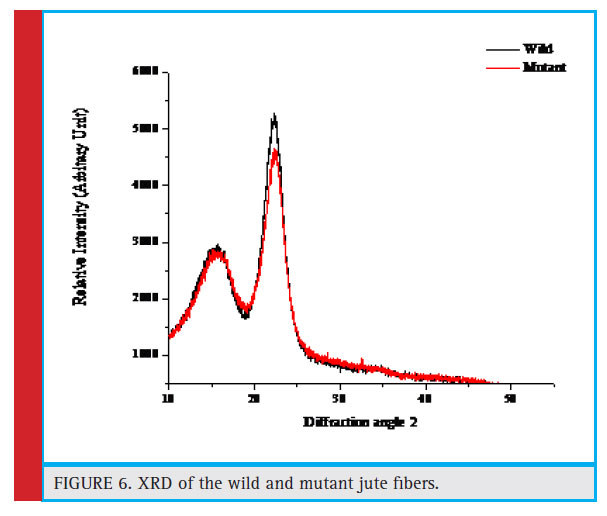 |
Figure 6: XRD of the wild and mutant jute fibers. |
Histobiochemical evidence of jute stem of cultivated variety JRC 321 demonstrates that the development of the secondary cell wall in phloem was initiated at a particular stage of development of jute plant and matured well with deposition of cementing materials, particularly lignin (Figures 1 and 2) as evidenced in our previous studies (Samanta et. al. 2015). This deposition of the cementing materials was found to be prerequisite for generation of well-developed fibre revealed from the comparative study of the normal with mutant by SEM (Figures 3 and 4).
These data are supported by previous study of Meshram and Palit (2013) where the authors found that the cell wall thickness, strength of fibre and lignin content goes together and the high cellulose content results fibre fineness. Compositional analysis of the fibre forming components indicated that the fibre of normal jute contains 57% more lignin than mutant. The data are also supported by the earlier evidence that the secondary wall development and formation of compact group of the bast fibre cells involve deposition of lignin over the cellulose matrix (Priestley and Scott, 1936; Kundu et al., 1959; Preston, 1974; Grabber et al., 1991) though the presence of high amount of lignin was believed to be undesirable for fibre quality (Harlley, 1987). In contrast, the mutant fibre was found to contain a comparatively high amount of cellulose (Table 1) that could be expected to improve the quality and strength of the fibre. This observation was supported by another study in (Corchorus olitorius L.) mutant with low lignin (7.23%) in phloem fibre being compared to wild-type JRO 204 (13.7%) was identified and characterized (Choudhary et al 2017).
However, a contrary result was observed in continuity and tensile properties of the fibre. The fibre generated from mutant on retting was found to be discontinuous or fragmented. It is difficult to conclude whether this fragmented nature of fibre was due to less deposition of lignin or due to poor development of the cell wall. The fibre of the normal showed to be stronger (tensile strength of 952.4 MPa) with an elongation percentage at break of 2.512 % than the mutant jute (tensile strength of 600.8 MPa) with an elongation percentage at break of 1.676 % (Figure 4). This data was supported by a previous study in which the authors have used different natural fibres and studied the effect of fibre morphology on the tensile strength (Fidelisa et. al. 2013).
They found that the increased secondary cell-wall thickness causes increase in both the fiber strength and Young’s modulus. This observation was further supported by the FTIR analysis and XRD analysis of both normal and mutant fibres (Figures 5 & 6). The XRD analysis of normal fibre revealed more crystallinity than that in mutant. Due to higher crystallinity (20.53%) of normal, it is stronger than the mutant jute having lower crystallinity (13.9%), which supports the experimental observation of strength property. This result was supported by a previous study in which the authors found similar result (Saha et. al. 2010). Further to this, the higher crystallinity in JRC 321 fibre can be ascribed to the presence of more intermolecular H-bonding compared to that in mutant jute. FTIR analysis results (Figure 5 and Table 2) indicate the shifting of O-H stretching band from 3382.98 cm-1 in JRC321 to 3415.74 cm-1 in mutant. This confirms the influence of H-bonding on the higher crystalline nature of JRC 321.
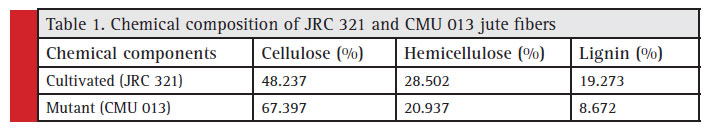 |
Table 1: Chemical composition of JRC 321 and CMU 013 jute fibers |
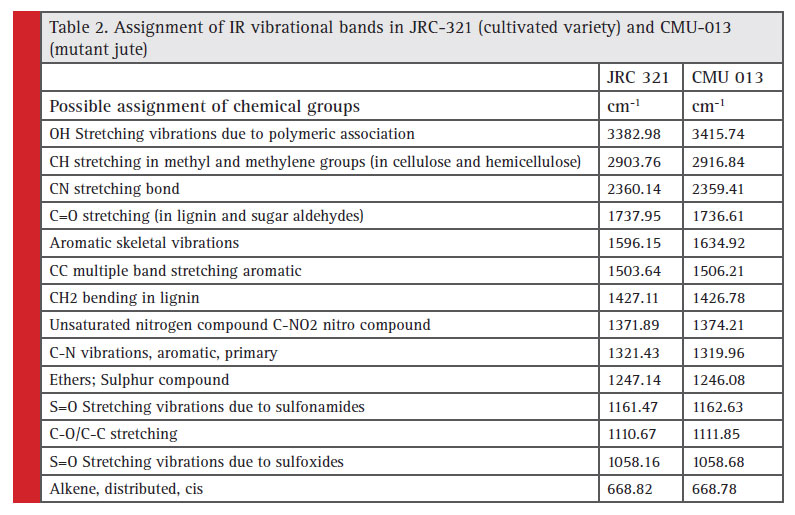 |
Table 2: Assignment of IR vibrational bands in JRC-321 (cultivated variety) and CMU-013 (mutant jute) |
Conclusion
It could be concluded that the molecular interaction of cellular components determines the fibre quality in jute in spite of the developmentally regulated synthesis of lignin only as evidenced earlier (Sengupta and Palit, 2004). Moreover, it could be expected that this interaction was genetically regulated as it was found to be hampered in the mutant. This mutant may serve as a model to understand the molecular mechanism that organizes the development of secondary wall thickening in jute bast fibre cells. The study will help in the development of desired jute fibre through genetic modifications.
Acknowledgement
Financial assistance in the form the grant support from the Department of Biotechnology, Government of India is acknowledged. Author would like to thank Dr. Jayanta Maity, Polymer and Textile Research Laboratory, Department of Chemistry, Sidho Kanho Birsha University for interpreting some of the data.
Strain is defined as the ratio of change in length to the original length and a ratio do not have any unit. But in the present study it was calculated in “mm/mm”.
References
Bera, A.K., Bandyopadhyay, S., Sen, S.K., Ghosh, S., Banerjee, A., (2002). Structural quality assessment of different cellulosic jute fi bres by X-ray diffraction, Indian Journal of Fibre & Textile Research 27: 65-71.
Choudhary, S.B., Chowdhury, I., Singh, R.K., Pandey, S.P., Sharma, H.K., Anil Kumar, A., Karmakar, P.G., Kumari, N., Souframanien, J., Jambhulkar, S. J. (2017). Morphological, Histobiochemical and Molecular Characterisation of Low Lignin Phloem Fibre (llpf) Mutant of Dark Jute (Corchorus olitorius L.), Applied Biochemistry and Biotechnology. 183(3):980– 992.
Crang, R., Lyons-Sobaski, S., Wise, R. (2018). Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants, 1st edn. Chapter 5:155-179.
Springer. Das, S., Majumdar, B., Saha, A.R., Sarkar, S., Jha, S.K. Sarkar, S.K., Saha, R. (2018). Comparative Study of Conventional and Improved Retting of Jute with Microbial Formulation, Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 88(4):1351–1357.
Day, A., Ruel, K., Neutelings, G., Crônier, D., David, H., Hawkins, S., Chabbert, B. (2005). Lignifi cation in the fl ax stem: evidence for an unusual lignin in bast fi bers, Planta. 222(2): 234–245.
Edwards, H.G.M., Farwell, D.W., Webster, D. (1997). FT Raman microscopy of untreated natural plant fi bers, Spectrochimica Acta Part A, 53: 2383-2392.
Evert, R.F. (2006). Esau’s plant anatomy, 3rd edn. John Wiley & Sons, Inc., Publication. Fahn, A. (1990). Plant anatomy, 4th edn. Oxford: Pergamon Press.
Fidelisa, M.E.A., Pereira, T.V.C., Gomes, O. da Fonseca M., Silva, F. de Andrade., Filho, R.D.T. (2013). The effect of fiber morphology on the tensile strength of natural fibers, J Mater Res Technol. 2(2):149–157.
Gorshkova, T., Brutch, N., Chabbert, B., Deyholos, M., Hayashi, T., Lev-Yadun, S., Mellerowicz, E.J., Morvan, C., Neutelings, G., Pilate, G. (2012). Plant fibre formation: state of the art, recent and expected progress, and open questions, Crit Rev Plant Sci 31:201–228
Grabber, J.H., Jung, G.A., and Hill, R.R.Jr. (1991). Chemical composition of parenchyma and sclerenchyma cell walls isolated from orchardgrass and switchgrass, Crop Sci. 31: 1058–1065.
Guerriero, G., Behr, M., Legay, S., Mangeot-Peter, L., Zorzan, S., Ghoniem, M., Hausman, J.F. (2017). Transcriptomic profi ling of hemp bast fi bres at different developmental stages, Sci Rep. 7(1):4961.
Harlley, R.D. (1987). The chemistry of lignocellulosic materials from agricultural wastes in relation to processes for increasing their biodegradability, In Meer JM, van der Rijkens BA, Ferranti MP, eds. Degradation of lignocellulosics in ruminants and in industrial processes. London and New York: Elsevier Applied Science Publishers.
Kharkwal M.C., Pandey R.N., Pawar S.E. (2004). Mutation Breeding for Crop Improvement, In: Jain H.K., Kharkwal M.C. (eds) Plant Breeding. Springer, Dordrecht Kundu B.C. (1944). Anatomy of jute stem with special reference to cambial activity and distribution of fi bres in relation
to leaf-trace system, Journal of Royal Asiatic Society, Bengal (Science) 10: 27–52.
Kundu, A., Sarkar D., Mandal, N.A., Sinha, M.K., Mahapatra, B.S. (2012) A secondary phloic (bast) fi bre-shy (bfs) mutant of dark jute (Corchorus olitorius L.) develops lignifi ed fi bre cells but is defective in cambial activity, Plant Growth Regulation 67(1):45–55.
Kundu, B.C., Basak, K.C., and Sarkar, P.B. (1959). Jute in India (ed.) M.G. Kamath. The Indian central jute committee, Calcutta.
McGovern, J. N., Coffelt, D. E., Hurter, A. M., Ahuja, N. K., and Weidermann, A, (1983). Other fibres, in Pulp and Paper Manufacture,
Vol. 3, Secondary Fibres and Agro-based Pulping, Hamilton F, Leopold B, Eds., TAPPI Press, Atlanta, Chapter IX.
Meredith R. (1956). Mechanical properties of Textile Fibres, Interscience Publisher, Inc., New York, Chaps. VI and VII.
Meshram, J.H., Palit, P. (2013) On the role of cell wall lignin in determining the fineness of jute fibre, Acta Physiologiae Plantarum 35(5):1565–1578.
Palit, P. (1999). Jute. In: Smith DL, Hamel C. eds. Crop yield, physiology and processes. Berlin: Springer-Verlag 271–286.
Palit, P., Sengupta, G., Datta, P., and Meshram, J. (2001). Lignin and lignifi cation with special reference to its down regulation for the improvement of wood and bast fi bre quality, Indian Journal of Plant Physiol. 6: 217–228.
Pan, N.C., Dey, A., Mahalanabish, K.K. (1999). Infrared spectra of bleached jute, Colourage 46(1): 15-19.
Preston, R.D. (1974). The physical biology of plant cell walls, London: Chapman and Hall. Priestley, J.H., and Scott, L.I. (1936). Vascular anatomy of Helianthus annus L., Proceedings of the Leeds Philological and Literary Society Science Section 3: 159–173.
Rana, A.K., Basak, R.K., Mitra, B.C., Lawther, M., Banerjee, A.N. (1997). Studies of acetylation of jute using simplified procedure and its characterization, Journal Applied Polymer Science 64:1517- 1523.
Ray, P.K. (1969). On the degree of crystallinity in jute and mesta fibres in different states of purifi cations and moisture conditions, Journal Appl Polym Sci. 13(12): 2593-2600.
Rials T.G. (1997). Wolcott MP in Paper and Composites from Agro-Based Resources, Rowell RM, Young RA, Rowell JK, editors, Lewis Publishers, New York, P.73.
Roy, M.M. (1953). Mechanical properties of jute. II. The study of chemically treated fibres, Text Inst J. 44:T44.
Sadasivam S, Manickam A. (2008). Biochemical Methods, 3rd Edn., New Age International (P) Ltd. Publishers, New Delhi, pp 12-13, 208.
Saha, P., Manna, S., RoyChowdhury, S., Sen, R., Roy D., Adhikari, B. (2010). Enhancement of tensile strength of lignocellulosic jute fibers by alkali-steam treatment, Bioresource Technology. 101(9):3182-3187.
Samal, R.K., Mohanty, M., Panda, B.B. (1995). Effect of chemical modification on FTIR spectra: Physical and chemical behavior of jute-II, Journal of Polymer Materials 12(3): 235-240.
Samanta, P., Sadhukhan, S., Basu, A. (2015). Identifi cation of differentially expressed transcripts associated with bast fibre development in Corchorus capsularis by suppression subtractive hybridization, Planta. 241(2):371-385.
Sengupta, G., and Palit, P. (2004). Characterization of lignifi ed secondary phloem fibre-deficient mutant of jute (Corchorus capsularis), Annals of Botany 93: 211-220.
Snegireva, A., Chernova, T., Ageeva, M., Lev-Yadun, S., Gorshkova, T. (2015). Intrusive growth of primary and secondary phloem fi bres in hemp stem determines fi bre-bundle formation and structure, AoB Plants. 7: plv061
Srivastava LM. (1966). Histological studies on lignin, Tappi 49:173-183
Varma DS, Varma M, Varma IK. (1984). Coir Fibers: Part I: Effect of Physical and Chemical Treatments on Properties, Journal of Textile Research Inst 54:349.
Young, R. A. (1993). Kirk-Othmer Encyclopedia of Chemical Technology, 4th edition Vol.10, John Wiley and Sons, New York.