Department of Chemistry, Hemchandracharya North Gujarat University, Patan, Gujarat, India.
Corresponding author email: drkap_chem@yahoo.com
Article Publishing History
Received: 17/09/2021
Accepted After Revision: 21/11/2021
The advancement of green nanotechnology has piqued the interest of researchers into the environmentally responsible production of nanoparticles. Conventionally used chemical methods for the synthesis of the nanoparticles have shown adverse effect on environment due to the use of highly toxic chemicals. They are also expensive as they utilize costly chemicals as a reducing and capping agent. Use of plant extract can be an environment friendly and cost-effective approach for the synthesis of nanoparticles. Copper is the metal which humans utilize from the ancient time period and it doesn’t show any adverse effect on humankind as well as on environment. Leaf extract of Ocimum sanctum was employed with CuSO4 (1:9, v/v) to synthesize stable copper nanoparticles (CuNPs) that were then functionalized with Polyvinyl Pyrrolidone (PVP) polymer.
Characterization of synthesized copper nanoparticles was carried out using UV–Visible spectroscopy, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and transmission electron microscopy (TEM). Synthesized CuNPs were subjected against the human pathogenic bacterial strain to evaluate their antibacterial potency. Antioxidative characteristics of CuNPs were analyzed using DPPH free radical scavenging activity. The UV–visible spectra of CuNPs showed unique peaks at 322 and 247 nm indicates the stable formation of nanoparticles. X-ray diffraction pattern suggest the face cubic centered (FCC) structure of copper nanoparticles.
FTIR analysis revealed the presence of biomolecules attached on the surface of CuNPs. TEM analysis proven the synthesis of spherical shaped CuNPs with the average particle size of 73.50 ± 1.78 nm. Biosynthesized CuNPs showed maximum zone of inhibition against E. coli which was tends to be 20 mm. 51.48 % of DPPH free radical scavenging activity was observed by synthesized PVP coated CuNPs. As a result, this technology can be employed for the quick and environmentally friendly biosynthesis of stable copper nanoparticles with antibacterial and antioxidant activities with the size range from 10 to 100 nm, implying their potential application in the healthcare, clinical as well as pharmaceutical fields.
Antibacterial, Antioxidant, Copper Nanoparticles, Ocimum Sanctum, Polymer Functionalized
Makvana C, Arodiya F, Parmar K. Green Synthesis of Polymer-Capped Copper Nanoparticles Using Ocimum sanctum Leaf Extract: Antibacterial and Antioxidant Potential. Biosc.Biotech.Res.Comm. 2021;14(4).
Makvana C, Arodiya F, Parmar K. Green Synthesis of Polymer-Capped Copper Nanoparticles Using Ocimum sanctum Leaf Extract: Antibacterial and Antioxidant Potential. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3DgVtjw“>https://bit.ly/3DgVtjw</a>
Copyright © Makvana et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Nanostructures with varying physical, chemical, and electrical properties enable a wide range of applications which includes potent antimicrobial and antioxidative agents, optical and electrical materials. Copper and its alloys, which have increased in value among Nobel/transition metals, have been used efficiently in a variety of applications such as electrical, catalytic, optical, and as an antibacterial and antifungal agent (Bhatti et al. 2015; Ashar et al. 2016; Cao et al. 2016; Iqbal et al. 2017; Khalil et al. 2019; Punniyakotti et al. 2020). Copper nanoparticles have been described as a potential substitute for gold, palladium, silver, and platinum nanoparticles due to their use as an antibacterial agent, and they also have quality properties in bioscience, biomedical, catalytic, dielectric, imaging, magnetic, and other fields.
In terms of benefits, medicinal plants are cost-effective, abundantly available, safe to handle, non-toxic, and this biological technology may be utilised for the industrial scale production of copper nanoparticles (Akbari et al. 2021). In recent years, algae, bacteria, fungi, mushrooms, enzymes and plant leaf extracts have been used to create non-toxic, energy-efficient, cost-effective, and environmentally friendly metallic nanoparticles (Rajeshkumar et al. 2019; Mumtaz et al. 2021).
Plants are better platform for nanoparticles manufacturing since they are free of hazardous chemicals and contain natural capping agents. Furthermore, the use of plant extracts lowers the cost of microbe isolation and culture media, improving the cost-competitive viability of microorganism-based nanoparticles synthesis. We report on the fast production of copper nanoparticles utilising Ocimum sanctum leaf extract in this paper.
Tulsi is an Indian traditional medicinal plant that is high in bio-reduction and stabilisers. Tulsi contains alkaloids, glycosides, tannins, saponins, and aromatic compounds, as well as minerals such as Ca, Mn, Cu, Zn, P, K, Na, and Mg, with tulsi leaves having a higher concentration of Cu than other leaves. It has a Cu content of 12.31 mg/kg (Chowdhury et al. 2008; Dubey and Pandey 2018; Wang et al. 2021).
Urosolic acid is the primary active ingredient of tulsi leaves. Tulsi is employed in ayurvedic medicine due of its therapeutic properties. Tulsi is also an excellent reducer. In an aqueous chemical approach, gallic acid was responsible for the reduction of silver ions into silver nanoparticles (Soundarrajan et al. 2012). Ocimum sanctum leaf extracts have recently been employed in the manufacture of silver and gold nanoparticles. Tulsi contains bio-reducers and stabilisers (Vennila et al. 2016).
Copper is extremely harmful to microorganisms like bacteria. Lemon fruit extract, Green coffee bean extract, Neem flower extract, Citrus Paradisi fruit peel extract, Hibicus rosasinensi, Ocimum santanum leaf extract, Syzygium aromaticum (Cloves), Vitis vinifira extract, Eucalyptus, Cassia alata, Centella asiatica, Malva sylvestris, Leaf extract of capsicum frutescens and other plant extracts were used to create copper nanoparticles (Hariprasad et al. 2016; Awwad et al. 2021; Ghaffar et al. 2021; Gopalakrishnan et al. 2021; Velsankar et al. 2021; Wang et al. 2021).
MATERIAL AND METHODS
Leaf extract was prepared accordingly method described by Khan et al. 2016. In brief, 5 g of fresh Ocimum sanctum leaves were taken and rinsed thoroughly with distilled twice. Leaves were then dried on filter paper to eliminate any remaining moisture. 100 mL of distilled water was added using a measuring cylinder and heated for 10 min. Then the leaf extract was filtered through Whatman’s filter paper no. 1 and the filtrate was kept at 4 °C till the use.
For the synthesis of copper nanoparticles under continually stirring condition, 25 mL plant extract was added to the 100 mL of 1 mM aqueous CuSO4·5H2O solution dropwise. After thoroughly combining the leaf extract with the precursor, the mixture was incubated at 31 °C for 24 h. Continues synthesis of CuNPs was observed by a change in colour from pale green to light yellow. After 24 h CuNPs were then centrifuged at 6000 rpm for 30 min at room temperature. The obtained pellets were then re-dispersed into the deionized water to eliminate any undesired biological elements (Khan et al. 2016).
For the synthesis of polymer functionalized copper nanoparticles in 100 mL of ultra-pure water, 0.2 g of PVP (Polyvinyl pyrrolidone) was dissolved and agitated for 1 h at 80 °C. After that, the solution was gradually added to the homogenous solution of CuNPs generated from the leaf extract. After 1 h, the mixture’s light colour yellowish was converted to a light brown colour. The reaction mixture was allowed to cool for 10 min before being centrifuged at 10000 rpm for 15 min.
The precipitates formed were washed with deionised water and dried in a 70 °C oven for 24 h (Aisida et al. 2019). For the characterization of green CuNPs and PVP functionalized CuNPs different typical current techniques were used to characterise synthesised CuNPs and PVP functionalized CuNPs. A UV-visible spectrophotometer (Perkin Elemer USA) was used to confirm the production of CuNPs and polymer functionalized CuNPs (Hulikere et. al. 2019).
FTIR analysis in the 500 – 4000 cm-1 range was used to confirm the functional bio molecules associated with the produced CuNPs and PVP functionalized CuNPs. XRD analysis was performed to obtain unique diffraction pattern of synthesized CuNPs using a Rigaku D/max 40 kv X-ray diffraction spectrometer. High resolution transmission electron microscopy was used to analyse the structural morphology of CuNPs (HR- TEM) (Hulikere et. al. 2019). For the antibacterial activity of synthesized AgNPs was carried out via a well diffusion method reported in previous studies with some modifications (Hulikere et. al. 2019).
All the test bacterial strains were grown in nutrient broth at 37 °C overnight and adjusted to 0.5 as per McFarland standards. Under sterile conditions, 100 μL of two Gram-positive (Bacillus subtilis and Staphylococcus aureus) and three Gram-negative strains (Pseudomonas aeruginosa and Escherichia coli) were spread on each nutrient agar plate. A diameter well of 10 mm was punched on the agar plate using a cork borer and the synthesized CuNPs and PVP CuNPs were inoculated in each well. Similarly, 100 μL of streptomycin (1 mg/mL) served as a positive control. Plates were incubated at 37 °C for 24 h and the antibacterial activity was evaluated by measuring the diameter of the inhibition zone using zone scale (HiMedia) (Hulikere et. al. 2019).
For the antioxidant activity, antioxidant properties of synthesized CuNPs and PVP functionalized CuNPs was examined using DPPH method. Ascorbic acid was taken as standard due to its high antioxidant properties. Standard solution of ascorbic acid as well as various concentration (10, 20, 30, 40, 50, 75, 100 μg/mL) were prepared. DPPH was prepared by weight of 20 mg was taken and dissolved in 100 mL methanol.
1 mL of various concentration CuNPs and PVP functionalized CuNPs and standard ascorbic acid solution were mixed separately with 1ml of DPPH solution and incubated for 30 min. The absorbance was measured by UV- Visible Spectrophotometer at 517 nm. The free radical scavenging activity was represented as the % of inhibition, calculated by using following formula (Mistry et al. 2021).
![]()
RESULTS AND DISCUSSION
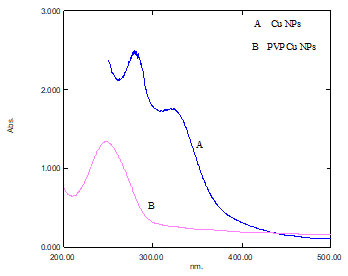
UV-visible spectroscopic analysis: To confirm the generation of CuNPs, the aqueous reduction reaction mixture was tested by a UV-visible spectrophotometer. The reduction of Cu+2 to Cu+1 detected by a colour change from light green to brownish was caused by PVP CuNPs excitation surface plasmon resonance (SPR) which finally proved the creation of CuNPs. The observed results are substantially consistent with recent research (Wang et al. 2015; Aswathanarayana et al. 2017).
The absorbance peaks of CuNPs and polymer functionalized CuNPs formed successfully are at 322 and 247 nm. According to research, the SPR of most metallic compounds varies with size and shape (Oberdörster et al. 2005; Soylu et al. 2006; Zhang et al. 2010; Aswathanarayana et al. 2017).
Figure 1: Colour Change from light green to yellowish [A] After 24 h [B] After adding PVP solution

Figure 2: UV Visible spectrum of [A] Cu NPs [B] PVP Cu NPs
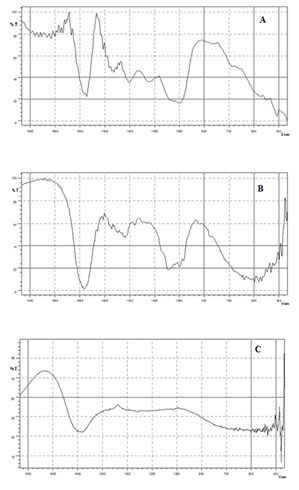
Fourier Transforms Infrared Spectroscopy (FTIR) analysis: The FTIR measurements of biosynthesized copper nanoparticles were carried out to identify the possible interaction between protein and copper nanoparticles. Results of FTIR study showed absorption peaks located at about 1600,1024, 1480,1377 and 449 cm-1 (Fig.3). The band at 1653 cm-1 attributed to C = C stretching. In addition, peaks at 1100 and 1700 cm-1 corresponds to C-O and C=O stretching, respectively of leaf extracts. Additionally, the observed peak at 610 cm-1 correspond to the formation of CuNPs, which is in accordance with previous finding (Zowalaty et al. 2013; Yugandhar et al. 2017).
The band at 1480-1320cm-1 attributed to C-H bending. The band at 1024 cm-1 for C-X stretching. These IR spectroscopic studies confirmed that carbonyl group of amino acid residues have strong binding ability with metal suggesting the formation of layer covering metal nanoparticles and acting as capping agent to prevent agglomeration and providing stability to the medium. These results confirm the presence of possible proteins acting as reducing and stabilizing agents (Varghese et al. 2020).
Figure 3: FT-IR Spectrum of [A] Ocimum sanctum leaf extract [B] CuNPs [C] PVP CuNPs
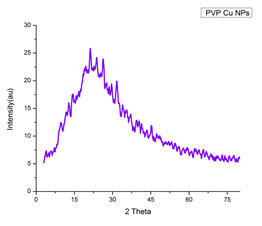
X-RAY diffraction analysis: The XRD pattern of polymer functionalized CuNPs (shown in Fig.4) revealed a well-crystallized sample with prominent diffraction peaks at 2 theta values of 19.64, 41.46, 45.47 and 72.28 corresponding to planes (100), (111), (200), and (311) (Sengottuvelan et al. 2014; Soomro et al. 2014). The bioconjugate between the polymer component and the formed polymer capped CuNPs may be attributed to the modulation of the phase transition by PVP. The Debye-Scherer formula was used to calculate the mean particle size of PVP CuNPs, which is given as,
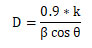
Where D is the crystalline size (nm), k is the X-ray wavelength (0.1541 nm), β represents the angular line full width at half maximum (FWHM) of the peak (in radians), and θ is the Braggs angle (in radians) (Guo, 2018). The PVP CuNPs were calculated to have an average particle size of 70.20 nm, which is in good agreement with the HR-TEM average particle size of 73.50 nm (Sengottuvelan et al. 2014; Soomro et al. 2014; Varghese et al. 2020).
Figure 4: XRD pattern of PVP functionalized CuNPs
HR TEM analysis: The H-7500 model was used for high resolution transmission electron microscopy (HR TEM). HR TEM was used to study the size and morphology. The polymer functionalized CuNPs had a spherical shape uniform size with an average particle size of 73.50 nm. The crystallinity of the biosynthesized polymer functionalized CuNPs was demonstrated using the selected area electron diffraction (SAED) pattern with bright circular spots.
Figure 5: HR-TEM images [A], [B], [C] and SAED image [D] of PVP CuNPs
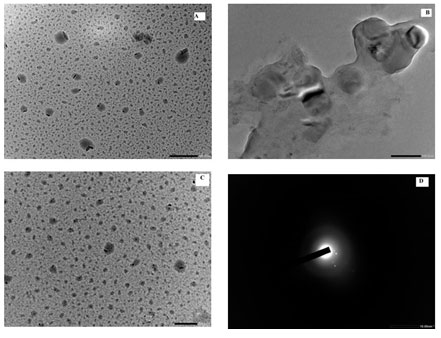
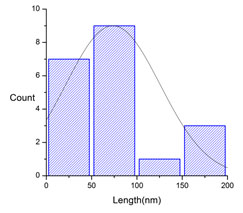
Figure 6: The size distribution curve from the TEM analysis of PVP functionalized CuNPs
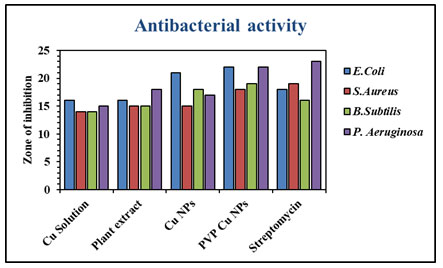
Antibacterial activity of CuNPs and Polymer functionalized CuNPs: The antibacterial potential of CuNPs was assessed by measuring the Inhibition zone of plant extract, Copper nanoparticles and polymer functionalized nanoparticles was summarised in table 1. The PVP CuNPs inhibit growth of Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Pseudomonas aeruginosa shown in figure 7. It also shows good activities then the all the organism in comparison with standard drug. Because of its lower concentration activities, PVP CuNPs has a higher possibility of becoming a possible alternative for conventional antibacterial medicines (Mistry et al. 2021).
Figure 7: Antimicrobial study of biosynthesized CuNPs and PVP CuNPs against pathogenic bacteria [A]
Escherichia coli [B] Pseudomonas aeruginosa [C] Bacillus subtilis [D] Staphylococcus aureus
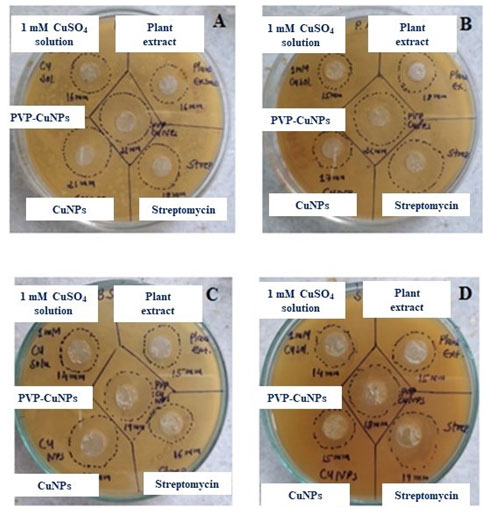
Table 1. Antibacterial activity of CuNPs and PVP CuNPs
| SR.NO. | Organism
|
Zone of Inhibition (In mm) | ||||
| CuSO4 Solution
(1 mM) |
Plant extract | Cu NPs | PVP-CuNPs
|
Streptomycin
(1 mg/ml)
|
||
| 1 | Escherichia coli | 16 | 16 | 21 | 22 | 18 |
| 2 | Staphylococcus aureus | 14 | 15 | 15 | 18 | 19 |
| 3 | Bacillus subtilis | 14 | 15 | 18 | 19 | 16 |
| 4 | Pseudomonas aeruginosa | 15 | 18 | 17 | 22 | 23 |
Figure 8: Antibacterial zone of inhibition of CuNPs and PVP CuNPs in
comparison with standard streptomycin
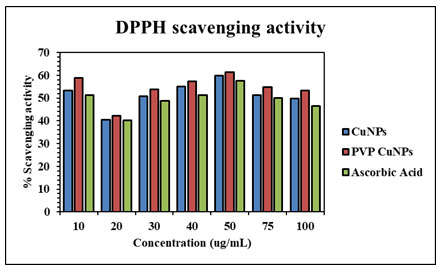
Antioxidant activity of CuNPs and Polymer functionalized CuNPs: DPPH is a stable molecule that can be reduced by taking hydrogen or electrons and has been commonly used to assess antioxidant activity (Mistry et al. 2021). CuNPs demonstrated effective antioxidant capability, with their radical scavenging capacity rising with concentration. The figure depicts the antioxidant activity of CuNPs, which is around 51.48 %. The percentage of PVP-CuNPs was around 54.54 %. The findings confirmed that polymer-capped CuNPs have higher antioxidant activity than CuNPs. The antioxidant property of CuNPs is due to plant component absorption on the copper nanoparticles (Keshari et al.2021).
Figure 9: Antioxidant activity (%) of synthesized CuNPs and PVP CuNPs in
comparison with standard ascorbic acid
CONCLUSION
The findings of the present study describe an environmentally friendly and cost-effective biological technique for producing polymer-capped nanoparticles with antibacterial and antioxidant activities. Using an extract of Ocimum sanctum (Tulsi) leaves and CuSO4·5H2O salt solution, copper nanoparticles were successfully produced. The generated CuNPs were further functionalized with PVP to improve biocompatibility without the addition of any harmful or toxic materials.
The UV visible confirmed the creation of CuNPs trolls by changing the visible colour to dark brown after 24 h, with a peak at 247 nm. The FTIR spectra revealed the various functional groups in the Ocimum sanctum extract that were responsible for the biogenic synthesis of CuNPs and polymer generated CuNPs. XRD examination confirmed the crystalline nature and average particle size of 70.20 nm of polymer capped CuNPs. HR-TEM imaging microscopy revealed a spherical form with particle sizes ranging from 10 to 100 nm. CuNPs and polymer capped CuNPs shown good antibacterial and antioxidant activities.
ACKNOWLEDGEMENTS
This study was supported by the Department of Chemistry, Hemchandracharya North Gujarat University, Patan, Gujarat, India.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests: Authors declare no conflict of interests to disclose.
REFERENCES
Aisida, S.O., Ugwoke, E., Uwais, A., et al. (2019). Incubation period induced biogenic synthesis of PEG enhanced Moringa oleifera silver nanocapsules and its antibacterial activity. Journal of Polymer Research, 26(9), pp.1-11.
Akbari, R., (2021). Green synthesis and catalytic activity of copper nanoparticles supported on TiO2 as a highly active and recyclable catalyst for the reduction of nitro-compounds and degradation of organic dyes. Journal of Materials Science: Materials in Electronics, pp.1-13.
Amer, M.W. and Awwad, A.M., (2021). Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. Chem Int, 7(1), pp.1-8.
Ashar, A., Iqbal, M., Bhatti, I.A., et al. (2016). Synthesis, characterization and photocatalytic activity of ZnO flower and pseudo-sphere: Nonylphenol ethoxylate degradation under UV and solar irradiation. Journal of Alloys and Compounds, 678, pp.126-136.
Bhatti, I.A., and Iqbal, M. (2015). Gamma radiation/H2O2 treatment of a nonylphenol ethoxylates: degradation, cytotoxicity, and mutagenicity evaluation. Journal of hazardous materials, 299, pp.351-360.
Bhowmik, S., Chowdhury, S.D., Kabir, M.H. et al. (2008). Chemical composition of some medicinal plant products of indigenous origin. Bangladesh Veterinarian, 25(1), pp.32-39.
Cao, J., Sun, X., Lu, C., et al. (2016c). Homogeneous synthesis of Ag nanoparticles-doped water-soluble cellulose acetate for versatile applications. International journal of biological macromolecules, 92, pp.167-173.
Dubey, R. and Pandey, S.K., (2018). Medicinally important constituents of tulsi (Ocimum spp.), In Synthesis of Medicinal Agents from Plants. Elsevier, pp. 151-176.
Ghaffar, A., Kiran, S., Rafique, M.A., et al. (2021). Citrus paradisi fruit peel extract mediated green synthesis of copper nanoparticles for remediation of Disperse Yellow 125 dye. DESALINATION AND WATER TREATMENT, 212, pp.368-375.
Gopalakrishnan, V. and Muniraj, S., (2021). Neem flower extract assisted green synthesis of copper nanoparticles–Optimisation, characterisation and anti-bacterial study. Materials Today: Proceedings, 36, pp.832-836.
Guo, R. (2018). Analysis of cation-treated clay microstructure using zeta potential and x-ray diffraction (Doctoral dissertation, University of Alaska Fairbanks).
Hariprasad, S., Bai, G.S., Santhoshkumar, J., et al. (2016). Green synthesis of copper nanoparticles by Arevalanata leaves extract and their anti-microbial activites. International Journal of ChemTech Research, 9(02).
Hulikere, M.M. and Joshi, C.G., (2019). Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus-Cladosporium cladosporioides. Process biochemistry, 82, pp.199-204.
Iqbal, M., Nisar, J., Abbas, M., et al. (2017). Mutagenicity and cytotoxicity evaluation of photo-catalytically treated petroleum refinery wastewater using an array of bioassays. Chemosphere, 168, pp.590-598.
Keshari, A.K., Srivastava, A., Chowdhury, S. et al. (2021). Green synthesis of silver nanoparticles using Catharanthus roseus: Its antioxidant and antibacterial properties. Nanomedicine Research Journal, 6(1), pp.17-27.
Khalil, I., Yehye,A. W., Etxeberria, A. E., et al. (2019). Nanoantioxidants: Recent trends in antioxidant delivery applications. In: Antioxidants. 9 (1), pp. 24, DOI: 10.3390/antiox9010024.
Khan, F.A., Zahoor, M., Jalal, A. et al. (2016). Green synthesis of silver nanoparticles by using Ziziphus nummularia leaves aqueous extract and their biological activities. Journal of Nanomaterials, 2016.
Li, Q., Zhang, Z., Haque, S.S., et al. (2010). Localized surface plasmon resonance effects by naturally occurring Chinese yam particles. Journal of Applied Physics, 108(12), p.123502.
Mistry, H., Thakor, R., Patil, C., et al. (2021). Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnology Letters, 43(1), pp.307-316.
Mumtaz, S., Nadeem, R., Sarfraz, R.A. et al. (2021). Synthesis, Characterization, and Evaluation of Antimicrobial and Antioxidant Potential of Polyalthia longifolia Mediated Copper Nanoparticles. In Journal of Nano Research, Vol. 68, pp. 35-51.
Oberdörster, G., Oberdörster, E. and Oberdörster, J., (2005). Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental health perspectives, 113(7), pp.823-839.
Punniyakotti, P., Panneerselvam, P., Perumal, D., et al. (2020). Anti-bacterial and anti-biofilm properties of green synthesized copper nanoparticles from Cardiospermum halicacabum leaf extract. Bioprocess and biosystems engineering, 43(9), pp.1649-1657.
Rajeshkumar, S., Menon, S., Kumar, S.V., et al. (2019). Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. Journal of Photochemistry and Photobiology B: Biology, 197, p.111531.
Sampath, M., Vijayan, R., Tamilarasu, E., et al. (2014). Green synthesis of novel jasmine bud-shaped copper nanoparticles. Journal of Nanotechnology, 2014.
Soomro, R.A., Sherazi, S.H., Memon, N., et al. (2014). Synthesis of air stable copper nanoparticles and their use in catalysis. Advanced Materials Letters, 5(4), pp.191-198.
Soundarrajan, C., Sankari, A., Dhandapani, P., et al. (2012). Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess and biosystems engineering, 35(5), pp.827-833.
Soylu, E.M., Soylu, S. and Kurt, S., (2006). Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia, 161(2), pp.119-128.
Usha, S., Ramappa, K.T., Hiregoudar, S., et al. (2017). Biosynthesis and characterization of copper nanoparticles from tulasi (Ocimum sanctum L.) leaves. International Journal of Current Microbiology and Applied Sciences, 6(11), pp.2219-2228.
Usman, M.S., El Zowalaty, M.E., Shameli, K., et al. (2013). Synthesis, characterization, and antimicrobial properties of copper nanoparticles. International journal of nanomedicine, 8, p.4467.
Varghese, B., Kurian, M., Krishna, S. et al. (2020). Biochemical synthesis of copper nanoparticles using Zingiber officinalis and Curcuma longa: Characterization and antibacterial activity study. Materials Today: Proceedings, 25, pp.302-306.
Velsankar, K., Suganya, S., Muthumari, P., et al. (2021). Ecofriendly green synthesis, characterization and biomedical applications of CuO nanoparticles synthesized using leaf extract of Capsicum frutescens. Journal of Environmental Chemical Engineering, p.106299.
Vennila, S. and Nithya, T., (2016). Green synthesis of copper, silver nanoparticles using Ocimum tenuiflorum leaf extract. world journal of pharmaceutical research, 5(1), pp.257-265.
Wang, G., Zhao, K., Gao, C., et al. (2021). Green synthesis of copper nanoparticles using green coffee bean and their applications for efficient reduction of organic dyes. Journal of Environmental Chemical Engineering, 9(4), p.105331.
Wang, Y., Ou, J.Z., Chrimes, A.F., et al. (2015). Plasmon resonances of highly doped two-dimensional MoS2. Nano letters, 15(2), pp.883-890.
Yugandhar, P., Vasavi, T., Devi, P.U.M. et al. (2017). Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Applied Nanoscience, 7(7), pp.417-427.


