1Department of Microbiology, Acharya Bangalore B-School, Bengaluru, Karnataka, India.
2*Department of Studies in Microbiology, University of Mysore, Manasagangotri, Karnataka, India.
Corresponding author email: keshava.micro@gmail.com
Article Publishing History
Received: 11/03/2021
Accepted After Revision: 19/06/2021
In the present investigation, a detailed study on the synthesis, characterization and application of Gold Nanoparticles (GNPs) using medicinally important plant Salacia fruticosa is reported for the first time. The aqueous leaf extract of S. fructicosa was used as reducing agent for more rapid, facile, cost-effective and eco-friendly synthesis of metallic GNPs. The synthesis of gold nanoparticles was done by treating the different concentrations of aqueous Gold (III) chloride trihydrate (HAuCl4) solution with the plant leaf extract at physiological condition (pH 7.4). The formation of GNPs was studied by varying the metal salt concentrations in the reaction medium and was initially confirmed by UV-visible spectroscopy by measuring the peak between 400-700 nm. The GNPs synthesised at optimised gold salt concentration showed a peak at 545 nm.
X-ray diffraction (XRD) analysis displayed Bragg’s peak conferring the 310, 310, 330, 420 and 422 facets of the face centered cubic symmetry of nanoparticles suggesting that these nanoparticles were crystalline in nature. Possible interaction of phytochemicals in mediating and stabilization of nanoparticles was confirmed with Fourier transform infrared spectroscopy (FTIR). Size and shape of the nanoparticles was determined using Transmission electron microscopy (TEM) microgram with size ranging from 20-50 nm. The plant S. fructicosa was found to be quite competent for the purpose of commercial gold nanoparticles production, since it is synthesized extracellular as well as rapidly. This work also presents a scientific support for the antibacterial activity of the gold nanoparticles against bacterial pathogens Staphylococcus aureus and Pseudomonas aeruginosa and consequently it may be used to discover the potential applications in the treatment of the infection caused by other microbial pathogens.
Antibacterial Activity, Green Synthesis, Gold Nanoparticles, Salacia Fruticosa, Transmission Electron Microscopy.
Keshavamurthy M, Rai V.R. Green Synthesis, Characterization and Screening for Antibacterial Activity of Gold Nanoparticles Produced by Salacia Fruticosa Leaf Extract. Biosc.Biotech.Res.Comm. 2021;14(2).
Keshavamurthy M, Rai V.R. Green Synthesis, Characterization and Screening for Antibacterial Activity of Gold Nanoparticles Produced by Salacia fruticosa Leaf Extract. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=https://bit.ly/3xHEW6x“>https://bit.ly/3xHEW6x</a>
Copyright © Keshavamurthy et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Nanotechnology is emerging as a rapidly growing field and has received enormous amount of interest in recent years. This has developed as a part of material science which deals with the development of new improved materials in nanometre scale. Nanoparticles (1-100 nm) being a fundamental building blocks of nanotechnology exhibits unique physicochemical properties compared to the bulk materials. Among noble metal nanoparticles, GNPs has an increasing interest and is considered an important area of research due to unique properties which made them use in divergent field of biological sciences (Vishwanatha et al. 2018; Yousaf et al. 2020).
GNPs have a wide application in drug delivery, tissue or tumour imaging, photo thermal therapy, diagnostic and in hyperthermia. Therefore, there is a lot of scope to develop eco-friendly process for the GNPs synthesis which has advantageous over physical and chemical methods of synthesis (Keshavamurthy et al. 2017). Biological synthesis of GNPs from plant materials, microorganisms and enzymes is simpler and eco-friendlier when compared to physical and chemical methods. Among the different synthetic methods in biological synthesis, phytochemicals mediated approach has been gaining significance in the recent years due to its renewable and eco-friendly nature (Nishanthi et al. 2019; Akintelu et al. 2020; Vaid et al. 2020).
The synthesis of GNPs involves the bioreduction of Au (III) to Au (0) by plant metabolites in aqueous medium with mild reaction conditions (Awad et al. 2019; Balasubramanian et al. 2020). Plant mediated nanoparticles synthesis is rapid and is cost effective in comparison to microbial mediated synthesis of nanoparticles which are slow rate of synthesis (Srinath and Ravishankar 2014). Biosynthesis of GNPs by plants such as Azadirachta indica, Emblica officianalis, Aloe vera, Cinnamomum camphora, Magnolia kobus, Mangifera indica, Ocimum sanctum, Amaranthus spinosus, Garcinia combogia, Lantana camara, Pterocarpus santalinus, Terminalia bellerica have been previously reported. However, standardization of experimental conditions is necessary to control the size, shape and dispersity of the nanoparticles (Shankar et al. 2004; Ankamwar et al. 2005; Chandran et al. 2006; Huang et al. 2007; Song et al. 2009; Philip et al. 2010; Daizy et al. 2011; Ratul et al. 2012; Anish et al. 2014; Dash et al. 2014; Keshavamurthy et al. 2017; Kumar et al. 2018; Awad et al. 2019; Sherin et al. 2020)
Salacia fruticosa is an angiospermic herb belongs to the family Celastraceae. It is important medicinal plant which is traditionally being used in Ayurveda and is found in western ghats of India and mainly seen in the states Karnataka, Kerala and Tamil Nadu. Their leaves are simple, opposite, entire estipulate, 2-3 cm broad, 4-8 cm long and fruits are widely consumed by Kanis, the primitive indigenous tribal community residing in the Agasthyamala Biosphere Reserve of Southern Western Ghats (Saravanan et al. 2015; Subin et al. 2018). The research community in India has not given due emphasis to this plant due to limited literature on the diversity, distribution, phenology and its uses. Hence the present investigation was undertaken to explore the potential use of this plant in the synthesis and formation of gold nanoparticles deserves a special attention.
In the present study, we have reported simple, rapid, facile, stable, eco-friendly and cost-effective method for the biosynthesis of GNPs by using aqueous leaf extract of medicinally important plant S. fructicosa as a reducing and stabilizing agent. We also investigated the stability of GNPs, therefore carried out green synthesis of GNPs by treating the aqueous leaf extract with varied gold salt concentrations at physiological conditions (pH 7.4). The GNPs synthesised at optimised gold salt concentration was further characterized by using UV-visible spectroscopy, XRD, FTIR and TEM. The present study also emphasizes on the screening of GNPs against selected human pathogenic bacteria (Dudhane et al. 2019; Akintelu et al. 2020; Rautray and Rajananthini 2020).
MATERIAL AND METHODS
Gold (III) chloride trihydrate (HAuCl4.3H2O, 99.99%) and other components were purchased from Hi Media Laboratories Pvt. Ltd. and Sigma-Aldrich, Mysuru, India. Taxonomically authenticated fresh leaves of Salacia fruticosa was collected from Western Ghats of Subramanya (12°39′38″ North Latitude 75°36′32″ East Longitude) near Sakaleshpura, Karnataka, India. About 10g of fresh S. fruticosa leaves were weighed and washed twice with tap water and followed by distilled water to remove mud, dust particles and other pollutants. The leaves (Figure 1 a) were cut into small pieces and boiled with 90 mL of double distilled water and kept on boiling water bath (Keshavamurthy et al. 2017).
Figure 1: a. Salacia fruticosa plant b. Leaf extract

The temperature was maintained at 60ºC for 15 min to facilitate the formation of aqueous leaf extract. The extract was filtered using Whatmann No. 1 filter paper. The filtrate (Figure 1 b) was stored at 4ºC until further use (Keshavamurthy et al. 2017). The fresh leaf extract was used as a reducing and capping agent for the synthesis of GNPs. The green synthesis approach employed for the synthesis of GNPs is explained briefly as follows; 1 mL of aqueous leaf extract was added to 4 mL of gold salt solution (0.5 mM, 1 mM, 1.5 mM and 2 mM concentration) and the solutions were incubated at room temperature (28ºC) under physiological condition (pH 7.4).
The bioreduction of the gold ions was monitored by measuring the solutions in UV-visible spectroscopy. Two controls were maintained at the same experimental conditions, one leaf extract without HAuCl4 and another control was gold salt without leaf extract. The UV-visible spectrophotometer (Thermo Scientific, Multiskan Spectrum) was used to record the colloidal suspension of GNPs in the range of 400-700 nm. The surface plasmon resonance peaks were assessed for size and distribution of synthesised GNPs. Deionised water was used as a blank. The crystalline nature of GNPs was studied by X-ray diffractometer (RigakuminiFlex 11) by operating at 30 kV and a current of 15 mA with Cu Kα radiation (λ= 1.5406 Å) and the 2θ scanning range was of 6-60° at 5° min-1. The FT-IR measurements of the synthesized GNPs was used to analyze the presence of surface functional groups on GNPs.
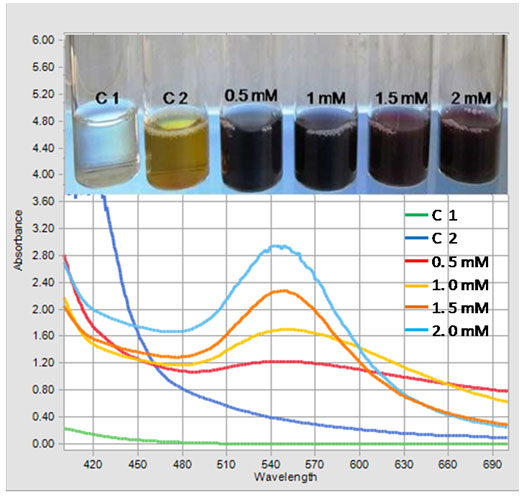
Figure 2: UV-Visible spectrum of Gold nanoparticles showing effect of different concentrations of HAuCl4 (0.5 mM to 2.0 mM) on GNPs biosynthesis. Inset shows the colloidal suspension of GNPs at different concentrations of HAuCl4.
Samples were recorded in a Perkin Elmer spectrophotometer at room temperature by scanning it in the range of 4000 cm-1 to 400cm-1 at a resolution of 4 cm-1. TEM studies were performed to elucidate the size and distribution of the biosynthesised GNPs by using a TecnaiG2 spirit BioTWIN, Netherland, operating at an accelerating voltage of 20-120 kV. For TEM analysis samples were prepared by placing a drop of colloidal suspension of GNPs on carbon coated copper grids.
The films on the TEM grids were then allowed to dry at room temperature before analysis (Srinath and Ravishankar 2014; Keshavamurthy et al. 2017). The GNPs synthesized from S. fruticosa leaf extract was tested for its antibacterial activity using standard disc diffusion method against pathogenic bacteria such as gram-positive Staphylococcus aureus and gram negative Psuedomonas aeruginosa the overnight inoculated test bacterial cultures were swabbed uniformly on the freshly prepared Mueller-Hinton agar (MHA) plates using sterile cotton swab.
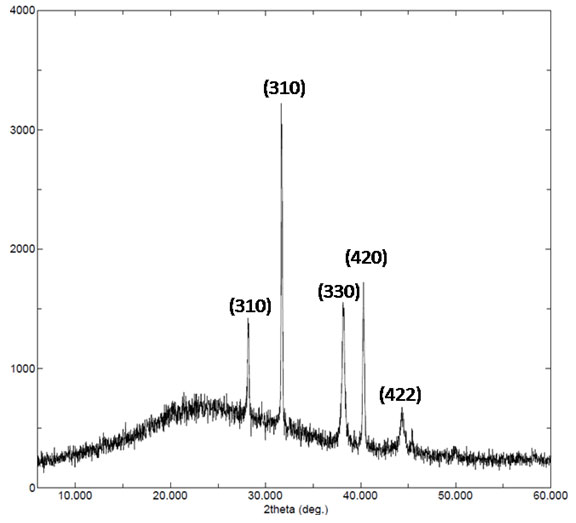
Figure 3: X-ray diffraction pattern of gold nanoparticles synthesized by leaf extract of Salacia fruticosa.
The 6 mm sterile discs were placed on solidified media and 50 µg/mL concentrations of GNPs were poured over the test discs. The control disc containing deionized water was also kept on the plate and incubated at 37 °C for 24 h. The antibacterial property of GNPs was determined by measuring the zone of inhibition around the discs in diameter (millimeter) after incubation. Chloramphenicol, a standard antibiotic of concentration 1mg/ml was used as positive control (Keshavamurthy et al. 2017; Al Saqr et al. 2021).
RESULTS AND DISCUSSION
Visual Observations: In the current study, the aqueous leaf extract of S. fruticosa was treated with different gold salt concentrations at physiological condition (pH 7.4) and the GNPs formation was indicated by a change in color from yellow to pinkish red or ruby red depending upon the size, shape and dispersity of GNPs in the colloidal suspension (Figure 2). The reaction took only two minutes for conversion of gold ions into gold nanoparticles. There was no color change in both the controls (C1 and C2) and therefore no nanoparticles synthesis occurred in control tubes. The color change remained stable for several months at 4°C. Previously, it was reported that the wine red color of GNPs in aqueous solution is due to vibrations of Surface Plasmon exhibited by GNPs. Similar changes in color during were reported for the synthesis of metallic nanoparticles in previous studies (Shankar et al. 2004; Bonigala et al. 2018; Gomathi et al. 2019; Nayeem et al. 2020).
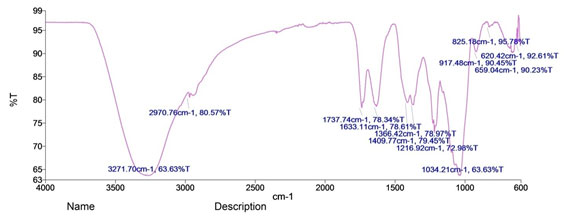
Figure 4: FTIR spectra of Gold nanoparticles synthesised by leaf extract of S. fruticosa
UV-visible spectroscopy: The bioreduction of gold ions to GNPs was preliminarily observed by appearance of red color and was confirmed by UV-visible spectroscopic studies. The UV-Visible spectrum of GNPs colloidal solution is shown in the Figure 2. The Surface Plasmon Resonance (SPR) bands centred between 545-560 nm confirmed the formation of GNPs in the solution. The salt control and the cell control tube did not show the peak characteristic for GNPs.
The height of the peak indicates the concentration of the GNPs produced and the shift of the peak towards higher wavelength indicates the larger size of the particles. Further, the sharp peaks and broader peaks indicate the monodispersed and polydispersed nature of GNPs respectively. The spectrum of the reaction mixture containing 1.5 mM HAuCl4 showed a sharp peak in range of 545 nm with high intensity indicating that this concentration is optimum for GNPs synthesis. At concentrations of 0.5, 1.0 and 2 mM HAuCl4, broader peaks were observed at these salt concentrations with less intensity.
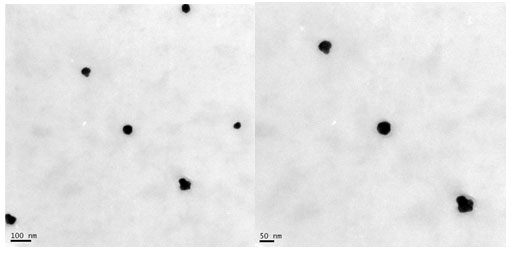
Figure 5: TEM image of gold nanoparticles formed at 1.5 mM HAuCl4 by leaf extract of S. fruticosa.
With 1.5 mM gold salt, the peak was sharp and intensity was high indicating that this concentration was able to bring about a reduction of gold salt over almost the whole range. It is hypothesized that; this concentration was optimum for efficient activity of the biomolecules that were involved in the synthesis and stabilization of GNPs. The SPR of GNPs was attributed to the interaction between free electrons on the metal surface and incident light (Song et al. 2009; Pimprikar et al. 2009; Aromal et al. 2012; Francis et al. 2017; Nayem et al. 2020).
X-ray diffraction analysis: The crystalline nature of GNPs synthesized was determined by using X-ray diffraction analysis. Figure 3 show XRD data of the synthesized GNPs. For GNPs sharp intense peaks observed at 2θ values of 28.19°, 31.70°, 38.19°, 40.30°, 44.37° correspond to the Braggs plane (310), (310), (330), (420) and (422), which confirmed that synthesized GNPs had a face-centered cubic structure. Thus, the XRD pattern indicates that the GNPs were crystalline in nature.
The average crystallite size of the GNPs was found to be 25 nm and further confirmed by TEM analysis. The mean crystallite size was calculated by applying the Scherrer formula: D=0.9λ/β1/2 cosθ, where D is the average crystal size, λ is the X-ray wave length (λ=1.5406A°), θ is Bragg’s angle (2θ), β1/2 is full width at half maximum (FWHM) in radians. The unassigned peaks on the surface of GNPs in XRD analysis might come from the crystallization of bioorganic molecules (Borchert et al. 2005; Keshavamurthy et al. 2017; Vishwanath et al. 2018; Umamaheswari et al. 2018; Doan et al. 2020).
Fourier transform infrared spectroscopy: FTIR spectroscopy was employed to deduce the possible biomolecules involved reduction of Au3+ to Au0 in the formation of GNPs. Figure 3 shows the presence of different functional groups involved in leaf extract of S. fruticosa for the bioreduction of gold salt into biocompatible GNPs. The intense broad absorbance at 3271 cm-1 is the characteristic of the hydroxyl functional group in alcohols and phenolic compounds. The bands at 2970 cm-1 are corresponding to C-H group, 1737 cm-1 is C=O in esters 1633 cm-1 corresponds to N-H bending modes and 1216 cm-1 is indicative of the presence of ester carbonyl and phenol.
The bands at 1034 cm-1 corresponds to C-O stretch. This indicates the GNPs synthesized from S. fruticosa leaf extract are surrounded by some proteins and plant metabolites like terpenoids, flavonoids and phenolic compounds. The shifting and reduction in peak intensity of main absorbance band of GNPs revealed that biomolecules present in leaf extract were responsible for the reduction in gold salt which was reported in previous studies (Zhao et al. 2004; Ramamurthy et al. 2007; Naraginti et al. 2017; Ahmad et al. 2019).
TEM analysis: TEM analysis was employed to investigate the morphology, shape, and size of GNPs synthesized at optimized gold salt concentration (1.5 mM). TEM micrographs (Figure 5) depicted that GNPs were spherical in shape and uniformly distributed without significant agglomeration. Our result was in accordance with the previous studies done using different plant extract. The size of GNPs determined by TEM was in the range of 20 to 50 nm. Our results are un accordance with the previously published reports, where 13-26 nm, 22 nm size of GNPs were synthesised using different plant extract (Keshavamurthy et al. 2017; Mollick et al. 2019; Al Saqr et al. 2021).
Antibacterial activity: The antibacterial activity of GNPs was tested by disk diffusion method against the pathogenic bacteria, S. aureus and P. aeruginosa. Antibacterial property of GNPs reveals the diameter of zone of inhibition of 9 mm for S.aureus and 11 mm for P.aueroginosa. Positive control disc (Chloramphenicol) shows a zone of 15 mm. The tested bacterial strains were selected to represent different bacterial machineries that are present in different arsenals of virulence factors, besides their noticeable pathogeneses and high prevalence in human and animal life. The possible reason for the difference in the diameter of zone of inhibition observed might be due to their cell wall composition. Similar findings were found by GNPs prepared by aqueous extract of Benincasa hispida, against bacterial pathogens by well diffusion technique (Al Saqr et al. 2021).
This part of study is a preliminary time bound approach on the possible utilization of GNPs as an antibacterial agent. The results obtained in this investigation could be in support of developing an efficient, alternative, stable and biocompatible antibacterial agent from metal nanoparticles to combat drug resistance pathogens. The antibacterial mechanism exhibited by the GNPs is subject to the degree of susceptibility of microorganisms. The GNPs binds to cell wall of bacteria due to electrostatic interaction thereby penetrates inside the bacterial cell causes DNA damage and leakage of cell components is well documented by previous studies. Further it has also been reported that the interaction of various metal nanoparticles including GNPs with the amino acid cysteine results in generation of reactive oxygen species, which also promotes bacterial cell death (Nikparast and Saliani 2018; Selvaraj et al. 2019; Akintelu et al. 2020).
CONCLUSION
The present investigation envisions the emerging role of plants for synthesis of metallic nanoparticles by employing a medicinally important plant S. fruticosa. The study highlights low cost, reproducible and rapid method to produce stable GNPs at room temperature. The reaction rate achieved in the synthesis of nanoparticles is faster than microorganisms mediated synthesis. As no toxic reducing agents were used and the GNPs were synthesized at physiological conditions (pH 7.4) this method is environment friendly and the synthesized GNPs could be used in biomedical applications. The study also highlights effect of GNPs as antibacterial agent against bacterial pathogens conferring the emerging strategy to combat multidrug resistant microorganisms.
ACKNOWLEDGEMENTS
Authors wish to acknowledge the Department of Studies in Microbiology, University of Mysore for providing Laboratory facility and C-CAMP, National Centre for Biological Sciences, Bangalore, Karnataka, India for TEM analysis of nanoparticles.
Conflict Of Interests: Authors declare no conflict of interests among themselves during the publication.
REFERENCES
Ahmad, T., Bustam, M.A., Irfan, M., Moniruzzaman, M., Asghar, H.M.A. and Bhattacharjee, S. (2019). Mechanistic investigation of phytochemicals involved in green synthesis of gold nanoparticles using aqueous Elaeis guineensis leaves extract: Role of phenolic compounds and flavonoids. Biotechnology and Applied Biochemistry, 66, pp. 698–708.
Akintelu, S. A., Olugbeko, S.C. and Folorunso, A.S. (2020). A review on synthesis, optimization, characterization and antibacterial application of gold nanoparticles synthesized from plants. International Nano Letters. 10, pp.237–248
Albrecht, M.A., Evans, C.W. and Raston, C.L. (2006). Green chemistry and the health implications of nanoparticles. Green Chemistry, 8(5), p.417.
Al Saqr, A., Khafagy, E.-S., Alalaiwe, A., Aldawsari, M.F., Alshahrani, S.M., Anwer, Md.K., Khan, S., Lila, A.S.A., Arab, H.H. and Hegazy, W.A.H. (2021). Synthesis of gold nanoparticles by using green machinery: characterization and in vitro toxicity. Nanomaterials, 11(3), p.808.
Ankamwar, B., Chaudhary, M. and Sastry, M. (2005). Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synthesis and reactivity in inorganic, metal-organic, and nano-metal chemistry, 35(1), pp.19–26.
Aromal, A S., Dinesh Babu, K.V. and Philip, D. (2012). Characterization and catalytic activity of gold nanoparticles synthesized using ayurvedic arishtams. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 96, pp.1025–1030.
Awad, M.A., Eisa, N.E., Virk, Promy., Hendi, A.A., Ortashi, K.M.O.O., Mahgoub, A.S.A., Elobeid, M.A. and Eissa, F.Z. (2019). Green synthesis of gold nanoparticles: Preparation, characterization, cytotoxicity, and anti-bacterial activities. Materials Letters, 256, pp.126608.
Balasubramanian, S., Kala, S.M.J. and Pushparaj, T.L. (2020). Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. Journal of Drug Delivery Science and Technology, 57, pp.101620.
Bonigala, B., Kasukurthi, B., Konduri, V.V., Mangamuri, U.K., Gorrepati, R. and Poda, S. (2018). Green synthesis of silver and gold nanoparticles using Stemona tuberosa Lour and screening for their catalytic activity in the degradation of toxic chemicals. Environmental Science and Pollution Research. 25, pp. 32540–32548.
Borchert, H., Shevchenko, E. V., Robert, A., Mekis, I., Kornowski, A., Grubel, G., and Weller, H. (2005). Determination of nanocrystal sizes: a comparison of TEM, SAXS, and XRD studies of highly monodisperse CoPt3 particles. Langmuir: The ACS journal of surfaces and colloids, 21(5), pp.1931–1936.
Chandran, S.P., Chaudhary, M., Pasricha, R., Ahmad, A. and Sastry, M. (2006). Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnology Progress, 22(2), pp.577–583.
Dash, S.S., Bag, B.G. and Hota, P. (2014). Lantana camara Linn leaf extract mediated green synthesis of gold nanoparticles and study of its catalytic activity. Applied Nanoscience, 5(3), pp.343–350.
Doan, V.-D., Huynh, B.-A., Nguyen, T.-D., Cao, X.-T., Nguyen, V.-C., Nguyen, T.L.-H., Nguyen, H.T. and Le, V.T. (2020). Biosynthesis of Silver and Gold Nanoparticles Using Aqueous Extract of Codonopsis pilosula Roots for Antibacterial and Catalytic Applications. Journal of Nanomaterials, 2020, pp.1–18.
Dudhane, A. A., Waghmode, S. R., Dama, L. B., Mhaindarkar, V. P., Sonawane, A. and Katariya, S. (2019). Synthesis and characterization of gold nanoparticles using plant extract of Terminalia arjuna with antibacterial activity. International Journal of Nanoscience and Nanotechnology.15, pp.75–82
Francis S, Joseph, S., Koshy, E.P. and Mathew, B. (2017). Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environmental Science and Pollution Research, 24 (21), pp.17347–17357.
Geetha, R., Ashokkumar, T., Tamilselvan, S., Govindaraju, K., Sadiq, M. and Singaravelu, G. (2013). Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnology, 4(4-5), pp.91–98.
Gomathi, M., Prakasam, A. and Rajkumar, P. V. (2019). Green synthesis, characterization and antibacterial activity of silver nanoparticles using Amorphophallus paeoniifolius leaf extract. Journal of Cluster Science, 30, pp.995–1001.
Huang, J., Li, Q., Sun, D., Lu, Y., Su, Y., Yang, X., Wang, H., Wang, Y., Shao, W., He, N., Hong, N.J. and Chen, C. (2007). Biosynthesis of Silver and Gold Nanoparticles by Novel Sundried Cinnamomum camphora Leaves. Nanotechnology, 18, pp. 105104-105115.
Kumar, H. K., Venkatesh, N., Bhowmik, H. and Kuila, A. (2018). Metallic nanoparticles: a review. Biomedical Journal of Scientific and Technical Research, 4(2) pp.1–11,
Keshavamurthy, M., Srinath, B.S. and Rai, V.R. (2017). Phytochemicals-mediated green synthesis of gold nanoparticles using Pterocarpus santalinus L. (Red Sanders) bark extract and their antimicrobial properties. Particulate Science and Technology, 36(7), pp.785–790.
Mohanpuria, P., Rana, N.K. and Yadav, S.K. (2007). Biosynthesis of nanoparticles: technological concepts and future applications. Journal of Nanoparticle Research, 10(3), pp.507–517.
Mollick, M.M.R., Rana, D., Dash, S.K., Chattopadhyay, S., Bhowmick, B., Maity, D., Mondal, D., Pattanayak, S., Roy, S. and Chakraborty, M. (2019). Studies on green synthesized silver nanoparticles using Abelmoschus esculentus (L.) pulp extract having anticancer (in vitro) and antimicrobial applications. Arabian Journal of Chemistry, 12, pp.2572–2584
Naraginti, S. and Li, Y. (2017). Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. Journal of Photochemistry and Photobiology B: Biology, 170, pp.225–234.
Nayem, S.M.A., Sultana, N., Haque, Md.A., Miah, B., Hasan, Md.M., Islam, T., Hasan, Md.M., Awal, A., Uddin, J., Aziz, Md.A. and Ahammad, A.J.S. (2020). Green synthesis of gold and silver nanoparticles by using Amorphophallus paeoniifolius tuber extract and evaluation of their antibacterial activity. Molecules, 25(20), p.4773.
Nikparast Y. and Saliani, M. (2018). Synergistic effect between phyto-synthesized silver nanoparticles and ciprofloxacin antibiotic on some pathogenic bacterial strains. Journal of Medical Bacteriology. 7, pp.36-43
Nishanthi, R., Malathi, S. and Palani, P. (2019). Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Materials Science and Engineering: C, 96, pp.693–707
Philip, D. (2010). Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 77(4), pp.807–810.
Philip, D. and Unni, C. (2011). Extracellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf. Physica E: Low-dimensional Systems and Nanostructures, 43(7), pp.1318–1322.
Pimprikar, P.S., Joshi, S.S., Kumar, A.R., Zinjarde, S.S. and Kulkarni, S.K. (2009). Influence of biomass and gold salt concentration on nanoparticle synthesis by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Colloids and Surfaces B: Biointerfaces, 74(1), pp.309–316.
Rajan, A., Kumari, M.M. and Philip, D. (2014). Shape tailored green synthesis and catalytic properties of gold nanocrystals. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 118, pp.793–799.
Ramamurthy, N., and Kennan, S., (2007). Fourier transform infrared spectroscopic analysis of a plant (Calotropis gigantea Linn) from an industrial village, Cuddalore .. Tamilnadu, India. Romanian Journal of Biophysics. 17 (4), pp. 269 276.
Ratul, K. D., Nayanmoni, G., Punuri, J. B., Pragya, S., Chandan, M., and Utpal, B. (2012). The synthesis of gold nanoparticles using Amaranthus spinosus leaf extract and study of their optical properties. Advances in Materials Physics and Chemistry, 2, pp. 275–281.
Rautray, S. and Rajananthini, A.S. (2020). Therapeutic potential of green, synthesized gold nanoparticles. Bio Pharm International.33(1), Pp.29–38
Saravanan, V. S., Mohamed Ismail, M. And Manokara S. (2015). Pharmacognostic studies and phytochemical analysis of Salacia fruticosa. International Journal of Pharmacognosy and Phytochemical Research.7(4); pp.656-660
Selvaraj, V., Sagadevan, S., Muthukrishnan, L., Johan, Mohd.R. and Podder, J. (2019). Eco-friendly approach in synthesis of silver nanoparticles and evaluation of optical, surface morphological and antimicrobial properties. Journal of Nanostructure in Chemistry, 9(2), pp.153–162.
Shankar, S. Shiv., Rai, A., Ahmad, A. and Sastry, M. (2004). Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. Journal of Colloid and Interface Science, 275(2), pp.496–502.
Sherin, L., Sohail, A., Amjad, U.-S., Mustafa, M., Jabeen, R. and Ul-Hamid, A. (2020). Facile green synthesis of silver nanoparticles using Terminalia bellerica kernel extract for catalytic reduction of anthropogenic water pollutants. Colloid and Interface Science Communications, 37, pp.100276.
Song, J. Y., Jan, H. K., and Kim, B. S. (2009). Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochemistry. 44, pp.1133-1138.
Srinath, B.S. and Ravishankar Rai, V. (2014). Biosynthesis of highly monodispersed, spherical gold nanoparticles of size 4–10 nm from spent cultures of Klebsiella pneumoniae. 3 Biotech, 5(5), pp.671–676.
Subin, K., Jose, P.A. and Sarath, T.V. (2018). On the reproductive biology of Salacia fruticosa Wall. ex M.A. Lawson – an endemic medicinal plant of the Western Ghats, India. Journal of Threatened Taxa, 10(15), pp.13002–13005
Umamaheswari, C., Lakshmanan, A. and Nagarajan, N.S. (2018). Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange. Journal of Photochemistry and Photobiology B: Biology, 178, pp.33–39.
Vaid, P., Raizada, P., Saini, A.K. and Saini, R.V. (2020). Biogenic silver, gold and copper nanoparticles – A sustainable green chemistry approach for cancer therapy. Sustainable Chemistry and Pharmacy, 16, pp.100247.
Vishwanatha, T., Keshavamurthy. M., Mallappa. M., Murugendrappa, M. V., Nadaf, Y. F., Siddalingeshwara, K. G., and Dhulappa, A. (2018). Biosynthesis, characterization, and antibacterial activity of silver nanoparticles from Aspergillus awamori. Journal of Applied Biology and Biotechnology. 6(05), pp.12-16.
Yousaf, H., Mehmood, A., Ahmad, K.S. and Raffi, M. (2020). Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agent. Materials Science and Engineering: C, 112, p.110901.
Zhao, Y., Ma, C.-Y., Yuen, S.-N. and Phillips, D.L. (2004). Study of succinylated food proteins by Raman spectroscopy. Journal of Agricultural and Food Chemistry, 52(7), pp.1815–1823.


