1 Department of Biotechnology, Jawaharlal Nehru Technological University, Anantapur, India.
2 Department of Biotechnology, Osmania University, Telangana, India.
Corresponding author email: daddala.gunabhushana@gmail.com
Article Publishing History
Received: 15/07/2021
Accepted After Revision: 19/09/2021
The objective of this study was to determine the uptake of glucose in L6 cell lines and alpha glucosidase inhibition activity of Secoisolariciresinol diglucoside (SDG) involved in glucose utilization. Diabetes mellitus is a clinical disorder characterized by hyperglycemia, an elevated amount of glucose circulates in the blood plasma. Different concentrations of SDG were analyzed for glucose uptake activity and alpha glucosidase inhibition activity. The results found to be significantly comparable with Metformin, the highest value was 68.41 ± 0.80 at 100 µg/ml dose for SDG and 85.50 ± 0.83 value for Metformin at the same concentration. The alpha glucosidase inhibition activity for SDG was found to be 79.67 ± 0.40 % and for Voglibose it was 96.33 ± 0.40 % at 2000µg/mL concentration.
The results of the current work therefore clearly indicate the potential of SDG to manage hyperglycemia. Elevation of the glucose uptake by SDG in association with glucose transport supported the up regulation of glucose uptake, through alpha glucosidase inhibition activity it delays the carbohydrate digestion which leads to decreases the post prandial blood glucose levels. The present study shows that SDG activate glucose uptake in L-6 cell line of skeletal muscles, which can correlate to that of standard Metformin used by diabetic patients. Currently increasing usage of Nutraceuticals is more evident in order to stay away from the synthetic drugs and its side effects. Our research can be considered as a step towards a more profound understanding of hypoglycemic activity of SDG as there is no much data available.
Alpha Glucosidase, Glucose Uptake, Hyperglycemia, L-6 Cells, SDG.
Daddala G.B, Rani. A.S, Kumar A.K. Glucose Uptake and α-Glycosidase Inhibition Activities of Secoisolariciresinol Diglucoside Isolated from Linum usitatissimum: An In vitro Study. Biosc.Biotech.Res.Comm. 2021;14(3).
Daddala G.B, Rani. A.S, Kumar A.K. Glucose Uptake and α-Glycosidase Inhibition Activities of Secoisolariciresinol Diglucoside Isolated from Linum usitatissimum: An In vitro Study. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3jOJSjV“>https://bit.ly/3jOJSjV</a>
Copyright © Daddala et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Diabetes mellitus is a metabolic disease categorized by hyperglycemia, occurs when defects in insulin secretion, insulin action, or both. The diabetes associated with the consequences of long-term damage, malfunction and failure of the various organs, specifically the eyes, kidneys, nerves, heart, and blood vessels (Diabetic care 2007). It is a serious metabolic disorder affecting the reasonable number of people globally, in which the effected person experiences high blood sugar, either may be because of the pancreas does not produce enough insulin or the produced insulin will not be effectively used by the body cells. Causes for enhancing the growth of its epidemic are the variances in social structure, intellectual stress, obesity, hormonal imbalance and heredity (Amos et al. 2010; Mukhtar et al. 2019).
Diabetes is the most common endocrine disorder and by the year 2010, also it is estimated that, more than 200 million people will comprise diabetes and 300 million will subsequently have the disease worldwide by 2025 (King et al. 1998; Amos et al. 2010). The percentage of the population is expected to increase by 55% with diagnosed diabetes between 2015 and 2040, may reaches to 642 million people by the majority of cases in low and middle-income countries (Eddouks and Maghrani 2004). Cumulative epidemiological studies have suggested that, the consumption of fruits, vegetables and few medicinal herbs to decrease the incidence of diabetes. The plant kingdom expresses a large pool of biologically active compounds which has not been explored yet (Amos et al. 2010; Mukhtar et al. 2019).
According to bygone literature, more than 800 plants are reported to have anti-diabetic properties (Kesari et al. 2007). More than 1200 plants are used in traditional medicine for their alleged hypoglycemic activity as per ethno pharmacological surveys (Fang etal. 2008). Inhibition of the carbohydrate digestive enzymes (α-glucosidase and α-amylase), improved peripheral uptake of glucose by hemidiaphragm are the potential preliminary mechanisms that are involved in hypoglycemic activity.
Phenolic compounds or polyphenols are a group of chemicals that are extensively distributed through the plant kingdom and thus form an essential part of the human diet. The α-glucosidase inhibition in our experiments could be due to the presence of these compounds. These compounds have been found to be responsible for blood glucose reducing activity and have also been reported to activate GLUT1-mediated glucose uptake (Kim et al. 2001; Eid et al. 2010; Ali et al. 2019).
Skeletal muscle is the major site for the disposal of ingested glucose in a healthy normal glucose tolerance (NGT) individual, Impaired glucose uptake in skeletal muscle is present in insulin resistance diabetes (Defronzo et al. 1988; Zisman et al. 2000). In skeletal muscle the glucose uptake is decreased by Insulin by increasing functional glucose transport molecules (GLUT-4) in the plasma membrane (Dachani et al. 2012). Insulin resistance and chronic hyperglycemia are caused by the reduction of GLUT4 (Zierath et al. 1996; Kim et al. 2001; Raghad et al. 2019). This triggers a lot of signaling cascades, inducing biological responses like glucose uptake into the cell, and glycogen synthesis (Binhthi et al. 2017). Skeletal muscle is the major tissue accountable for insulin stimulated glucose disposal and the main site of peripheral insulin resistance (Defronzo et al. 1981; Defronzo et al. 1985; Chadt and Al-Hasani 2020).
L6 cell lines are derived from the skeletal muscle, used in antidiabetic research, they are good model for the glucose uptake because they have been used extensively to elucidate the mechanism of glucose uptake in the muscle, have an intact insulin signalling pathway and express the insulin sensitive GLUT-4 (Ruddich et al. 1998). Secoisolariciresinol diglucoside (SDG), is a known antioxidant found in flaxseed (Prasad 2000; Kezimana et al. 2018). The present study was carried out, to study the hyperglycemic activity of Secoisolariciresinol diglucoside (SDG) through in vitro α-glucosidase activity and glucose uptake activity as there are no ample experimental studies have been carried so far.
MATERIAL AND METHODS
Samples of flax seeds (whole grains) were obtained from a local store in Chennai, India. p-nitrophenyl glucopyranoside (pNPG) procured from Sigma Aldrich, α-glucosidase enzyme from Sigma Aldrich. L-6 cell lines purchased from MCCS Pune. All other chemicals /reagents and solvents used in this study were purchased from Loba Chem, Merck, India Pvt. Ltd as analytical reagent grade materials and applied without subsequent purification. Isolation and purification of SDG described in, the extract of L. usitassimum was prepared by 80 % Aqueous methanol (Daddala et al. 2018).
For the α-glucosidase inhibition assay, the effect of SDG and its extract on α-glucosidase activity was determined as per the method described by Apostolid using α-glucosidase enzyme (Apostolid et al. 2007). The p-nitrophenyl glucopyranoside (pNPG) as a substrate solution was prepared in 100mM phosphate buffer (pH 6.8). 200 µL of the α-glucosidase was pre-incubated with different concentrations (10,20,40,80,160 and 320) of the extract for 10min. Then, dissolved 400µL of 5.0mM (pNPG) in 100mM phosphate buffer (pH 6.8) was added to start the reaction.
The reaction mixture was incubated at 37°C for 20min and the reaction will be stopped by adding 1 mL of Na2CO3 (0.1M). The yellow-colored reaction mixture, 4-nitrophenol, released from pNPG was measured at 405nm using UV – VIS spectrophotometer. A blank and the sample blanks were also prepared by adding 5 µL of deionized water instead of the plant extract and 20 µL of deionized water instead of the enzyme, respectively. Voglibose was used as a positive control and the inhibitory activity of α-glucosidase was calculated using the following formula,
% Inhibition = [(Abs Control – Abs Sample) / Abs Control] x 100
For the glucose utilization experimental procedure on L-6 cell lines, the glucose utilization was determined by the minor modification method defined by van de Venter et al. (2008). The L-6 cells were dislodged by the brief exposure to 0.25% Trypsin in phosphate-buffered saline, counted and suspended in the new growth medium. Then seeded at a density of 6000 cells per well into a 96-well culture plate and allowed to adhere and grow in a humidified incubator with 5% CO2 at 37°C for three days. Two cell-free rows also included to serve as blanks. On day three after seeding, without changing the medium, different concentrations (3.125, 6.25, 12.5, 25, 50 and 100 𝜇g) of test samples and standard Metformin were added to each well.
After 48 h incubation, the spent culture medium was removed by aspiration and replaced with a 25 𝜇l incubation buffer (DMEM medium diluted with PBS, 0.1% BSA and 8 mm of glucose) and further incubated for an additional time of 3 h at 37°C. The negative control (untreated) which contains only the incubation buffer without samples. After incubation, 10 𝜇l of the incubation medium was removed from each well and transferred into a new 96-well plate, to this added 200 𝜇l of glucose oxidase reagent to determine the concentration of glucose in the medium. After 15min of incubation at 37°C, measured the absorbance at 492 nm using a microtitre plate reader. The amount of glucose utilized was calculated by using the below mentioned formula, the difference between the untreated control and treated wells.
% Glucose uptake = 100 x [Absorbance control untreated cells – Absorbance sample treated cells /Absorbance control untreated cells]
RESULTS AND DISCUSSION
α-GLUCOSIDASE INHIBITION ASSAY: In this study, the results indicated that SDG exhibited significant effect on alpha-glucosidase than extract at all the tested concentrations, illustrated in Table -1. At the highest concentration (2000µg/ml) investigated, the SDG, extracts displayed appreciable effect on alpha-glucosidase by 68.7% and 79.7 % respectively. However, voglibose as positive controls, was more effective in the respective assays than the extract and SDG, exhibiting percentage inhibitory activity of 96.3% against alpha-glucosidase.
Table 1. In vitro α-Glucosidase Inhibition assay
| Concentration (µg) | Voglibose | Extract | SDG |
| 50 | 52.01±0.31 | 21.65 ± 0.32 | 31.94 ± 0.58 |
| 100 | 67.40 ± 0.38 | 24.66 ± 0.40 | 47.27 ± 0.15 |
| 250 | 73.15 ± 0.47 | 30.67 ± 0.44 | 60.93 ± 0.23 |
| 500 | 79.47 ± 0.47 | 53.39 ± 0.61 | 68.31 ± 0.62 |
| 1000 | 91.90 ± 0.31 | 59.30 ± 0.84 | 69.69 ± 0.47 |
| 2000 | 96.33 ± 0.46 | 68.72 ± 0.23 | 79.67 ± 0.40 |
Figure 1: The effect of SDG, extract of L. usitassimum and Voglibose on alpha-glucosidase activity. Indicates a significant increase relative to the control. Data expressed as mean ± SD (n = 3).
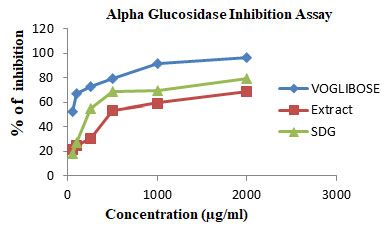
Currently, there are numerous antidiabetic drugs available to deal with diabetes and the mechanisms of action may be the inhibition of alpha-amylase, alpha-glucosidase, lipase, and DPP-IV enzyme. Our results indicated that the SDG demonstrated significant inhibition on alpha-glucosidase. However, the mild inhibition observed by the extract of L. usitassimum. Whereas alpha-amylase inhibition has been reported in our previous publication. This suggests that the antidiabetic mechanism of SDG may also through the inhibition of these enzymes (Daddala et al. 2020).
Glucose Utiliastion In L6 Cell Lines: The results obtained for glucose uptake in L6 cells in the presence of the SDG, extract of L. usitassimum at 3.125µg to 100 µg/ml are presented in table 2. The SDG caused a significant higher increase in glucose uptake in both L6 cells at all the concentration tested in a concentration-dependent manner when compared to the un treated control and metformin. On the other hand, the crude extract of L. usitassimum also exhibited glucose uptake at 100 µg/ml but lower than observed for SDG.
The L6 cell lines, the extract of L. usitassimum showed some significant potential in lowering blood glucose levels at 50, 100 μg/ml concentrations tested (Figure 2). However, at all the concentrations the extract also caused a slight increase in glucose uptake in L6 cells but less significant than SDG. The highest activity of glucose uptake was for extract 52.16 ± 0.41 and for SDG 68.41 ± 0.80 at 100 μg/ml concentration. Whereas slightly lower than metformin 85.50 ± 0.83. These results were nearly correlated with metformin, which were used as the standard antidiabetic drugs.
The present study has employed to identify the potential mechanism of probable antidiabetic actions of SDG. The result obtained in this study on glucose uptake using L6 cells demonstrated that SDG increased glucose uptake in L6 cells when compared to extract but somewhat lesser than Metformin (Figure 3). Metformin lowers glucose, sensitizes insulin by reducing gluconeogenesis and opposing glucagon-mediated signalling in the liver and in a lesser extent by increasing glucose uptake in skeletal muscle. It exerts its hypoglycemic effect through activation of the AMP activated protein kinase (AMPK) in the liver (Viollet et al. 2012).
Most abundant tissue in the whole body is skeletal muscle. Hence, proper function of skeletal tissue is important to maintain normal blood glucose level (Koncic et al. 2010; Chadt and Al-Hasani 2020). Insulin increases the glucose uptake in the skeletal muscle by increasing functional glucose transport molecules in the plasma membrane. Common pathological condition in non-insulin dependent diabetes mellitus is, the defect in insulin stimulated skeletal muscle glucose uptake. SDG, a lignan of flaxseed upregulated GLUT4 protein expression in the skeletal muscle (Yanwen et al. 2015; Chadt and Al-Hasani 2020).
Our experimental outcome suggested that the SDG, therefore, masquerade as metformin by increasing glucose uptake in the skeletal muscle. SDG is a phytochemical lignan, the presence of phytochemicals suppresses glucose release and also enhances glucose uptake. Therefore, it may be hypothesized SDG could be linked to activation of the insulin signalling cascade, resulting in stimulation of GLUT4 that facilitates the translocation of glucose into the cell (Hanhineva et al. 2010; Rosenzweig and Sampson 2021).
Table 2. In vitro Glucose uptake studies in L6 cells
| Concentration (µg) | Extract | SDG | Metformin |
| 3.125 | 2.86 ±0.40 | 11.33 ± 0.84 | 13.54 ± 0.35 |
| 6.25 | 7.52 ± 0.57 | 20.45 ± 0.61 | 22.21± 0.76 |
| 12.5 | 10.99 ± 0.63 | 29.07 ± 0.84 | 36.25 ± 0.86 |
| 25 | 25.07 ± 0.84 | 44.26 ± 0.52 | 57.54 ± 0.86 |
| 50 | 37.70 ± 0.89 | 57.84 ± 0.40 | 71.92 ± 0.46 |
| 100 | 52.16 ± 0.41 | 68.41 ± 0.80 | 85.50 ± 0.83 |
Figure 2: The effect of SDG, extract of L. usitassimum and Metforminon glucose utilization inL6cells. Cells were treated for 48 h in the presence or absence of varying concentration of the SDG and extract. Data expressed as mean ± SD (n = 3).
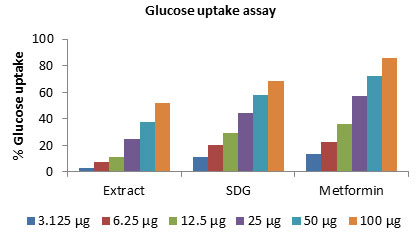
Figure 3: A- Extract of L. usitassimum; B- SDG, C-Metformin, D-Control
The toxicity assay revealed that L. usitassimum extract, SDG were not toxic to L-6 cells, producing less than 10% cell death at the concentrations investigated. However, metformin also displayed no significant toxicity but rather proliferated the cells. Furthermore, the low level of cell death exhibited by this extract, SDG and the positive controls most likely explains the significant reduction in glucose uptake in the cells (Rosenzweig and Sampson 2021). To our knowledge no prior studies have examined the in vitro glucose uptake assay, α-glucosidase inhibitory activity of SDG. Therefore, this will be a foremost in vitro approach on cell lines.
CONCLUSION
The findings of the present study suggests that SDG enhances the glucose uptake under in vitro conditions, exerts its hypoglycemic activity by reducing the post prandial glucose levels by possessing alpha-glucosidase inhibition activity. The antidiabetic activity might be due to the phyto constituents of L. usitassimum. Moreover, SDG itself is a phytochemical lignin. It is also concluded that based on the toxicity assay SDG found to be nontoxic and safe to the L6 cells. However, in vivo studies have to be carried out to validate the in vitro results by employing different in vivo models and clinical trials for their effective utilization as therapeutic agents.
Conflit of Interests: Authors declare no conflict of interests.
REFERENCES
Chadt A and Al-Hasani H (2020) Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch – Eur J Physiol 472: 1273–1298.
Alqahtani AS, Hidayathulla S and Rehman MT et al (2019) Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2020 10: 61.
Amos A, McCarty D and Zimmet P (2010). The rising global burden of diabetes and its complications, estimates and projections to the year 2010. Diabetic Med 14: S1-S85.
Apostolidis E, Kwon YI and Shetty K (2007). Innovative Food Science and Emerging Technologies 8: 46 – 54.
Trinh BTD, Jager AK and Staerk D (2017). High Resolution Inhibition Profiling Combined with HPLCHRMS-SPE-NMR for Identification of PTP1B Inhibitors from Vietnamese Plants. MDPI 17: 00057.
Dachani SR, Avanapu SR and Ananth PH (2012). In vitro antioxidant and glucose uptake effect of Trichodesma indicum in L-6 cell lines. J Pharm Bio Sci 3(4): 810 – 819.
DeFronzo RA, Jocot E, Jequier E et al. (1981). The effect of insulin on the disposal of intravenous glucose. Diabetes 30: 1000-1007.
DeFronzo RA, Gunnarsson R, Bjorkman O et al. (1985). Effects of insulin on peripheral and splanchnic glucose metabolism in non-insulin-dependent (Type II) diabetes mellitus. J Clin Invest 76(10): 149-155.
De Fronzo RA (1988). The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM, Diabetes 37(6): 667–687.
DIABETES CARE (2007) -American Diabetes Association, VOLUME 30, SUPPLEMENT 1, JANUARY.
Eddouks M and Maghrani M (2004). Phlorizin-like effect of Fraxinus excelsior in normal and diabetic rats. J Ethnopharmacol 9: 149-54.
Eid HM, Martineau LC, Saleem A et al. (2010). Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitisidaea. Mol Nutr Food Res. 54: 991-1003.
Fang XK, Gao J and Zhu DN (2008). Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 82: 615-622.
Daddala G, Swarooparani A (2020). In vitro cytotoxic activity of secoisolariciresinol diglucoside on HT-29, PA-1 cell lines, and α-amylase inhibitory activity, Asian J Pharm Clin Res, 13(2): 168-173.
Hanhineva K, T¨orr¨onen R and Bondia-Pons. I (2010). Impact of dietary polyphenols on carbohydrate metabolism. International Journal of Molecular Sciences 11(4): 1365–1402.
Kesari AN, Kesari S, Santosh KS et al. (2007). Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J Ethnopharmacol 112(2): 305-311.
Kezimana P, Dmitriev AA, Kudryavtseva AV et al. (2018) Secoisolariciresinol Diglucoside of Flaxseed and Its Metabolites: Biosynthesis and Potential for Nutraceuticals. Front. Genet. 9: 641.
Kim J. K, A. Zisman, J. J. Fillmore, O. D. et al (2001). Glucose Toxicity and the Development of Diabetes in Mice with Muscle-Specific Inactivation of GLUT4 The Journal of Clinical Investigation 108(1): 153-160.
King H, Aubert R, Herman W (1998) Global burden of diabetes, 1995-2025. Prevalence, numerical estimates and projections. Diabetes Care 21: 1414-1431.
Koncic MZ, Kremer D, Gruz J et al. (2010). Antioxidant and antimicrobial properties of Moltkia petraea (Tratt.) Griseb. flower, leaf and stem infusions. Food Chem Toxicol 48(6): 1537-1542.
Mukhtar, Y., Galalain, A.M. and Yunusa, U.M (2019). European Journal of Biology ISSN 2520-4738 l4(1): 1-14.
Prasad K (2000) Antioxidant activity of secoisolariciresinol diglucoside derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol 9: 220-225.
AL-Ishaq RK, Abotaleb M, Kubatka P et al (2019) Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 9: 430.
Rosenzweig T and Sampson S.R (2021) Activation of Insulin Signaling by Botanical Products. Int. J. Mol. Sci. 22: 4193.
Ruddich A, Tirosh A, Ruth P et al. (1998). Prolonged oxidative stress impairs insulin induced GLUT4 translocation 3T3-L1 adipocytes. Diabetes 47: 1562-1569.
Venter MV, S. Roux, and Bungu LC (2008). Antidiabetic screening and scoring of 11 plants traditionally used in South Africa, Journal of Ethno pharmacology 119(1): 81–86.
Viollet BB, Guigas N. and Garcia S et al. (2012). Cellular and molecular mechanisms of metformin: an overview, Clinical Science 122(6): 253–270.
Wang Y, Fofana B, Roy M et al. (2015) Flaxseed lignan secoisolariciresinol diglucoside improves insulin sensitivity through upregulation of GLUT4 expression in diet-induced obese mice. Journal of functional foods 18: 1-9.
Zierath JR, He L, Guma A, et al. (1996) Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia 39: 1180-1189.
Zisman. A, Peroni O. D., Abel E. D. et al. (2000) Targeted Disruption of the Glucose Transporter 4 Selectively in Muscle Causes Insulin Resistance and Glucose Intolerance. Nature Medicine 6 (8): 924-928.


