1Department of Biology, Faculty of Science, King Abdulaziz University Jeddah, Saudi Arabia,
2Department of Biology, College of Science Jeddah University of Jeddah, Saudi Arabia
Article Publishing History
Received: 24/10/2020
Accepted After Revision: 07/12/2020
Nowadays, feathers are a major by-product of the poultry industry. Due to the increasing production of feathers from poultry industries, the untreated feathers could become pollutants. Feathers account for 5-7% of the total weight which is constituted of 90% keratin. Bioconversion is widely accepted as a low-cost and environmentally gentle process but limited by the availability of safe and highly efficient feather degrading bacteria. In this study, 15 actinomycete isolates were isolated, purified and screened for keratinase production using solid and broth media. Out of the 15 isolates, 9 recorded keratinase activities. The isolate AM1 was the most active one, thus it was selected for further studied. Using morphological, physiological and biochemical characters in addition to 16S rRNA, it was identified as Streptomyces enissocaesilis AM1with 95% similarity level to S. enissocaesilis. This isolate can efficiently degrade feathers. Keratinase enzymes from Streptomyces enissocaesilis AM1 showed optimal activity at pH 7 and 50°C. Mechanism of degradation includes, sulfitolysis, proteolysis, followed by deamination. In conclusion, Streptomyces enissocaesilis AM1 can grow on keratin as a carbon sourceand secret keratinase which degrade keratin tosmall peptide chains, amino acids, and minerals which can be used as organic fertilizer for enhancing plant growth.
Feather, Degradation, Streptomyces enissocaesilis, Keratinase, Plant Compost.
Khalel A, Alshehri W, Aly M. Enhancing Plant Growth by Chicken Feather Compost Obtained from Feather Degradation by Streptomyces enissocaesilis. Biosc.Biotech.Res.Comm. 2020;13(4).
Khalel A, Alshehri W, Aly M. Enhancing Plant Growth by Chicken Feather Compost Obtained from Feather Degradation by Streptomyces enissocaesilis. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: <a href=”https://bit.ly/33Sxet4″>https://bit.ly/33Sxet4</a>
Copyright © Khalel et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Feather waste, generated in large quantities as a byproduct of commercial poultry processing, is nearly pure keratin protein (Moran et al., 1966). Keratin in its native state is not degradable by common proteolytic enzymes such as trypsin, pepsin and papain. However, keratin does not accumulate in nature and keratinolytic activity has been reported for many bacterial and fungal genera like Bacillus sp. (Aly et al, 2019) and Streptomyces (Fuhong et al., 2010, Tork and Aly, 2019), Aspergillus and Ctenomyces (Gupta et al., 2002). Importance enzymes were reported from bacteria (Aly et al., 2020, Bahamdain et al., 2020, Tork et al., 2020a, b).
Currently, feather waste is utilized on a limited basis as a dietary protein supplement for animal feed stuffs. A current value-added use for feathers is the conversion to feather meal, a digestible dietary protein for animal feed, using physical and chemical treatments. These methods can destroy certain amino acids and decrease protein quality and digestibility (Moritz and Latshaw, 2001). The nutritional inferiority and insolubility of native feather protein derive from the composition and molecular configuration of constituent amino acids that ensure the structural rigidity of feathers (Parry and North, 1998). Resistance to proteolytic enzymes has been attributed to the complex structure of keratin filaments. In addition, disulfide cross-links produce a compact three dimensional network, as a result of intermolecular disulfide bonds between rod domains and terminal domains of the constituent molecules (Parry and North, 1998). The nutritional upgrading of feather meal through microbial or enzymatic treatment has been described.
Feather meal fermented with Streptomyces fradiae and supplemented with methionine resulted in a growth rate of broilers comparable with those fed isolated soybean protein (Elmayergi and Smith, 1971). The crude keratinase enzyme increased the digestibility of commercial feather meal and could replace as much as 7% of the dietary protein for growing chicks (Odetallah et al., 2003). Keratinolytic microorganisms and their enzymes may be used to enhance the digestibility of feather keratin. They may have important applications in processing keratin-containing wastes from poultry and leather industries through the development of non-polluting methods (Onifade et al., 1998). Generally, an increase in keratinolytic activity is associated with thermophilic organisms, which require high energy, inputs to achieve maximum growth and the decomposition of keratin wastes (Nam et al., 2002, Tork and Aly, 2019). Actinobacterial isolates can degrade raw feathers and therefore useful to develop efficient processes involving keratin substrates. In this study, we described the collection of feather dumping soil from several areas, isolation of Actinobacteria from feather dumping soil and selection of keratinolytic actinobacterial isolates by performing primary and secondary screening.
MATERIAL AND METHODS
Keratinolytic strains and culture medium:The chicken feather-degrading strain Streptomyces enissocaesilisAM1 was identified by screening. In this study, Streptomyces enissocaesilis AM1 cultured in a chicken feather medium (initial pH 8.0) comprising (g/L): chicken feathers 50, yeast extract 1.5, glucose 3.0, KH2PO4 0.7, K2HPO4 1.4, NaCl 0.5, and MgSO4 0.1.
Screening of keratinolytic actinobacteria:Screening of keratinolytic actinobacteria First, the keratinolytic activity of the isolated actinobacteria was determined in Milk agar medium (Riffel and Brandelli, 2006). The isolates that showed efficient keratinolytic activity were subjected to a second screening in modified basal liquid medium supplemented with raw chicken feather, MgSO4 , 7H2O 0.2 g/l; K2HPO4 0.3 g/l; KH2PO4 0.4 g/l; CaCl2 0.22 g/l and Yeast extract 0.1 g/l were used to prepare the modified basal liquid medium (Aly et al., 2019).
Keratinase assay:Keratinase activity was determined by the modified method of Letourneau et al. (1989). Keratin azure (Sigma-Aldrich, USA) was used as the substrate. It was first frozen at − 10°C and then ground into fine powder by using Oscillating mil mm400 retch (Figure 3.1). Keratin azure powder (5mg) was suspended in 1 ml of 50 mM Tris-HCl buffer (pH 8.0). The reaction mixture contained 1 ml keratin azure suspension and 1 ml crude enzyme. The reactions were carried out at 50°C in a water bath for 30 min. After incubation, the reactions were stopped by adding 2 ml 0.4 M trichloroacetic acid (TCA) and followed by centrifuging at 3000×g for 5 min to remove the substrate. The supernatant was spectrophotometrically measured for the release of azo dye at 595 nm. One-unit (U) keratinase activity was defined as the amount of enzyme causing 0.01 absorbance increase between the sample and control at 595 nm under given conditions.
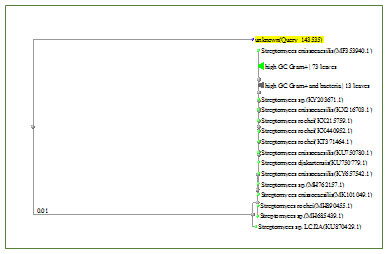
Characterization of keratinolytic isolate AM1 :Morphological and biochemical characterization of the keratinolytic isolate was carried out. The morphology of the spore-bearing hyphae with the entire spore chain, and the substrate and aerial mycelium of the strain were examined by light microscope. The isolate was compared and identified according to Bergey’s Manual of Determinative Bacteriology. The second step is DNA sequence which was compared to the GenBank database at the National Center for Biotechnology Information (NCBI) using the BLAST program.
Enzyme purification: Solid ammonium sulphate (80% w/v) was used to the filtrate with gentle stirring at 4ºC overnight. The mixture was then centrifuged at 8000 rpm for 30 minutes at 4ºC. Both enzyme activity and protein content were determined in the precipitate. This purification step was carried out to remove the traces of ammonium sulphate. The resultant precipitate was dissolved in 5 mℓ 0.02 M tris-HCl buffer pH 8.5 and dialyzed overnight against 2 liters of the same buffer in a cellophane bag (Aly et al, 2020). The concentrated and dialyzed cell free supernatant became ready to be applied on further purification step. The dialyzed solution was concentrated under vacuum and applied to a column (30×1.5 cm) of Sephadex G100 column chromatography followed by diethylaminoethyl-cellulose (DEAE cellulose) and elution was with 1M NaCl in phosphate buffer at a flow rate of 80 ml/h and analyzed by UV spectrophotometer at 280 nm. The purity of the isolated protein was determined by the SDS-PAGE on 10% gel according to the method of Laemmli (1973). This method was used to determine the molecular weight of the purified keratinase enzyme. The molecular weight of enzyme was determined by standard protein markers (low molecular weight 14-60 kDa) with different molecular weights.
Effects of pH and temperature on keratinase activity: The effects of pH and temperature were assayed with keratinazure as substrate. Keratinase activity was studied in the pH range of 5·0–9·0. Optimum temperaturekeratinase activity was determined by varying the incubationtemperature between 20 and 80 °C. After growth period, the AM1 isolated keratinase activity were measured.
Molecular detection of Keratinase gene: In order to confirm the presence or absence of Keratinase gene inthe isolations strain by PCR technique for amplifying, the primer sequences were ordered and used. Gel electrophoresis was used to analyses the PCR yields.
Preparation of Feather Compost:For preparing soil Fertilizer from chicken feather, 20 g of sterile chicken feather were mixed in plastic bag with 1000 g sterile soil each which was pre-autoclaved at 121 °C for 15 min. Similarly, 20 g of non-sterile chicken feather was mixed with 1000 g of non-sterile soil. The preparations were then uniformly inoculated with 200 ml overnight culture suspension of isolated bacteria. Soil mixing was done aseptically, and each bag was labelled appropriately. A separate control without bacterial culture addition was prepared. The feathers were kept for degradation for 30 days, respectively. After 30 days degradation process, the treatments showing ≥ 95% feather degradation were selected for the physicochemical parameter analysis and for further pot study experiments.
RESULTS AND DISCUSSION
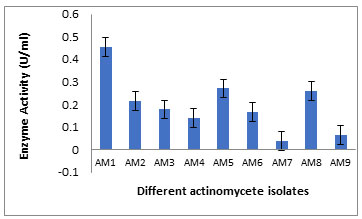
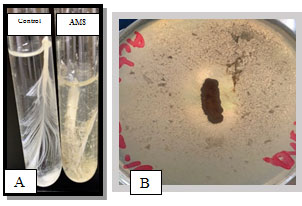
Isolation of Keratinase Producing Bacteria :A total of nine of the actinobacterial strains (AM 1-9) were isolated from the poultry waste and evaluated for their Keratinolytic activity on skim milk agar media and screened at raw feather broth medium for feather degrading property.Among the nine isolates the maximum keratinase enzyme production, observed at the 5th day incubation, was obtained byAM1 isolate as recorded in Table 1 and Figure 1. Out of the 9 isolates, isolate AM1 produced 0.456±0.038U/ml and it was the most active on solid and in broth medium. (Figure 2 A, B) showed the keratin degradation by the isolate AM1 in liquid and solid media. Growth profile of strain AM1 indicated that the selected strain efficiently utilized chicken feather as sole source of carbon and nitrogen. The strain AM1 isolated in the present study has an increased keratinolytic activity which is a desired potential characteristic feature. Thus, it was identified and characterized.
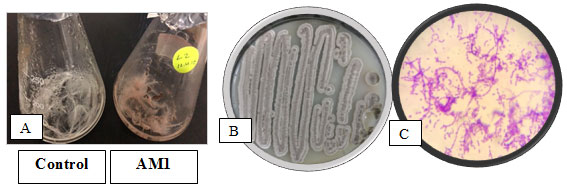
Identification of the Selected Bacterial strain:The keratin degrading actinobacterial isolate AM1 was Gram positive,filamentous bacterium, non-motile in nature and colony characteristics and morphology were examined (Figure 3). The biochemical testes of keratinolytic culture were also studied and the results were shown (Table 2).
Table 1. Keratinase production (U/ml) by the different actinomycete isolates grown in Mineral feather broth medium.
| Actinomycete isolates | Enzyme Detection on solid medium | Enzyme Activity |
Actinomycete isolates |
Enzyme Detection on solid medium
|
Enzyme Activity | ||
| (A595) |
U/ml |
(A595) |
U/ml |
||||
| AM1 | +++ | 0.656±0.038 | 0.494 | AM6 | + | 0.045±0.133 | 0.032 |
| AM2 | ++ | 0.117±0.144 | 0.073 | AM7 | + | 0.047±0.002 | 0.039 |
| AM3 | ++ | 0.178±0.019 | 0.159 | AM8 | ++ | 0.451±0.224 | 0.370 |
| AM4 | ++ | 0.151±0.023 | 0.118 | AM9 | + | 0.064±0.009 | 0.055 |
| AM5 | ++ | 0.371±0.05 | 0.221 | ||||
+++: high production, ++: moderate production, + Low: production
Figure 1: Keratinase production (U/ml) by the different actinomycete isolates grown in Mineral feather broth medium.
Figure 2: A) Secondary screening of keratinolytic actinobacteria on modified basal liquid medium. B) Primary screening.
Figure 3: The selected actinobacterium AM1in A: Broth feather medium, B:on Starch agar c: under light microscope x 1000 after Gram staining.
Table 2. Some physiological and chemical characters of the selected actinomycete isolate AM1
| Character | AM1 | Character | AM1 |
| Gram stain | + | keratinase | + |
| Catalase | + | Growth at 45°C | + |
| Oxidase | + | H2S production | + |
| Urease | + | Melanin production | + |
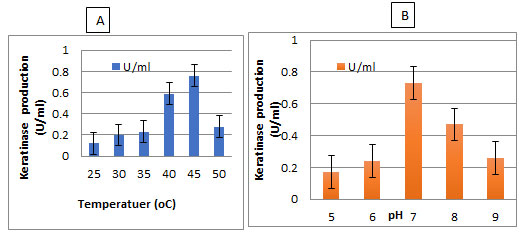
The enzyme production by the selected isolate was carried out between temperature 25- 50°C and pH 5-9. It was found that the highest enzyme production by the selected bacterium was observed at 50°C and pH 7 (Figure 5).
Figure 4: Phylogenetic tree of the isolate AM1 and most related genera.
Figure 5: Effect of Temperature and pH on keratinase production by the isolate AM1 in broth medium
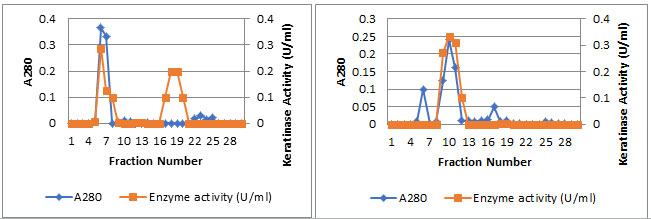
Purification of keratinase: The Streptomyces enissocaesilisAM1 was cultivated for 5 days for all subsequent experiments. Purification of the keratinase was then undertaken. The crude enzyme, which was concentrated by centrifugation and precipitation with 80% saturation of ammonium sulfate. The precipitate was dialyzed and subjected to gel filtration on a Sephadex G-75 column. The elution profiles for keratinase and protein from the Sephadex G-75 column. Two protein peaks were obtained. The second peak shows the highest specific keratinase activity (243.2 U/mg of protein). The most active fractions (numbers 16-22) from the Sephadex G-75 column were pooled and further purified by DEAE-Sephadex A-50 column chromatography.
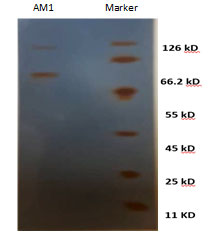
Enzyme detection in the eluate revealed an active peak with high keratinase activity in the active fractions numbers 9-13. An overall recovery of 11.53-fold with a recovery of 32.36% and a specific activity of 316.15 U/mg protein were obtained (Data not shown). Analysis by SDS-PAGE revealed a single protein band. The molecular mass of keratinase of Streptomyces enissocaesilisAM1was estimated to be 66kDa by SDS-PAGE gel electrophoresis (Figuer6 and 7).
Figure 5a: Purification of the Keratinase using two different columnschromatography
Figure 6: SDS-PAGE analysis of keratinase obtained from bacteria, Lane 1: purified keratinase by column chromatography, Lane 2: Marker.
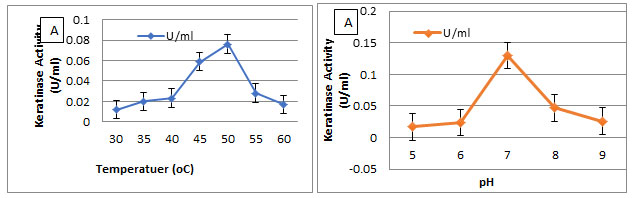
Effect of temperature and pH and on keratinase activity:The optimal temperature and pH for keratinase activity were determined (Figure 7). The optimal temperature for keratinase activity was determined by varying the reaction temperatures, between 30℃ and 60℃, at pH 7. The optimal temperature was 50℃ for keratinase activity, but above 60℃ the activity sharply decreased, as shown in Figure7A. However, the enzyme was completely inactivated at more than 60℃. The optimal pH for keratinase activity was 7 andthe enzyme activity declined rapidly at a pH higher than 8.0.
Figure 7: Effect of Temperature and pH on keratinase activity by the isolate AM1 Molecular detection of Keratinase gene in Streptomyces enissocaesilisAM1 by PCR.
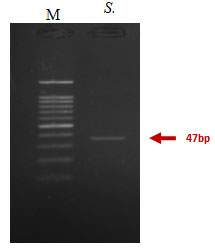
The gel electrophoresis image showed that Keratinase gene was detected in Streptomyces enissocaesilis AM1. Keratinase gene presence in Streptomyces enissocaesilis AM1was detected by PCR yield of Keratinase genes at 476bp (Figure 8).
Figure 7: PCR amplification of Keratinase gene from Streptomyces enissocaesilis AM1 Lane 1:100bp ladder; Lane 2; 47bp partial Keratinase genes.
Preparation of Feather Compost: After 30 days of degradation process, it was observed that there was no visible feather degradation in the control and cultureless treatment. It is indicative that during direct application of raw feather waste to soil, their degradation is augmented by the addition of microbial culture, else the process is hindered. The 10 g of feather treatment were completely degraded (Figure 8). The results indicated that for the effective degradation of keratin rich chicken feather, presence of specific Streptomyces enissocaesilis AM1 is required for enhancing degradation Composting of residual feather seems to require the presence of a co-substrate for composting and nitrogen conservation. Recently many works have been published on the biodegradation of animal wastes using specific microbial populations. Gushterova et al. (2005), Tiquia et al, (2005) obtained 50% carbon conversion when composting the wastes from the poultry industry with high nitrogen content indicating high biodegradability of protein of animal origin. The selected treatment soil sample which showed complete feather degradation were analyzed for physiochemical properties. An increase in N, P, K content was obtained with increase in feather compost percentage in soil.
Figure 8. A) The feather compost after 30 days of degradation. B) the feather compost after drying and prepared for growth plating.
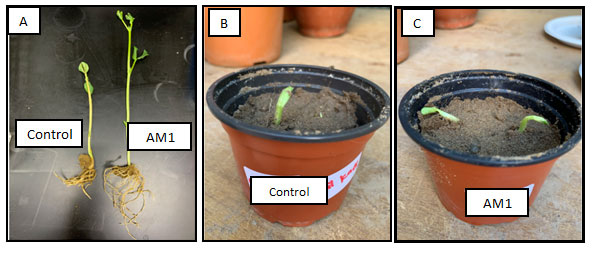
Plant Growth Experiment: After 7 days of seed germination, it was observed that the plant growth in treatment sample exhibited high plant growthparameters when compared to control (Figure 9). The plant growth measurements namely shoot, root and leaf length were shown in Figure 9A.
Figure 9: Plant growth under feather treatment of soil; A) broad bean growth, B) control plant, C) treated soil with feather compost.
Actinomycetes may have important role in processing keratin-containing wastes. Biodegradation of feathers by keratinase from Streptomyces may provide viable alternative sources. Various species of Actinomycetes have been reported for feather degrading activity by keratinase (Singh et al., 2012). As it was reported that proteases specially keratinase were secreted by Genus Streptomyces and these enzymes presented activity at a wide range of pH (7.0 to 9.0) and temperature (30°C to 40°C). The added value of feather is its conversion by using physical and chemical treatments to dietary meal for animal feed or as a fertilizer for poor soil. These methods can destroy and decrease protein quality and digestibility of meal. Keratinase may be used to digest keratin. Ability of genus Streptomyces to degrade keratin into economically useful keratin product i.e. nitrogenous fertilizers, biodegradable films, glues and foils are well known. The enzymatic ability of the genus Streptomyces in large scale for decomposing feathers was not studied well while they were playing many important roles in carbon cycle in the environment.
All studies revealed the use of actinomycetes in degradation of keratin waste through is much safe and friendly for human and the environment than other commercial used methods. The aim of this study was to identify and isolate the keratinolytic actinomycetes from poultry farms wastes of Jeddah region. The most active isolates was identified using morphological and molecular methods as a spices belong to genus Streptomyces. Poultry feather degradation property of Streptomyces enissocaesilis AM1could be efficiently utilized in feather waste management. This study is useful in rapid removal of the recalcitrant feather content with the release of valuable by products acceptable in land use application. Microbial augmentation to compost at correct inoculum ratio can bring rapid and complete feather reduction to support increase in the quality of soil and growth of plants. The compost prepared from feather degradation along with bacterial strain could be successfully employed as an economic source of nitrogen fertilizers for plants. Addition of composts increases the nutritive value of soil. These feather compost characteristics thus increase the value of feather waste in agricultural field.
CONCLUSION
Keratinase enzymes from Streptomyces enissocaesilis AM1 showed optimal activity at pH 7 and 50°C. Mechanism of degradation includes, sulfitolysis, proteolysis, followed by deamination. In conclusion, Streptomyces enissocaesilis AM1 can grow on keratin as a carbon sourceand secret keratinase which degrade keratin tosmall peptide chains, amino acids, and minerals which can be used as organic fertilizer for enhancing plant growth.
ACKNOWLEDGEMENTS
The authors are grateful to King Abdulaziz University, Department of Biology, Faculty of Science for providing laboratory facilities.
REFERENCES
Bahamdain L A , Abo Aba S E., Sabry2 A, Amasha R H. , Noor SO and Aly M M (2020) Molecular Identification and Phylogenetic Analysis of Some Rare Actinomycetes Obtained from Al-Lith Hot Spring Area of Saudi Arabia. Biosc. Biotech. Res. Comm. Vol 13 (3) Pp-1037-1049.
Elmayergi HH and Smith RE (1971) Influence of growth of Streptomyces fradiae on pepsin-HC1 digestibility and methionine content of feather meal. Can J Microbiol 17:1067-1072.
Fuhong X, Yapeng C, Xiuqing Y, Jing Y, Zhiquan X, Yuanming L and Shijun Q (2010) Purification and characterization of four keratinases produced by Streptomyces sp. strain 16 in native human foot skin medium. Biores Tech 101: 344-350.
Gupta R, Beg QK and Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15-32.
Gushterova A, Braikova, D, Goshev, I, Christov, P, Tishinov, K, Vasileva-Tonkova, E, Haertle, T, Nedkov, P, Degradation of keratin and collagen containing wasted by newly isolated thermoactinomycetes by alkaline hydrolysis, Letters, Appl. Microbiol, 2005, 40: 335-340.
J.S. Moritz, J.D. Latshaw,Indicators of nutritional value of hydrolyzed feather meal,Poultry Science,Volume 80, Issue 1,2001,Pages 79-86,ISSN 0032-5791.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685.
Letourneau F, Soussotte V, Bressollier P, Branland P and Verneuil B (1998) Keratinolytic activity of Streptomyces sp. SK1-02: a new isolated strain. Lett Appl Microb 26:77-80.
M M. Aly , Alsiny M N., AbuRas M M. and Jastaniah S D. (2020) Antitumor L-Glutaminase Production by Rare Actinomycetes Obtained from Marine Water Using Submerged Fermentation. Bioscience Biotech, Research Combination, 13 (3) Available from: https://bit.ly/2D4wRBe
Magda M. Aly.Isolation, Identification, And Characterization Of A Keratolytic Bacterium From Poultry Wastes. IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) 14.5 (2019): 46-50.
Moran ET, Summers JD and Slinger SJ (1966) Keratin as a source of protein for the growing chick. Amino acid imbalance as the cause for inferior performance of feather meal and the implication of disulfide bonding in raw feathers as the reason for poor digestibility. Poul Sci 45:1257-1266.
Nam GW, Lee DW, Lee HS, Lee NJ, Kim BC, Choe EA, Hwang JK, Suhartono MT and Pyun YR (2002) Native feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase producing thermophilic anaerobe. Arch Microbiol 178:538-547.
Odetallah NH, Wang JJ, Garlich JD and Shih JCH (2003) Keratinase in starter diets Improves growth of broiler chicks. Poul Sci 82:664-670.
Onifade Okami Y (1952) Utilization of nitrogen compounds by streptomycetaceae and its application to classification. Jap J Med Sci Biol 5:265.
Parry DAT and North ACT (1998) Hard α-keratin intermediate filament chains: substructure of the N and C-terminal domains and the predicted structure and function of the C-terminal domains of type I and type II chains. J Struct Biol 122:67-75.
Parry, D.D.D, North, A.C.T., 1998. Hard -keratin inter- mediate filament chains: substructure of the N- and C-terminal domains and the predicted structure and func- tion of the C-terminal domains of type I and type II chains. J. Struc. Biol., 122, 279, 290.
Riffel A and Brandelli A (2006) Keratinolytic bacteria isolated from feather waste.Braz J Microb 37:395-399.
Singh S, Shrivastava A R , Gupta A , Singh A K , Gopalan N and Chaudhary H S (2012). Keratinloytic Actinomycetes Isolated From Poultry Waste. Journal of Chemical and Pharmaceutical Research, 4(9):4107-4111.
Tiquia SM, Ichida JM, Keener HM, Elwell DL, Burttt EH, Michel FC, Bacterial community profiles on feathers during composting as determined by terminal restriction fragment length polymorphism analysis of 16S rDNA genes, Appl, Microbial, Biotechnology, 2005, 67: 412-419.
Tork SE, Qari H, Zainal H M, Aly M M. (2020b). Biodegradation of tannins from polluted sources using natural enzyme. Kuwait J. Sci. 47 (4) pp. 82-91.
Tork S E., Aly M M., Al-Fattani S Q. (2020a). A new uricase from Bacillus cereus SKIII: Characterization, gene identification and genetic improvement. International Journal of Biological MacromoleculesVolume 165, Part B: 3135-3144.