1University of Science, Vietnam National University Ho Chi Minh City.
2University of Food Industry Ho Chi Minh City Viet Nam
Corresponding author email: thoatta@hufi.edu.vn
Article Publishing History
Received: 10/09/2022
Accepted After Revision: 25/11/2022
Water hyacinth is an aquatic plant, of which proliferation rate is extremely rapid as a weed, causing great economic, social and environmental damage. On the other hand, the water hyacinth also has potential economic value, because it is used as food for livestock, gas, fertilizer, environmental treatment, art crafts, decoration items, as well as herbal medicine. This research investigated the effects of concentrations of BA (Benzyl adenine) and NAA (Naphthylacetic acid) on in-vitro bud and root formation of Eichhornia crassipes [Mart.] Solms to create in-vitro sample source which is initially used in subsequent researches of water hyacinth. After 4 weeks of in-vitro culture, the results showed that the two-layer MS (Murashige Skoog) medium – the lower solid agar medium and the upper liquid medium – accompanied by aerobic culture conditions supplemented with 0.5 mg/L BA were suitable for bud proliferation. Next, these explants after destroying shoot apical meristem were transferred to MS medium supplemented with 0.75 mg/L BA suitable for bud development. The mature buds were transferred to MS medium supplemented with 0.25 mg/L NAA, suitable for rooting of water hyacinth and gave high survival rate (83.00%) when planted in the garden on hydroponic nutrient medium Howard of 600ppm.
BA, NAA, in-vitro, Water hyacinth (Eichhornia crassipes [Mart.] Solms).
Tran T. A. T, Do T. K, Bui T. V. Effect of Phytohormones on In-vitro Bud and Root Formation of the Water Hyacinth, Eichhornia crassipes. Biosc.Biotech.Res.Comm. 2022;15(4).
Tran T. A. T, Do T. K, Bui T. V. Effect of Phytohormones on In-vitro Bud and Root Formation of the Water Hyacinth, Eichhornia crassipes. Biosc.Biotech.Res.Comm. 2022;15(4). Available from: <a href=”https://bit.ly/3DNSpNW“>https://bit.ly/3DNSpNW</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
The dense development of water hyacinth has caused serious economic, social and environmental problems for many countries around the world, especially riverine communities (Howard, 2003). Water hyacinth has become a major weed in more than 50 countries in the tropics and subtropics with strong and lasting effects (Holm et al, 1991). According to the most recent reports in 2017, the major effects of this weed include: Impact on the irrigation system; increase in epidemics such as malaria, flukes, worms, cholera; lack of clean water, effects to waterway traffic; loss of aesthetic value of water bodies, affecting tourism; reduction of capture fisheries and fishing; reduction of biodiversity; difficulties in the hydropower sector; floods; increase in snake and crocodile attacks (Gebregiorgis, 2017).
Regardless of its limitations, the water hyacinth also has advantages. Water hyacinth has potential economic, social and environmental values. Water hyacinth grows widely with a yield of 400 tons/ha, which will meet the requirements of biogas production for cooking, silage for ruminant cattle, mushroom cultivation or fuel (coal briquettes) (Suthar,2022). In the alkaline soil, water hyacinth juice is put into the pond to raise the pH from 3.2 to 4.5, creating more nutrients in the water, especially creating a good environment for Chlorella algae – which is the food of fry, especially flounder and marble goby (Jimenez, 2020). In its natural form, water hyacinth has the effect of absorbing heavy metals (such as lead, mercury, strontium) and thus, can be used to eliminate environmental pollution (Mary P.N, 2011). According to Dan Viet newspaper, the current saltwater intrusion has caused death for water hyacinth, dozens of poor and disadvantaged households in Hoa Tien commune (Vi Thanh city, Hau Giang province) who live on this plant have lost their jobs.
The whole water hyacinth contains 92.6% water; 2.9% protein; 0.9% sugar; 2.2% fiber; 1.4% ash – with 40.8% calcium; 0.8% phosphorus; 0.86% carotenoids; 20% vitamin C and inorganic components such as SiO2, Mg, K, Na, Cl, Cu, Mn, Fe. Besides, it also contains vitamins such as B1, B2, B6, B12 and E. Its flower contains delphinidin diglucoside (Cheng, 2015). With a chemical composition rich in vitamins and fiber, the water hyacinth is also used as a green vegetable in the family’s diet. Young buds, petioles and flowers of water hyacinth are washed, boiled or cooked in half-done soup, people can eat this refreshing meal without itchiness.
In addition, the water hyacinth also works as a medicine, its extract has antibacterial properties, helping to inhibit the growth of gram-negative and gram-positive bacteria (Shanab, 2010). In addition, the scientists also indicated that some extracts of water hyacinth also have anti-candida albicans effects. Moreover, the antioxidant activity in water hyacinth has the effect of preventing liver and breast cancer cells (Aboul-Enein,2014). However, due to its ability to absorb heavy metals, the water hyacinth can be harmful to users. Thus, it is necessary to cultivate clean water hyacinth for food and medicine.
Besides, the water hyacinth is also used as art crafts, decoration items, ornamental plants. Dried water hyacinth can be used to braid into ropes and then weave into mats, crafts, or tables and chairs. Water hyacinth is also grown as a decorative ornamental plant in gardens, fish tanks, etc (Villamagna, 2010).
Along with the advantages and disadvantages of water hyacinth, the problem is to research the growth and development of water hyacinth so that it can be adjusted when necessary. Specifically, it is possible to increase growth and development in case of using water hyacinth as food, gas, fertilizer, environmental treatment, art crafts, decoration items, etc., or inhibit the growth and development of water hyacinth in case water hyacinth spreads as fast as weeds…, or adjust the growth and development of water hyacinth in the direction of creating a source of clean plants, or creating a source of plants with small beautiful shapes as ornamental plants. To solve this problem, the plant tissue culture can be used to create in vitro plants. The tissue culture is the culture of material completely free of microorganisms on artificial nutrient medium under sterile conditions (Soumare, 2021).
In vitro plant plays very important role in the research of plant growth and development, in understanding the physiological mechanisms of plants. In addition, the in vitro plant also makes a fast, abundant and clean sample source. However, in the in vitro plant researches, water hyacinth is most unconcerned because of its untapped economic value and difficulties in in vitro culture of group of aquatic plants. In this research, we focus on describing the process of creating in vitro samples of water hyacinth, Eichhornia crassipes [Mart.] Solms through the investigation of the effects of phytohormones on in vitro bud and root formation of water hyacinth.
MATERIAL AND METHODS
Research Materials
The research materials were 2-week-old water hyacinth buds grown in the experimental garden, the buds were removed from the leaves and cut into 3 cm long segments bearing the apical meristem, trimmed off the roots.
Cultivation conditions: Lighting of 12 hours/day, light intensity of 3000 lux; temperature of 25 °C ± 2 °C at the Cell Technology Laboratory, Ho Chi Minh City University of Food Industry.
Research Methods
Investigation of suitable in vitro culture mediums for bud viability of water hyacinth: Water hyacinth buds grown in the experimental garden with all leaves removed were disinfected by washing with soap for 5 minutes and putting under running water for 3 hours, disinfecting with alcohol 700 2 min and HgCl2 0.1% for 3 minutes. Then they are cultured on MS basic medium supplemented with 30g/l sucrose and solid MS medium conditions (4g/ml agar added), 2-layer MS medium- solid bottom and liquid top, and liquid MS medium with cotton wool. They are cultivated under in vitro conditions with lighting of 3000 Lux, temperature of 250C. The experiment consisted of 3 treatments, each treatment had 5 samples, each culture flask had 1 sample. The development of in vitro water hyacinth was monitored by imaging at 10, 20, 30, 40 days after culture.
Investigation of the effect of BA concentration on bud formation and bud proliferation for in vitro Eichhornia crassipes [Mart.] Solms: The experiment was designed to determine the optimal BA concentration for bud formation and bud proliferation of Eichhornia crassipes [Mart.] Solms. The in vitro buds are 2-2.5 cm in size, are not destroyed shoot apical meristem and subcultured to two-layer MS medium supplemented with BA with different concentrations of 0.0; 0.5; 1.0; 1.5; 2.0 mg/L for the purpose of investigating bud proliferation. The in vitro buds are 2-2.5 cm in size, after destroying the apex of the mother buds, subcultured to two-layer MS medium supplemented with BA with different concentrations of 0.0; 0.25; 0.5; 0.75; 1.0 mg/L for the purpose of investigating bud formation (new bud formation). Each treatment was repeated 3 times, with 5 flasks each replicate, and 1 sample each flask. After 4 weeks of culture, the parameters including number of buds (buds/sample), bud morphology under the in vitro conditions are monitored.
Investigation of the effect of NAA concentration on rooting ability for in vitro Eichhornia crassipes [Mart.] Solms: The experiment was designed to determine the optimal concentration of NAA for in vitro rooting of Water hyacinth Eichhornia crassipes [Mart.] Solms. The in vitro buds are 5-6 cm in size, 2-3 leaves were cultured on two-layer MS medium supplemented with 30g/L sucrose, 4g/L agar, supplemented with NAA with different concentrations of 0.0; 0.25; 0.50; 0.75; 1.0 mg/L respectively for rooting investigation purposes. Each treatment was repeated 3 times, with 5 flasks each replicate, and 1 sample each flask. After 4 weeks of culture, the parameters including number of roots (roots/sample), root morphology under the in vitro conditions are monitored.
Investigation of the effect of nutrient environment on the survival of seedlings in the nursery garden: The experiment was designed to investigate the appropriate nutrient environment for the survival of seedlings in the nursery garden. The in vitro water hyacinth samples are 8-10 cm in size, have healthy roots and buds. The in vitro water hyacinths are domesticated in glass bottles for 15 days, then cultured in 3 medium types including water, Howard Resh hydroponic solution 300ppm, and 600ppm Howard Resh hydroponic solution (Resh, 2022). After 10 days, the survival rate (%) of water hyacinth is monitored in the nursery garden.
Statistical processing: All experiments were repeated 3 times, data were recorded and statistically processed by Statgraphics Centurion XV software, the significant difference was at p ≤ 0.05.
RESULTS AND DISCUSSION
Investigation of suitable in vitro culture mediums for bud viability of water hyacinth: Water hyacinth grown in the experimental garden removed all the leaves, were disinfected and cultured on the conditions of solid MS medium (A), 2-layer MS medium- solid bottom and liquid top (B), and liquid MS medium with cotton wool (C). In the 2-layer medium, plants had the highest survival rate and well developed bud morphology. Meanwhile, in the solid MS medium, the new leaves of plants were formed in the early stage, but quickly absorbed and drained water, leading the drying of the agar medium and the yellowing of leaves after 15 days of culture. In liquid medium, cotton and in vitro buds are completely undeveloped and blackened at the 15th day (Table 3.1, Figure 3.1).
Table 3.1 . Survival rate of in-vitro water hyacinth in three culture conditions
| Treatment | Survival rate (%) |
| A medium | 44.44 b ± 11.11 |
| B medium | 77.78 c ± 11.11 |
| C medium | 0.00 a ± 0.00 |
Figure 3.1: In-vitro water hyacinth after 15 days of culture. A: solid MS medium, the buds form 3-4 leaves, have no stolon, the plant turns yellow and begins to die after 15 days. B: 2-layer MS medium, the buds form 4-5 leaves, have stolon, develop well. C: liquid MS medium with cotton wool, the plants do not produce buds, turn black and die after 15 days of culture.

However, under culture conditions in medium B, in vitro plants have yellowing phenomenon and die after 15 days. Subsequent observations on airtight and aerobic culture conditions resulted in the following: In an airtight culture, the in vitro water hyacinth formed buds from axillary buds after 15 days of culture and roots after 30 days of culture in semi-solid MS medium. After 30 days, the stolon extends from the lateral bud, the first leaf of the seedling also has a reduced leaf blade, the second and third leaves grow linearly embracing each other, roots form just below the base of the first leaf, the outer spongy petioles embrace the entire leaf and the buds inside, the top of the leaf sheath is a thin membrane embracing the entire inside. The in vitro complete plant has 4-5 young green leaves with spongy petioles. The rootlets arise in the upper position of the stolon extending from the axillary bud, the roots are dark gray in color. The in vitro plants live up to 30 days, then the leaves begin to show signs of yellowing. On the 45th day, the leaves turned yellow and died (Figure 3.2).
Under aerated culture conditions, water hyacinth plants absorb water and transpire strongly, causing the drying and cracking medium at the 7th day (Figure 3.3 A). When transplanted to 2-layer MS medium, the plants thrived with two lateral buds, young green leaves, and spongy petioles (Figure 3.3 B). The in vitro water hyacinth continued to absorb water and transpire strongly, causing the water level to dry up quickly on the 20th day (Figure 3.3 C). On the 30th day of culture, the in vitro water hyacinth increased leaf size and increased number of roots (Figure 3.3D).
From the 40th day onwards, new buds appeared at the roots and leaf axils. The stolon also stretches in the horizontal and vertical directions (Figure 3.3 E, F)
Figure 3.2: The in-vitro water hyacinth after 15,30,45 days of culture in semi-solid MS medium under airtight condition
A: The in-vitro water hyacinth after 15 days of culture, with 3 leaves and stolon extending from the lateral bud of the explant, B: Water hyacinth leaves with spongy petioles after 15 days of culture.
C: The in-vitro water hyacinth after 30 days of culture with leaves turning yellow
D: The in-vitro water hyacinth died on the 45th day of culture.
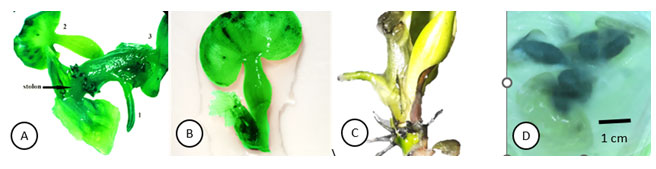
Figure 3.3: The in-vitro water hyacinth and days of culture in MS medium under aerated conditions
A: The in-vitro water hyacinth after 7 days of culture, there were two lateral buds and the medium was dry and cracked.
B: The in-vitro water hyacinth in liquid MS medium after 15 days of culture.
C: The in-vitro water hyacinth after 20 days of culture
D: The in-vitro water hyacinth after 30 days of culture.
E: The in-vitro water hyacinth after 40 days of culture, stolon appeared
F: The in-vitro water hyacinth after 45 days of culture with prolonged stolon
Investigation of the effect of BA concentration on bud formation and bud proliferation for in vitro Eichhornia crassipes [Mart.] Solms: The in vitro buds are 2-2.5 cm in size, are not destroyed shoot apical meristem and subcultured to two-layer MS medium under aerobic conditions supplemented with BA with different concentrations of 0.0; 0.5; 1.0; 1.5; 2.0 mg/L for the purpose of investigating bud proliferation. At a concentration of 0.5 mg/L, the highest number of buds were formed with a well-developed bud morphology with 2.33 buds/sample. Meanwhile, at concentrations of 1; 1.5; 2 mg/L BA the number of buds formed was lower than that of 0.5 mg/L BA and there was no statistical difference compared to the control (Table 3.2, Figure 3.4).
Table 3.2. Effect of BA concentration on bud formation in-vitro Eichhornia crassipes [Mart.] Solms
| BA concentration (mg/L) | number of buds | Length of buds (cm) |
| 0,00 | 1.00 a ± 0.00 | 5.20 b ± 0.97 |
| 0,50 | 2.33 b ± 0.53 | 6.80 d ± 1.31 |
| 1,00 | 1.66 ab ± 0.33 | 6.20 c ± 0.73 |
| 1,50 | 1.00 a ± 0.00 | 4.80 a ± 1.54 |
| 2,00 | 1.00 a ± 0.00 | 5.30 b ± 0.72 |
a, b, c: show differences in significant columns at confidence level p ≤ 0.05 in the Duncan test.
Cytokinin has a strong stimulant effect on bud differentiation, so under the basic MS medium supplemented with BA, all samples were induced increased biomass. According to Phillips, a high concentration of BA is suitable to stimulate bud formation, however, lower BA concentration is required at the bud proliferation stage because BA at high concentrations can inhibit the absorption of nutrients, creating abnormal buds. Experimental results of Jordan et al. showed that when using MS medium supplemented with BA for plant bud proliferation at a concentration of 0.5 µM, this result is similar to the research experiment. Thus, subculture to MS medium supplemented with 0.5 mg/L BA is suitable for bud proliferation.
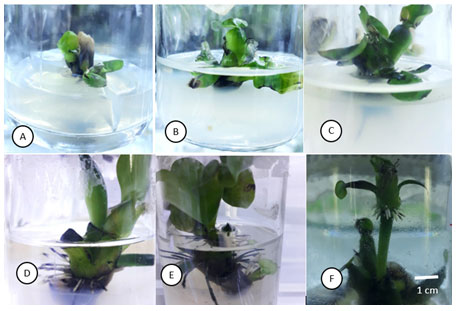
Figure 3.4. Effect of BA concentration on bud formation in-vitro Eichohonia crassipes [Mart.] Solms
A: 0.00 mg/L BA concentration, mother bud grows, no baby bud. B: 0.50 mg/L, mother bud grows, has a baby bud . C: 1.00 mg/L, mother bud grows, has a baby bud. D: 1.50 mg/L, mother bud grows, no baby bud. E: 2.00 mg/L, mother bud grows, no baby bud mother bud grows, no baby bud. E: 2.00 mg/L, mother bud grows, no baby bud The in vitro buds are 2-2.5 cm in size, after destroying the apex of the mother buds, subcultured to two-layer MS medium supplemented with BA with different concentrations of 0.0; 0.25; 0.5; 0.75; 1.0 mg/L for the purpose of investigating bud formation. Under the condition of destroying shoot apical meristem, the number of buds formed increased and reached the highest number of buds at the concentration of 0.75 mg/L BA with 7.6 buds/mother plant (Table 3.3, Figure 3.5).
Table 3.3. Effect of BA concentration on bud proliferation for in-vitro Eichhornia crassipes [Mart.] Solms
| BA concentration (mg/L) | number of buds | Length of buds (cm) |
| 0,00 | 2.00 a ± 0.29 | 5.20 ab ± 0.55 |
| 0,25 | 2.33 a ± 0.23 | 5.00 a ± 0.95 |
| 0,50 | 4.33 b ± 0.23 | 6.20 b ± 0.29 |
| 0,75 | 7.66 c ± 0.20 | 6.80 bc ± 1.33 |
| 1,00 | 3.66 b ± 0.29 | 5.10 a ± 0.84 |
a, b, c: show differences in significant columns at confidence level p ≤ 0.05 in the Duncan test.
Cytokinin has a strong stimulant effect on bud differentiation, corrects apical dominance, and releases lateral buds from the relative inhibition of apical buds. Therefore, cytokinins are often used to induce bud proliferation and increase the propagation coefficient in tissue culture. On MS medium supplemented with BA, the samples were induced and bud sprouted. However, when used at high concentrations, it often has an inhibitory effect (Phillips, 2019). In this experiment, the obtained results also correspond to the proven theory. The ability to sprout new buds increased in the treatments from 0.00-0.75 mg/L BA and reached the highest concentration at 0.75 mg/L with 7.66 buds/sample and then the number of new buds decreased at a concentration of 1.00 mg/L, this proves that it inhibits bud proliferation at high concentrations of Cytokinin.
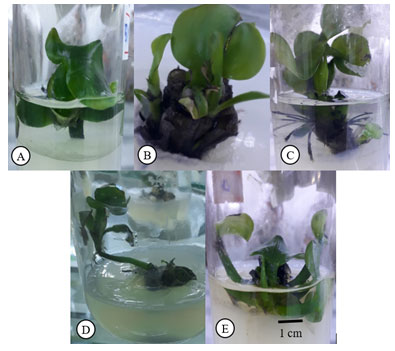
Figure 3.5. Effect of BA concentration on bud proliferation for in-vitro Eichhornia crassipes [Mart.] Solms
A: 0,00 mg/L BA concentration, 2 baby buds . B: 0,25 mg/L, 2-3 baby buds. C: 0,50 mg/L, 4-5 baby buds. D: 0,75 mg/L, 7-8 baby buds. E: 1,00 mg/L, 3-4 baby buds.

The in vitro buds are 5-6 cm in size, 2-3 leaves were cultured on two-layer MS medium supplemented with 30g/L sucrose, 4g/L agar, supplemented with NAA with different concentrations of 0.0; 0.25; 0.50; 0.75; 1.0 mg/L respectively for rooting investigation purposes. After 4 weeks of culture, at the concentration of 0.25 mg/L NAA, the highest number of roots were formed with stiff, black root morphology (Table 3.4, Figure 3.6).
Table 3.4. Effect of NAA concentration on rooting ability for in-vitro Eichhornia crassipes [Mart.] Solms
| NAA concentration (mg/L) | Number of roots | Length of roots (cm) |
| 0,00 | 10.00a ± 0.00 | 1.20 b ± 0.31 |
| 0,25 | 17.44 b ± 0.20 | 2.90 d ± 0.98 |
| 0,50 | 11.00 a ± 0.88 | 0.70 a ± 0.33 |
| 0,75 | 12.00 a ± 2.18 | 1.50 c ± 0.48 |
| 1,00 | 11.78 a ± 0.51 | 1.00 a ± 0.24 |
a, b, c: show differences in significant columns at confidence level p ≤ 0.05 in the Duncan test.
Auxin stimulates root formation, especially minor roots (Kumari, 2022). In tissue culture, auxin stimulates root differentiation and branching root development. At high concentrations, the auxin inhibited primary root elongation but initiated lateral root and indeterminate root formation (Bui Trang Viet, 2020). The MS medium supplemented with NAA stimulated root formation in water hyacinth buds. At the concentration of NAA 0.00-0.25 mg/L, the number of roots/sample increased, the roots were strong, black with hairy suckers. After that, at the concentration of 0.5 mg/L to 1.00 mg/L NAA, the rooting efficiency is not high, the number of roots is small. At a concentration of 1 mg/L NAA, the number of roots per sample decreased, and the roots were short and brittle. Thus, the best concentration is NAA 0.25 mg/L for roots with hairy suckers, with many black, strong roots.
Figure 3.6. Effect of NAA concentration on rooting ability for in-vitro Eichohonia crassipes [Mart.] Solms
A: 0,00 mg/L NAA concentration, black, small and weak. B: 0,25 mg/L, black, big .C: 0,50 mg/L, light brown, small and weak. D: 0,75 mg/L, light brown, small and weak. E:1,00 mg/L, light brown, small and weak.

Investigation of the effect of nutrient environment on the survival of seedlings in the nursery garden: We can clearly see that in water without adding nutrients, water hyacinth has browning and dies after 2 weeks. In contrast, in hydroponic solution with concentrations of 300 ppm and 600 ppm of dissolved inorganic substances, the plants tend to grow well, have long roots and generate new buds. However, in a hydroponic solution of 300 ppm, the plants have few buds and roots and do not grow as much as the plants in the 600 ppm hydroponic solution.
Table 3.5. Effect of nutrient environment on the survival of seedlings in the nursery garden
| Nutrient environment | Survival rate (%) |
| Water | 10.00a ± 2.00 |
| 300ppm hydroponic solution | 50.44 b ± 8.30 |
| 600ppm hydroponic solution | 83.00 c ± 9.87 |
a, b, c: show differences in significant columns at confidence level p ≤ 0.05 in the Duncan test.
Figure 3.7: Effect of nutrient environment on the survival of seedlings in the nursery
garden water hyacinth Eichhornia crassipes [Mart.] Solms
A: water solution, leaves turn yellow. B: 300ppm hydroponic solution, roots develop weakly, stems are submerged in water. C: 600ppm hydroponic solution, leaves and roots are well developed.

CONCLUSION
After 4 weeks of culture, the results have determined that two-layer MS (Murashige Skoog) medium – the lower solid agar medium and the upper liquid medium – under aerobic conditions with the addition of BA 0.5 mg/L is suitable for the growth of water hyacinth buds, then destroying shoot apical meristem and transferring to a medium supplemented with 0.75 mg/L BA suitable for bud growth, and supplemented with 0.25 mg/L NAA suitable for rooting. The survival rate at the nursery garden was 83 % on the hydroponic nutrient medium with TDS of 600ppm. The results of the research determined that exogenous sources of cytokinin and auxin have an impact on the micropropagation of water hyacinth, increasing the ability to create healthy buds and roots, creating a premise for the plant to have a high survival rate when transferred to nursery garden in hydroponic nutrient medium. This is the basis for continuing to research the effect of auxin and cytokinin coordination ratio on bud and root formation in order to improve the efficiency of the micropropagation process of Eichhornia crassipes [Mart.] Solms.
REFERENCES
Aboul-Enein, A. M., Shanab, S. M., Shalaby, E. A., Zahran, M. M., Lightfoot, D. A., & El-Shemy, H. A. (2014). Cytotoxic and antioxidant properties of active principals isolated from water hyacinth against four cancer cells lines. BMC complementary and alternative medicine, 14(1), 1-11. https://doi.org/10.1186/1472-6882-14-397
Bui Trang Viet (2020). Plant physiology. Ho Chi Minh City National University Publisher, pp. 248-249.
Cheng, Y. S., Chen, K. Y., & Chou, T. H. (2015). Concurrent calcium peroxide pretreatment and wet storage of water hyacinth for fermentable sugar production. Bioresource technology, 176, 267-272. https://doi.org/10.1016/j.biortech.2014.11.016
Gebregiorgis, F, Y, (2017), Management of water hyacinth (Eichhornia crassipes [Mart,] Solms) using bioagents in the Rift Valley of Ethiopia (Doctoral dissertation, Wageningen University). DOI http://dx.doi.org/10.18174/401611
Holm, L,G,, D,L, Plucknett, J,V, Pancho, and J,P, Herberger (1991), The World’s Worst Weeds: Distribution and Biology, Melbourne, FL: Krieger Publishing Company.
Howard, G,W, and S,W, Matindi (2003), Alien Invasive Species in Africa’s Wetlands, Some threats and solutions, IUCN Eastern African Regional Program, Nairobi, Kenya, pp 15.
Jimenez-Llanos, J., Ramirez-Carmona, M., Rendon-Castrillon, L., & Ocampo-Lopez, C. (2020). Sustainable biohydrogen production by Chlorella sp. microalgae: A review. International Journal of Hydrogen Energy, 45(15), 8310-8328. https://doi.org/10.1016/j.ijhydene.2020.01.059
Kumari, A., Gogna, M., Mehta, S., & Husen, A. (2022). Adventitious root formation in cuttings and effects of maturation. In Environmental, Physiological and Chemical Controls of Adventitious Rooting in Cuttings . Academic Press (pp. 397-420).
Mary Lissy P.N, & Madhu, G. (2011). Removal of heavy metals from waste water using water hyacinth. International journal on transportation and Urban Development, 1(1), 48. DOI: 01.IJTUD.01.01.39.
Phillips, G. C., & Garda, M. (2019). Plant tissue culture media and practices: an overview. In Vitro Cellular & Developmental Biology-Plant, 55(3), 242-257. https://doi.org/10.1007/s11627-019-09983-5
Resh, H. M. (2022). Hydroponic food production: a definitive guidebook for the advanced home gardener and the commercial hydroponic grower. CRC press.
Shanab, S. M., Shalaby, E. A., Lightfoot, D. A., & El-Shemy, H. A. (2010). Allelopathic effects of water hyacinth [Eichhornia crassipes]. PloS one, 5(10), e13200. https://doi.org/10.1371/journal.pone.0013200
Soumare, A., Diédhiou, A. G., Arora, N. K., Tawfeeq Al-Ani, L. K., Ngom, M., Fall, S. & Sy, M. O. (2021). Potential role and utilization of plant growth promoting microbes in plant tissue culture. Frontiers in Microbiology, 12, 649878. https://doi.org/10.3389/fmicb.2021.649878
Suthar, S., Sharma, B., Kumar, K., Banu, J. R., & Tyagi, V. K. (2022). Enhanced biogas production in dilute acid-thermal pretreatment and cattle dung biochar mediated biomethanation of water hyacinth. Fuel, 307, 121897. https://doi.org/10.1016/j.fuel.2021.121897
Villamagna, A, M,, & Murphy, B, R, (2010), Ecological and socio‐economic impacts of invasive water hyacinth (Eichhornia crassipes): a review, Freshwater biology, 55(2), 282-298. https://doi.org/10.1111/j.1365-2427.2009.02294.x


