Zoology Department, Lumbding College, Lumbding, Assam, India.
Corresponding author email: jashodeb@gmail.com
Article Publishing History
Received: 19/10/2021
Accepted After Revision: 28/12/2021
Aquatic ecosystem has been reported to be the universal sufferer of pollution caused by direct exposure of industrial discharges which causes severe genotoxic damages to aquatic flora and fauna. Researchers have found that fish have been extensively harmed by such exposure compared to other aquatic fauna. As living organisms directly depend on fish as a food resource, hence the study of mutagenicity induced have been extensively important not only for safety of aquatic organisms but also for safety of other living organisms too. Micronucleus (MN) assay has been continuously used in the evaluation of DNA damage. Mutagenic and genotoxic studies employed this methodology to evaluate possible carcinogenic risk due to exposure to harmful xenobiotics in including aquatic organisms.
The aim of this study was to monitor the level of genotoxicity induced in fishes due to exposure to local paper mill effluent by using micronucleus assay as a biomarker. Fish were exposed to different concentrations of PME as 10%, 25% and 50%. Variation of body weight, survivality rate and percentage of micronucleated PCEs were analyzed. One-way anova was performed and data were expressed as Mean± S.E. Consecutive dose dependent and time dependent increase of toxicity was recorded in PME compared to negative and positive control (Mitomycin C). Our study supported the carcinogenic and chromosomal damage induced in aquatic organisms specially in fishes due to direct exposure of industrial discharges; also, the importance of MN test as an effective indicator for testing genotoxicity in fishes was confirmed.
Micronucleus, Mitomycin C, Paper Mill Effluents.
Paul R, Jashodeb A. Ecotoxicological Risk Assessment of Paper Mill Effluent Waste Water. Biosc.Biotech.Res.Comm. 2021;14(4).
Paul R, Jashodeb A. Ecotoxicological Risk Assessment of Paper Mill Effluent Waste Water. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3xAcRhG“>https://bit.ly/3xAcRhG</a>
Copyright © Paul and Jashodeb This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Water pollution day by day has become a global matter of concern. Industrial, agricultural and domestic discharges find their ultimate recipient source as the water bodies leading to pollution in the aquatic environment, which in last few decades in researches have been reported to become a threat to aquatic ecosystem resulting in water quality damage and severe chromosomal abnormalities in aquatic organisms specially in fishes (Porto et al. 2005; Yadav et al. 2010).
Thus, Safety of aquatic environment is a major task as water resources covers two third of the globe and living organisms fully depend on it for their livelihood including humans (Krishnamurthi et al. 2003). The country administration has been continuously looking after this serious issue of cleanliness and safety of all water bodies starting from river Ganga upto the smallest stream or pond on which living organisms depends for their livelihood. Because in researches it has come to notice that people are not concerned for the cleanliness of such water bodies on which they actually depend (Ismail et al. 2014).
As a result, not only the water quality is degrading very fast but also the aquatic ecosystem is in danger including both its flora and fauna (Kohlpoth et al. 2009). Among all of the aquatic organisms, fish have been considered as an important species as it covers the majority of the aquatic fauna and it has been consumed by living organisms especially human beings for their survival (Minovski et al. 2019). So, to test the level of genotoxicity induced by industrial effluents in aquatic organisms, fish can accepted as a good test model. Simultaneously, fish have been successfully used in cytogenetic analysis, being easy to handle and adaptable with the laboratory environment, provides a relatively low-cost method (Hayashi et al. 1997; Singh et al. 2020).
Micronuclei (MN) were first described in the cytoplasm of erythrocytes more than a century ago and were called “fragment of nuclear material” by Howell or “intraglobu- laries corpuscules” in the terminology of Jolly in the late.
laries corpuscules” in the terminology of Jolly in the latMicronuclei (MN) were first described in the cytoplasm of erythrocytes a century ago and were called ‘ fragments of nuclear material’ by Howell or ‘ Intraglobularies corpuscules ‘ in the terminology of Jolly in the late 18th century and early 1900. Hematologisits called these structures as ‘Howell Jolly Bodies’ (Kirsch –Volders et al.., 2003)
Micronucleus assay has been successfully used as a mutagenic assay to test the induced genotoxicty in various organisms as fishes. Among the various mutagen tests preferred for bio-monitoring contaminated environments, micronuclei assay (MN) proved relatively simple, reliable and sensitive, and has been used to evaluate the effects of mutagen compounds induced by industrial pollution and domestic discharges in aquatic ecosystem (Al-Sabti and Metcalfe 1995). The use of fish erythrocytes to test the level of toxicity leads to quick results with less chances of suffering of the test organism selected (Minissi et al. 1995; Zúñiga-González et al. 2000; Venier and Zampieron 2005; Singh et al. 2020).
Micronuclei are cytoplasmic chromatin masses that look like small nuclei as a result of lesions at the chromosomes or DNA strains, or at the level of proteins directly involved in chromosome segregation; formation of MN originating from chromosome fragments or chromosome loss events requires a miotic or meiotic division (Heddle et al. 1983). Micronucleus is composed either of small chromatin fragments which arise as a result of chromosome breaks after clastogenic action, or of whole chromosomes that do not migrate during anaphase as a result of aneugenic affects (Cavas and Ergene Gozukara 2003).
Our work was conducted on the only paper mill situated in Barak valley region, Assam from where industrial effluents after paper composition have been observed to get liberated in local river Barak without proper treatment. During exposure a corrosive smell was also found to get spread in the neighboring atmosphere which can be easily felt by any passer by fellow causing air pollution too. The exposure in river Barak was expected to cause harm not only to water body but also to aquatic life. Before exposure fish were brought to laboratory environment and acclimatized. Fish were divided into groups and exposed to three different concentrations of both Paper Mill Effluent (PME) after proper trial and observation was recorded for three consecutive days (Singh et al. 2020).
Besides the average length of the fish, body weight and survivality rate were measured in three replicates. Erythrocyte smears were obtained with heparinized syringes by puncturing the gills on previously washed microscopic slides. The fish remained unharmed and were soon returned to their natural habitat. The slides were air-dried for 24 h, fixed in a 70% methanol solution for 7 min and then dried a further 24 h. Shortly after, they were stained with Giemsa (4%) for 15 min. 3.000 intact erythrocytes were counted from each fish. Only cells that were clearly visible and isolated under a Zeiss microscope with amplification of 1000 X, were counted. Cells with more than four micronuclei were discarded so as to exclude apoptotic phenomena.
Nuclear abnormalities were manifest as changes in the normal elliptic shape of nuclei (Ferraro et al. 2004; Bolognesi et al. 2006). For a detailed description on nuclear abnormalities see previous studies (Ayllón and Garcia-Vazquez 2000; Çavas and Ergene-Gözükara 2003; Çavas et al. 2005). Micronuclei were considered as small inclusions of nuclear material inside erythrocytic cytoplasm. Criteria for identification were a round or oval shape with a flat and well-defined outline, coloration similar to that of the main nucleus and a size from 1/3 to 1/20 in relation to that of the main nucleus (Al-Sabti and Metcalfe 1995; Singh et al. 2020).
MATERIAL AND METHODS
For the sampling site and preparation of water samples, major objective of this current piece of study was to evaluate the acute toxicity of a paper mill effluent of Barak valley region, Assam on the behavioral responses of a regularly consumed freshwater fish Channa punctatus. Raw effluent sample was collected from the outlet discharge pipes in the local river Barak in plastic containers. pH was measured on the very collection spot. Effluent sample was brought to laboratory and to prevent further microbial growth was stored at -20oC. For test model, among various water borne organisms fishes have been considered to be efficient model for studying the induced level of carcinogenesis by industries and domestic affairs.
Researches have reported remarkable dose and time dependent increase in the induction of micronucleus in peripheral blood of fishes (Chaudhury et al. 2006; Ali et al. 2009; Nwani et al. 2010; Saleh and Alshehri; 2011; Pandey et al. 2014). Around 200 fishes of species Channa punctatus were used for the selected bioassays. Fish weighing 15-20 g of weight were selected and before exposure were acclimatized in the laboratory environment for 2-3 weeks. This study was conducted with prior Institutional animal ethics clearance. Fish were given proper treatment and were fed with standard fish food.
For animal exposure and micronucleus assay, after acclimatization fishes were divided into five groups, each group including 40 fishes per aquaria were exposed to three selected concentrations (10%, .25% and 50%, v/v, effluent/distilled water) of the paper mill effluent against negative (distilled water) and positive (Mitomycin C, 2mg/lit) controls for 24, 48 and 72 hrs. 5 fishes per group of treatment were selected for micronucleus assay and blood erythrocyte smears were obtained.
Three slides per sample were prepared. 3000 erythrocytes were counted and scored by a single scorer to eliminate inter-observer variation Using a light-microscope (Leica DMLS) at 1000 magnification where criteria for identification were a round or oval shape with a flat and well-defined outline, coloration similar to that of the main nucleus and a size from 1/3 to 1/20 in relation to that of the main nucleus (Al-Sabti and Matcalfe 1995).
For scoring criteria for micronuclei, following criteria for identification were used (Al-Sabti and Matcalfe 1995):-
- MN must be smaller than one third of the main nuclei,
- MN must be clearly separated from the main nuclei,
- MN must be on the same plane of focus and have same color as of the original nuclei.
For statistical analysis, the data obtained were presented as Mean±SE. Data was analyzed using One Way ANOVA and were expressed as percentage frequency for MN test. Significance at different dose levels Were studied by using Graph Pad Prism Software (Graph Pad Inc., san Diego, CA, USA).
Results AND DISCUSSION
Table 1. Body weight variation in Channa punctatus due to Paper Mill Effluent exposure.
| Dose (mg/kg)
|
Concentrations | Initial | 24 hrs | 48 hrs | 72 hrs |
| Control | —– | 17.5±0.22
|
17.52±0.22
|
17.6±0.23
|
17.48±0.21
|
| Positive control | 2mg/lit | 17.88±0.19
|
17.8±0.19
|
17.8±0.28
|
17.7±0.28
|
| Paper Mill Effluent (PME) | 10% | 17.1±0.31
|
17.08±0.30 | 17.02±0.30 | 17.0±0.31 |
| 25% | 16.90±0.16
|
17.0±0.16
|
16.75±0.23
|
16.67±0.24
|
|
| 50% | 17.7±0.11
|
17.66±0.12
|
17.42±0.16
|
17.50±0.18
|
Figure 1: Body weight Variation in Channa punctatus due to Paper Mill Effluent exposure.
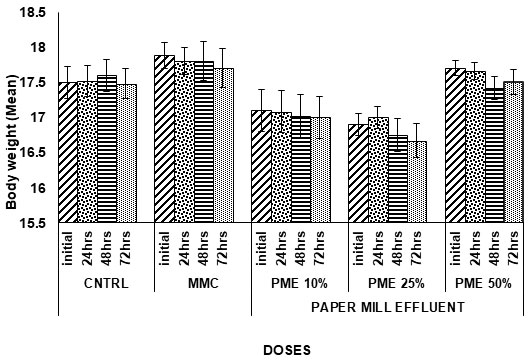
Table 2. Survivality rate of Channa punctatus exposed to different concentrations of Paper Mill Effluent.
| Treatment /DOSE | No. of Fish per aquarium | Concentrations | Survivality (in %) | ||
| 24 hrs | 48 hrs | 72 hrs
|
|||
| Control | 50 | – | 100
|
100 | 100 |
| MMC | 50
|
2mg/L | 100 | 80 | 80 |
| Paper Mill Effluent | 50 | PME 10% | 100
|
100 | 100 |
| 50 | PME 25% | 100
|
80 | 80 | |
| 50 | PME 50% | 100
|
80 | 60 | |
Figure 2: Survivality rate of Channa punctatus exposed to different concentrations of Paper Mill Effluent.
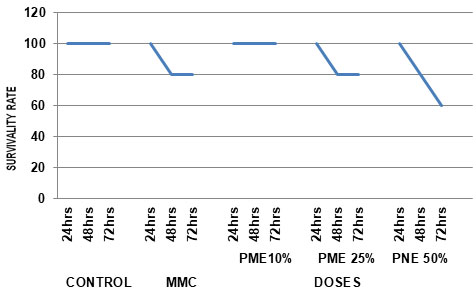
Table 3. Incidence of micronucleated PCEs in Channa punctatus induced by Paper Mill Effluent.
| Dose
(mg/kg)
|
Concentrations | Exposure Timing
(In hrs)
|
Total PCE/n | %PCEs With MN
(mean ±S.E) |
| Control | — | 24 hrs | 5001/5 | 0.01±0.004 |
| 48 hrs | 5002/5 | 0.03±0.01* | ||
| 72 hrs | 4017/4 | 0.04±0.01* | ||
| MMC | 2mg/L | 24 hrs | 5020/5 | 0.07±0.01 |
| 48 hrs | 4017/4 | 0.17±0.02* | ||
| 72 hrs | 4018/4 | 0.29±0.01 | ||
|
Paper Mill Effluent |
PME 10% | 24 hrs | 5019/5 | 0.07±0.01 |
| 48 hrs | 5022/5 | 0.19±0.01* | ||
| 72 hrs | 5025/5 | 0.23±0.03 | ||
| PME 25% | 24 hrs | 5021/5 | 0.27±0.01* | |
| 48 hrs | 4017/4 | 0.35±0.04* | ||
| 72 hrs | 4011/4 | 0.37±0.02***,**,* | ||
| PME 50% | 24 hrs | 5022/5 | 0.35±0.01***,**,* | |
| 48 hrs | 4015/4 | 0.71±0.03* | ||
| 72 hrs | 3011/3 | 0.79±0.02*** |
When compared PME with Control
P<0.05=*, P<0.01=**, P<0.001=***
Figure 3: Incidence of micronucleated PCEs in Channa punctatus induced by Paper Mill Effluent
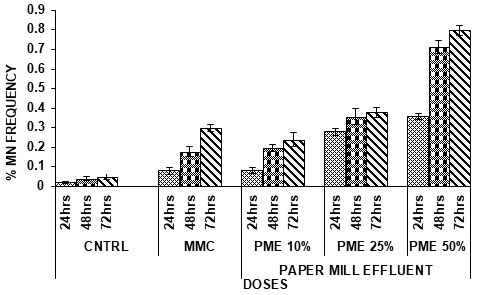
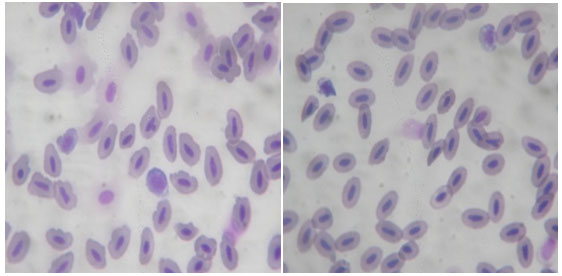
Figure 4: Pictorial depiction of Micronucleus in Channa punctatus
Industrial discharges are recognized as one of the major resources of toxic chemicals in the environment. In the present study, the mutagenic potentiality of an effluent from a paper mill industry located in the Barak valley region of Assam, India was assessed by using an in vivo assay in fish system.
Body weight and Survival rate variation in exposed groups: In current investigation, variation in body weight was measured in fishes exposed to different concentrations of PME. Minute variation in body weight of fishes were recorded in exposed groups compared to negative and positive control. Whereas Remarkable mortality rate was recorded in exposed sets where dose dependent and time dependent death was noted.
Micronuclei Formation: Micronuclei are chromatin masses in the form of small nuclei which appear within the cytoplasm and close to the main nucleus in interphase cells. They are originated spontaneously or as consequence of clastogenic and/or aneugenic effects, which generate acentric chromosomal fragments and/or lagging chromosomes during the mitotic anaphase (Fenech 2003). Due the nucleated nature of erythrocytes in fish, the practicality of MN test has gain high relevance in bio-monitoring of aquatic environments, also including assessment of water quality (Xing et al. 2012; Chen et al. 2016).
Originally the micronucleus test was developed for application in mammals, it was subsequently modified and used in fish (Schmid 1976; Houk 2007). Later, the analysis of MN was increasingly used for assessing environmental genotoxicity in fish (Al-Shabti and Metcalfe 1995; Ayllon and Garcia-Vazquez 2000; Jha 2000; Oost 2003). The micronuclei assay is one of the best biomarkers that clearly correlate with pollution load, as it has been shown in a number of studies (Belfiore et al. 2001; Kirch and Sofuni 2003; Cavas and Ergene-Gozukara 2005; Singh et al. 2020).
Several studies throughout years have demonstrated increases in micronuclei frequency in species of marine fish in polluted areas, and their use as genotoxicity markers in accordance with the present study, and also in the laboratory (Al-Sabti and Metcalfe 1995; Hayashi et al. 1998; Ateeq et al. 2002; Teles et al. 2003; Buschini et al. 2004; Çavas et al. 2005).
Many researchers have demonstrated the success of using micronucleus test in the evaluation of environmental quality, by using several freshwater fish (Hayashi et al. 1998). Group of researchers (Lopes-Poleza 2004) evaluated the genotoxic effect methylmercury (CH3Hg+) in Hoplias malabaricus, using chromosomal aberrations (anterior kidney), micronuclei, and DNA damage by the comet assay in erythrocytes (Claxton et al. 2008; Singh et al. 2020).
Just like previous, our study also confirmed the usefulness of the erythrocyte micronucleus as a powerful monitoring tool for detecting genotoxic agents in a coastal environment (Nunes et al. 2015; Singh et al. 2019). Significant increase in the frequency of micronucleated erythrocytes has been observed after exposing Channa sp. to various concentrations of industrial effluents. Industrial effluents have already been reported to have genotoxic effects on fishes which arise in the form of micronucleus (Serrano and Montero 2001; Araújo et al. 2006). Present investigation confirmed the importance of erythrocyte micronucleus assay as an effective tool for detecting genotoxic agents.
In present investigation, selected paper mill effluent has been observed to induce MN in the blood erythrocytes of fish. The frequency of micronuclei was significantly higher than in the negative control and Mitomycin C. There was a significant difference between frequency of miconuclei among the negative and positive control. PME lead to the formation of micronucleus were compared to 10% concentration, 25% and 50% concentration showed more micronucleus formation in both PME and DS (Singh et al. 2019).
Frequency of micronucleated PCEs increased from 24hrs to 48hrs and from 48hrs to 72hrs of time interval. (p<0.05, p<0.001) designating the time dependent increase of genotoxicity. With time the chances of toxicity increase leading to any kind of chromosomal abnormality which may ultimately lead to death of the aquatic organisms in long run. Same happened in our case too (Singh et al. 2020).
CONCLUSION
The findings of the present study suggests that the risk factor graph of pollution of water bodies due to direct industrial exposure is consistently affecting the aquatic ecosystem and human life. Based on previous research reports, current research was conducted using Micronucleus assay as an effective and verified model to monitor the level of induced toxicity which was found up to the mark, as it came out with remarkable results. Cachar paper mill effluent has been visualized to have genotoxic potentials via the tested toxicity parameter as Micronucleus assay.
Thus, we hope that we will try to get better details about the mutagenicity caused by applying other parameters and our piece of work will motivate other researchers to work on this issue from other aspects which will attract the attention of government for protection of such water resources from water pollution and make public aware about it , so that such exposures can be prevented and measures will be undertaken to aware people against direct use of untreated water from these polluted water bodies.
REFERENCES
Ali, D; Nagpure N.S; Kumar, S; et al. (2009). Assessment of Genotoxic and mutagenic effects of chlorpyrifos in fresh water fish Channa punctatus (Bloch) using micronuleus assay and alkaline single-cell gel electrophoresis. Food and Chemical Toxicology, 47, 650-656.
Al-Sabti, K and Metcalfe, C.D, (1995). Fish micronuclei for assessing genotoxicity in water, Mutat.Res. 343,121-135.
Araújo, C.V.M; Cohin-de-Pinho, S.J; Santos, J.S; et al. (2006). In situ and laboratory bioassays using Poecilia reticulata Peters, 1859 in the biomonitoring of an acidic lake at Camaçari, BA, Brazil. Chemosphere, 65, 599-603.
Ateeq, B; Farah, M.A; Ali, M.N et al. (2002). Induction of micronuclei and erythrocyte alterations in the catfish Clarias batrachus by 2,4-dichlorophenoxyacetic acid and butachlor. Mutat Res, 518,135-144.
Ayllon,F and Gari,F-Vazquez, (2000). Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna- as assessment of the fish micronucleus test, Mutat Res, 467, 177-186.
Barea LJ, (2004). Biomarkers to detect environmental pollution, Toxicol. Lett, 79-88.
Belfiore, N.M and Anderson, S.L, (2001). Effects of contaminants on genetic patterns in aquatic organisms a review, Mutat Res., 489, 97-122.
Bolognesi, C; Perrone, E; Roggieri, P; et al. (2006). Assessment of micronuclei induction in peripheral erythrocytes of fish exposed to xenobiotics under controlled conditions. Aquat Toxicol, 78S, S93-S98.
Buschini, A; Martino, A; Gustavino, B; et al. (2004). Comet assay and micronucleus test in circulation erythrocytes of Cyprinus carpio specimes exposed in situ to lake waters treated with desinfectants for potabilization. Mutat Res., 557, 119-129.
Cavas, T and Ergene, S.G, (2003). Evaluation of the genotoxic potential of lambd a-cyhalothrin using nuclear and nucleolar biomarkers on fish cells, Mutat Res., 534, 93-99.
Cavas, T and Ergene, S.G, (2003). Micronuclei nuclear lesions and interphase silver –stained nucleolar organizer regions (Ag NORs) as cyto-genotoxicity indicators in Oreochromis miloticus exposed to textile mill effluent, Mutat .Res.538,81-91.
Cavas, T and Ergene,S.G, (2001). Genotoxicity evaluation of metronidazole using the piscine micronucleus test by acridine orange fluorescent staining, Environ.Toxicol.Phar.19,107-111.
Cavas, T; Garanko,N.N and Arkhipchuk, V.V, (2005). Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subcronically exposed to cadmium chloride and copper sulphate, Food Chem. Toxicol., 43, 569-574.
Chaudhary, R; Pandey, S; Kushwaha, B; et al. (2006). Fish micronucleus assay: a sensitive tool for ecogenotoxicity studies. Journal of Ecophysiology and Occupational Health, 6, 143-147.
Chen, W; Gao, J; Huang, J; et al. (2016). Fate and removal of typical pharmaceutical and personal care products in a waste water treatment plant from Beijing: A mass balance study. Front Environ. Sci. Eng, 10, 491-501.
Claxton, L.D; Houk, V.S and Houghes, V.J, (2008). Genotoxicity of industrial wastes and effluents, Mutat Res., 410,237-243.
Fenech, M; Chang, W.P; Kirsch, M.V; et al. (2003). Human Micronucleus Project Human Project- detailed description of the scoring criteria for the cytokinesis bloch micronucleus assay using isolated human lymphocyte cultures, Mutat Res, 65-75.
Fenech, M; Kirsch-Volders, M; and Natarajan, A.T, (2011) Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells, Mutagenesis, vol. 26, (1), 125–132.
Ferraro, M.V.M; Fenocchio, A.S; Mantovani, M.S; et al. (2004). Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. Malabaricus as evaluated using the comet assay and piscine micronucleus and chromosome aberration tests. Genet Mol Biol, 27,103-107.
Fleeger, J.W; Carmon, K.R and Nisbet, R.M, (2003). Indirect effect of contaminants in aquatic ecosystem, Sci.Total Environ. 317(1-3). 207-233.
Fowler, B.A; Kahng, M.W; Smith, D.R, (1994). Role of Lead binding Proteins in Renal Cancer. Environ. Health Perspect.102(3),115-116.
Garciia, S.L and Montoya, M.R. (2001). Micronuclei and chromatine buds are related genotoxic events, Environ.Mol.Mutagen, 38-45.
Hatje, V; Barros, F; Figueiredo, D.G; et al. (2006). Trace metal contamination and benthic assemblages in Subaé estuarine system, Brazil. Mar Pollut Bull 52, 982-987i.
Hayashi, M; Ueda, T; Uyeno, K; et al. (1998). Development of genotoxicity assay systems that use aquatic organisms. Mutat Res., 399,125-133.
Heddle, J.A.; Hite, M; Jrkhart, B; et al. (1983). The induction of micronuclei as a measure of genotoxicity. Mutat. Res., 123: 61-118.
Houk,V.S, (2007). The genotoxicity of industrial wastes and effluents, Mutat Res., 277, 91-138.
Ismail, M; Khan, Q.M; Ali, R; et al. (2014) Evaluation of genotoxicity of chlorpyrifos in common Indus Valley Toad, Bufo stomaticus using alkaline single cell gel electrophoresis (Comet Assay), Agricultural Sciences, vol. 5, (4), 376–382.
Jha, A.N, Cheung, V.V, Foulkes, M.E, et al. (2000). Detection of genotoxins in the marine environment: adoption and evaluation of an integrated approach using the embryolarval stages of the marine mussel, Mytilus edulis. Mutation Research, 464: 213-228.
Kayhanian, M; Suverkropp, C; Ruby, A et al. (2007). Characterization and prediction of highway runoff constituent event mean concentration. J Environ Manage, 85:279-295.
Khanm, U.S; Masul, A.L.; Khurshed, T; et al. (2018). Antibiotics prescription pattern in rural area of Bangladesh, Across sectional study in Debidwar Upasila of Camilla district. Int. J. Pharm. Sci, 10, 36-40.
Kohlpoth, M; Rusche, M; Nusse, J, (2009). flow of cytometric measurement of micronuclei induced in a permanent fish cell lines as a possible screening test for the genotoxicity of the industrial waste waters, Mutagenesis, 14(4),397-402.
Krishnamurthi, K, (2008). Evaluation of DNA damage in workers occupationally exposed to pesticides using single-cell gel electrophoresis (SCGE) assay; pesticide genotoxicity revealed by comet assay; Mutat. Res., 469, 344-385.
Krishnamurthi, K; Devi,F and Chakraborti,T, (2003). Genotoxic effects of PAH containing sludge extracts in Chinese hamster ovary cell cultures, Biomed, Environ.Sci., 16,68-82.
Lemos, C.T; Iranc¸ O; Oliveira, N; et al. (2008). Fachel Biomonitoring of genotoxicity using micronuclei assay in native population of Astyanax jacuhiensis (Characiformes: Characidae) at sites under petrochemical influence. Sci. Total Environ., 406, 337–343
Li, S.W; and Lin, A.Y, (2015). Increased acute toxicity of fish caused by pharmaceuticals in hospital effluents in a pharmaceutical mixture and after solar irradiation. Chemosphere, 139;190-196.
Lima, D; Freitas, J.E.P; Araújo, M.E et al. (2005). Genetic detection of cryptic species in the frillfin goby Bathygobius soporator. J Exp Mar Biol Ecol 302:211-223.
Minissi, S; Ciccoti, E and Rizzoni, M, (1995). Micronucleus test in erythrocytes of Barbus plebejus (Teleostei, Pisces) from two natural environments: A bioassay for the in-situ detection of mutagens in freshwater. Mutat Res., 367, 245-251.
Minovski, N; Sacan, M.T; Eminolu E.M; et al. (2019). Revisiting fish toxicity of active pharmaceutical ingredients: Mechanistic insights from integrated ligand-/ structure-based assessments on acetylcholine esterase. Ecotoxicol Environ Saf., 170, 548-558.
Mortalmans, K and Zeiger, E, (2000). The Ames Salmonella/ microsome mutagenicity test, Mutat. Res. 455, 29-60.
Nunes, B; Antunes, S.C; Gomes, R; et al. (2015). Acute effects of tetracycline exposure in the freshwater fish Gambusia holbrooki: Antioxidant effects, neurotoxicity and histological alterations. Arch. Environ. Contam. Toxicol, 68, 371-381.
Nwami, C.D; Lakra, W.S; Nagpure, N.S; et al. (2010). Mutagenic and Genotoxic effect of Carbosulfan in fresh water fish Channa punctatus (Bloch) using micronucleus assay and alkaline single cell gel electrophoresis. Food and Chemical Toxicology, 48(1), 202-208.
Oost, RVD; Beyer J and Verneulen, N.P.E, (2003). Fish bioaccumulation and biomarkers in environmental risk assessment –a review, Environ.Toxicol.Phar.,13, 57-149.
Pandey, A.K; Nagpure, N.S and Trivedi, S.P, (2014). Evaluation of genotoxicity of profenofos to fresh water fish Channa punctatus (Bloch) using micronuleus assay. African Journal of Biotechnology, 13(39), 3985-3988.
Porto, J.I.R; Araujo, C.S.O and Feldberg, E, (2005). Mutagenic effects of mercury pollution as revealed by micronucleus test on three Amazonian fish species. Environmental Research, 97, 287-292.
Saleh, K.A and Alshehri, (2011). The intensity of pollutant genotoxicity in Lake Ulubat: investigation of peripheral erythrocytes of Cyprinus carpio. African Journal of Biotechnology, 10(71) 16045-16050.
Sanchez-Galan, S; Linde, A.R; Ayllon, F et al. (2001). Induction of micronuclei in eel (Anquilla anquilla L.) by heavy metals. Ecotoxicology and Environmental Safety, 49, 139-143.
Schmid, W, (1976). The micronucleus test for cytogenetic analysis. In: Hollander A(ed) Chemical Mutagens, Principles and Methods for Their Detection, Plenum Press, New York, 4, 31-53.
Sharma, M; Chadda, P, (2017). Widely used non-ionic surfactant 4-nonylphenol: Showing genotoxic effects in various tissues of Channa punctatus. Environ. Sci. Pollut. Res.Int., 24, 11331-11339.
Singh, U.S. and Tripathi, Y.C., (2019). Human Health Implications of pulp and Paper Mill Wastewater Toxicity in 18th Symposium on phytochemistry and Ayurveda: Potential and Prospects, 7th December, Dehradun, India.
Singh, U.S., Panwar, S., Jain R.K. et al. (2020). Assessment of Physicochemical Characteristics of Effluents from Paper Mill in the State OF Uttar Pradesh, India. Int.J. Eng. Res. Technol., 9: 313-318.
Singh, U.S., Panwar, S., Jain R.K. et al. (2020). Physicochemical analysis of Effluents from Agro Based Paper Mill in Uttarakhand State India. Int.J.Chem Tech. Res., 13: 174-180.
Stahl Jr, R.G., (1991). The genetic toxicology of organic compounds in natural waters and wastewaters. Ecotoxicology and Environmental Safety, 22(1), pp.94-125.
Teles, M; Pacheco, M and Santos, M.A, (2003) Anguilla anguilla L. Liver ethoxyresorufin O-deethylation, glutathione S-tranferase, erythrocytic nuclear abnormalities, and endocrine responses to naphthalene and β-naphthoflavone. Ecotoxicol Environ Saf, 55, 98-107.
Tolbert, P.E; Shy, A.C and Allen, J.W (1992). Micronuclei and other nuclear abnormalities in buccal smear-mathods development, Mutat.Res., 271,69-77.
Venier, P and Zampieron, C, (2005). Evidence of genetic damage in grass gobies and mussels from the Venice lagoon. Environ Int, 31, 1053-1064.
Volders K.M; Fenech, M, (2001). Inclusion of micronucleus in non-divided mononuclear lymphocytes and necrosis/apoptosis may provide a more comprehensive cytokinesis bloch MN assay for biomonitoring purposes, Mutagenesis, (1-2), 51-58.
Volders K.M; Sofuni M.T; Aardema M; et al. (2003). Report from the in vitro micronucleus assay working group. Mutat. Res., 540, 153-163.
Xing, H; Wang, X; Sun, G et al. (2012). Effects of atrazine and chlorpyrifos on activity and transcription of glutathione S-transferase in common carp (Cyprinus carpio L.), Environmental Toxicology and Pharmacology, vol. 33, no. (2), 233–244.
Yadav, A.S and Saini, M, (2015). Increased frequency of nuclear anomalies in exfoliated buccal mucosa of cigarette smokers, Journal of Entomology and Zoology Studies, vol. 3, (2), 7–10.
Yadav, A.S; Bhatnagar, A and Kaur, M, (2010). Assessment of genotoxic effects of butachlor in fresh water fish, Cirrhinus mrigala (Hamilton), Research Journal of Environmental Toxicology, vol. 4, (4), 223–230.
Zúñiga-González, G; Torres-Bugarin, O; Luna-Aguirre, J; et al. (2000). Spontaneous micronuclei in peripheral blood erythrocytes from 54 animal species (mammals, reptiles and birds): Part two. Mutat Res, 467, 99-103.


