1Institute of Biosciences and Technology, Shri Ramswaroop Memorial University, Deva Road, Barabanki, Uttar Pradesh, India.
2Bansal Institute of Engineering and Technology, Lucknow, Uttar Pradesh, India
Corresponding author email: ashishbiotech63@gmail.com
Article Publishing History
Received: 09/07/2020
Accepted After Revision: 14/09/2020
Meat is used as nutritious food, as it provides a sufficient amount of protein, vitamins, and minerals to be used for human consumption. In the present time, it is notable that meat and meat-related products are high in demand, consequent to the rise in the human population and their disposable income. Nowadays, consumers are concerned about the adulteration of meat or meat products and request accurate marking; therefore, to identify the meat correctly, proper checks are needed so that accurate identification can be made in a short time and the customer can be assured of the right meat. This study was carried out to differentiate Cow, Buffalo, Goat and Sheep meat by targeting 12S rRNA and cytochrome b gene. Mitochondrial Analysis of DNA was the most frequently used DNA because of its highly conserved sequences in various organism species. Two set of primers were utilized to amplify the 12S rRNA gene of cow and buffalo and another two set of primers were utilized to amplify the cyt b gene of goat and sheep. Initially species specificity of these primers was tested by running a conventional PCR using a pair of primers, the forward CYTCFP (for goat and sheep), 12SCFP (for cow and buffalo) and the reverse primers which were species specific.
The primers in the multiplex PCR amplified target sequences at the ability comparable to ordinary PCR. Amplified PCR products for four species ranged from 159 to 501bp (Cow 501bp, Buffalo 229bp, Goat159bp and Sheep 336 bp) respectively. It was observed that from amplified PCR product of cyt b gene and 12S rRNA gene by utilizing the protocol of conventional PCR and could be obtained a species-specific band from isolated genomic DNA from four meat species. Multiplex PCR was created and can be used for synchronous recognition of numerous species origin in meat by cyt b, and 12S rRNA gene-derived species-specific primers.
Multiplex PCR, Species Specific Meat Detection, Adulteration.
Singh A, Bhargava P, Rai A. K. Double Gene Targeting in Multiplex PCR for Discriminating Raw Meat of Cow, Buffalo, Goat and Sheep Species. Biosc.Biotech.Res.Comm. 2020;13(3).
Singh A, Bhargava P, Rai A. K. Double Gene Targeting in Multiplex PCR for Discriminating Raw Meat of Cow, Buffalo, Goat and Sheep Species. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/3bAPJET
Copyright © Singh et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
From the nourishing perspective, meat is a rich source of essential amino acids and provides some amount of minerals also. Organ part of meat like liver is a good source of nicotinic acid, Vitamin B1, and Vitamin A. Scientific exploration is still in progress for better understanding of the contradictions of variation in different animal breeds and species for good health. It is very apparent from the past research that meat having less connective tissue is probably going to have low scores of absorption and digestion. For decades, meat adulteration has been taken for granted as inevitable. The main reason for this is an excess of demand over supply, favoring the seller over the buyer (Li et al., 2019).
In present time food adulteration involving animal species is very common to the mixing of cow, buffalo, goat and sheep meat. Due to religious, health and economic value it is very important to identified origin of meat product (Shi et al., 2019). In current scenario, every person wants to know exact source of meat which they are getting for consumption; so, it is important to inform consumer’s choice, respect their lifestyle, religion, diet and health concerns. The price of meats from different species differs so substantially that meat vendors tend to misrepresent meats for economic gain. Buffalo are culturally and economically preferable meat having the large range of utilization in many countries. Indians prefer Sheep mutton because of their nutritious value, and avoid goat because of religious requirements. On the other hand, cow is totally unacceptable to the Hindus (Lin et al., 2019).
In earlier times, many morphological, protein, and lipid-based methods were used for the correct identification of animal meats or their associated food products, but these methods are not reliable due to the breakdown of the analyzed biomarker during food processing. Based on anatomical structures of various species of animals utilizing for meat generation, we can undoubtedly recognize the origin of meat species. If meat is in the lean, then the anatomical method is not a method for the specification of meat species (Song et al., 2019). Analytical methods are indispensable for labeling meat products because of the long time it takes, as this requires simple and rapid procedures so that precise labeling of meats or meat products can be easily done (Guo et al., 2019). Protein techniques are based on the expression of genes, and the immunoassay technique is used for detecting animal species in raw meat samples. But due to the possible similarity among antibodies in closely related meat species, these techniques are intense and followed by a lack of express to antibodies themselves, (Mansouri et al., 2020).
Food authenticity based on physical condition is either a tough task or impossible because of the damage in morphological parameters during processing and packaging (Taboada et al., 2014, Ali et al., 2015). PCR based techniques have shown great success in animal species identification due to its high level of specificity, sensitivity, accuracy and precision. PCR based detection techniques are reliable because this technique amplifies specific DNA targets from very less amount of sample. In present days an advance technique; PCR restriction fragment length polymorphism (PCR-RFLP) is very helpful in identifying meat authentication. It is identifying the meat species by cut the amplified PCR product at a specific position by using one or more restriction enzymes (Rashid et al., 2015). Using the cutting site variation that exists within a specific region of DNA, the differentiation of even closely related species is possible using a PCR-RFLP assay (Hsieh et al., 2016).
For reducing both time and cost Multiplex PCR technique with the use of species-specific primers are preferred since this technique offers multiple amplification in a single PCR (Qin et al., 2019). Multiplex PCR is a technique that includes the concurrent detection of various species. Both mitochondrial and genomic genes have been targeted for species detection by utilizing a multiplex PCR technique (Wang et al., 2019). Among the benefits of this technique are its efficiency, reliability, and sensitivity for mixed meat samples. In this regard, such advanced molecular methods like Multiplex PCR technique with the utilization of species-specific primer should be right-hand techniques for the detection of specific meat in a food product or unprocessed meat (Liu et al., 2019).
In the era of globalization and increased concern for animal food quality and safety, there is a great need to develop rapid, sensitive, specific and reproducible methods for authentication of meats (Cahyadi et al., 2019). Thus, this technique will also help to strengthen the growth and commercial potential of the nation’s well-organized meat industry by creating awareness between traders and consumers about the adulteration of meat. Hence, this study is proposed to develop simple, rapid and reliable speciation techniques for authentication of meat species like cow-buffalo and goat-sheep.
MATERIAL AND METHODS
Sample collection and preservation: Raw and fresh meat samples of goat, sheep and buffalo were collected from different butchery of Lucknow, in clean and sterilized containers. For DNA isolation cow samples were taken from biopsy method because in UP, India cow slaughter is totally banned and illegal. Preserve the meat samples at -200C until used for further analysis.
DNA Extraction: Genomic DNA was isolated from the meat samples by Phenol-chloroform method as described by Sambrook and Russel (2001) with slight modifications. The tissue samples (75 mg) were cut into very small pieces or pulverized in liquid nitrogen and 10 volumes (w/v) of DNA lyses buffer (fresh meat) (pH 8.0) containing Ribonuclease-A at 100 μg/ml (20 μg/ml) was added and incubated at 37° C for 1 h. Proteinase-K solution (20 mg/ml) was added at 200 μg/ml and again incubated at 50°C for not less than 3 h or overnight. Equal volume of Tris-saturated phenol (equilibrated with 0.1 M Tris-Cl, pH 8.0) was added and the contents of the tubes were subjected to gently mixing end to end for 10 min and centrifuged at 6,500 RPM for 15 min.
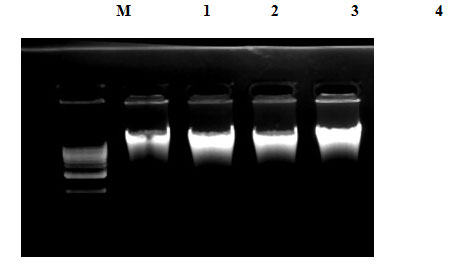
The upper aqueous phase collected was washed twice with equal volume of phenol: chloroform: isoamylalcohol (25:24:1) mixture. The upper phase was again collected in to a fresh tube containing 1/5 volume of 10 M ammonium acetate and double volume of absolute ethanol and was mixed well for precipitation of DNA. The mixture containing visible DNA threads was centrifuged at (10,000 RPM for 10 min). The DNA pellet was washed twice with 70 per cent alcohol by centrifugation (10,000 RPM for 5 min each), dried over a dry bath at 60° C and then dissolved in 1X TE (Tris-EDTA) buffer (50- 100 μl) or nuclease free water. Subsequently, the quality of DNA was checked on 1% agarose gel (Figure 4).
Primer Design: Mitochondrial sequences of cow, buffalo, goat and ship were downloaded from the NCBI database and aligned using M-Coffee alignment program. The NCBI accession numbers of cow (Bos Taurus), buffalo (Bubalus bubalis), goat (Capra hircus) and sheep (Ovis aries) were respectively AF492351.1, KX758401.1, KY662383.1 and KP229295.1.Specific primer sets (sequences are provided in Table 1) with similar annealing temperatures were designed with Primer3 on the basis of cyt b (goat and sheep) and 12S rRNA (cow and buffalo) gene sequences. The specific primers were verified insilico by SnapGene software.
Table 1. Sequences of primers used for Multiplex PCR of cyt b and 12S rRNA gene
| S. No | Name | Primer | Sequences (5’- 3’ direction) | No. of
Bases |
| 1 | 12SCFP | F | GTGACAAAAATTAAGCCATAAACG | 27 |
| 2 | CORP | R | TTTTTATGTATCATAATTACGCTTACTTTTT | 31 |
| 3 | BORP | R | GTGTGTCAGCTGTTATAGAGTCACTTTCGT | 30 |
| 4 | CYTCFP | F | GACCTCCCAGCTCCATCAAACATCTCATCTTGATGAAA | 38 |
| 5 | GRP | R | ATCTCGACAAATGTGAGTTACAGAGGAAAA | 30 |
| 6 | SRP | R | ATAGCCTATGAATGCTGTGGCTATTGT | 27 |
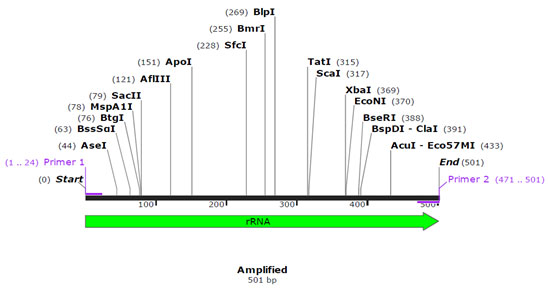
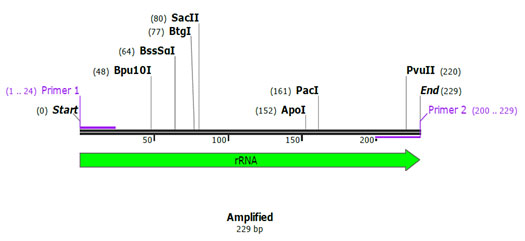
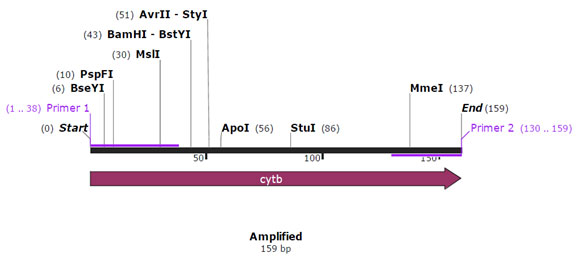
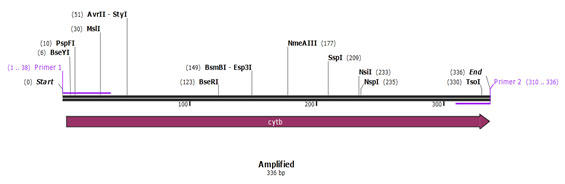
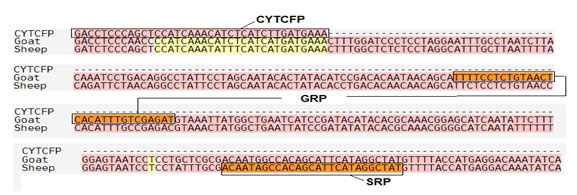
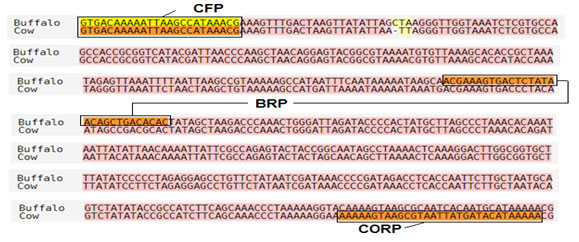
Primer validation by In silico method: SnapGene tool (http://www.snapgene.com) was used for validating species specific primers, by using species specific sequences retrieved from NCBI for cytochrome b and 12S rRNA gene. The sequences of 12S rRNA gene of cow (Accession no. – AF492351.1) and buffalo (Accession no. – KX758401.1) and for cytochrome b gene of sheep (Accession no. – KP229295.1) and goat (Accession no. – KY662383.1) were retrieved from NCBI, used for validation of designed species specific primers. In silico primer validation results are given in the figure 1, figure 2, figure 3 and figure 4 for cow, buffalo, goat and sheep respectively. Primer sequence and target region on cytochrome b gene and 12S rRNA gene checked by TCOFEE (multiple sequence alignment tool) are showing in figure 5 and figure 6 respectively.
Figure 1: Insilico Primer validation of Cow’s species-specific cytochrome b gene.
Figure 2: Insilico Primer validation of buffalo’s species-specific cytochrome b gene.
Figure 3: Insilico Primer validation of Goat’s species-specific cytochrome b gene.
Figure 4: Insilico Primer validation of Sheep’s species-specific cytochrome b gene.
Figure 5: Primer sequence and target region on cytochrome b gene. CYTCFP indicates common forward primer for cytochrome gene. GRP and SRP indicate specific reverse primer of goat and sheep respectively.
Figure 6: Primer sequence and target region on 12S rRNA gene. CFP indicates common forward primer for 12S rRNA gene. BRP and CORP indicates specific reverse primer of buffalo and cow respectively.
Primer Synthesis: Species specific designed primers were purchased from Micelles Life Sciences (P) Ltd. Initially primers were in desalted state, dilute the primers (in concentration 100pm/μl) with double distilled water as mentioned by manufacturer and stored at 20 0C for further analysis.
PCR amplification of the gene: For amplify 12S rRNA by conventional PCR, 0.5µl 12SCFP (12S r RNA common forward primer) and 0.5µl of reverse primer of cow and buffalo were used independently and for amplify cyt b gene by conventional PCR, 0.5 µM CYTCFP (Cytochrome common forward primer) and 0.5 µM of reverse primer of goat and sheep were used independently. 12SCFP was used as forward primer for cow and buffalo; and CYTCFP was used as forward primer for goat and sheep. After confirming all the species by using both gene primer individually, perform multiplex PCR by using multiplex PCR kit (Quiagen, Cat No.: 206143) by mixing all primers (conc. of each primer 0.5 µM) in single reaction with template DNA.
Firstly, mix all the primers in equal part and incorporate 4 µl in PCR reaction. Optimize the PCR reaction according to primer concentration. The PCR optimization used for 12S r RNA and cyt b gene consist of pre denaturation at 940C for the duration 5 min., denaturation (40 cycle) at 940C for the duration 30 sec., annealing (40 cycle) at 600C for 30 sec., elongation (40cycle) at 720C for 30 sec., and final elongation at 720C for 10 min.
Gel visualization of amplified PCR Product: Amplified DNA sample were separated by 1% agarose gel electrophoresis and each sample produced a characteristic band pattern at constant voltage 90V for 45 minutes. After running the gel, DNA bands with separate size were visualized under UV light and documented by gel doc.
RESULTS AND DISCUSSION
Meat is used as a nutritious food because it provides a sufficient amount of protein, vitamins, and minerals to be used for human consumption. In the present time, notably, meat and meat-related products are high in demand, consequent to the rise in the human population and their disposable income. From a nutritional point of view, meat is an excellent source of essential amino acids and also provides some amount of minerals, which are very important for the growth of the human body. Organ parts like liver is a good source of nicotinic acid, Vitamin B1 and Vitamin A. Scientific exploration is still in progress for better understanding of the contradictions of variation in different animal breeds and species for good health (Li et al., 2019).
Adulteration of meat species is a vast and serious problem around the world, which constitutes economic fraud in violation of food labeling laws that raise concerns about food security ethically and religiously (Hsieh et al., 2016). Therefore, to secure food security to the public as well as all over the world, fast and accurate methods for detecting the species of meat should be regulated by which quality control can be done (Manso uri et al., 2020). Species of cooked meat are difficult to identify because heat transmission during cooking is widespread, leading to changes within the meat tissue (Shi et al., 2019). Despite this change, short nucleic acid sequences are capable of surviving their processes even at maximum cooking temperature, allowing it to be employed to detect meat species (Cahyadi et al., 2019). Numerous researchers have shown interest in creating analytical methods and distinctive techniques for identification of specific meat species or their processed products, including a wide range of degraded and prepared materials that were predominantly based on estimating either DNA or protein (Qin et al., 2019).
Nucleic acid-based techniques popularly known as molecular techniques have been preferred over other techniques used in recent years because of the DNA composed of the organism’s complete genetic information of a person (Liu et al., 2019). The nucleic acid-based analysis has been widely used in many fields, and these techniques became very popular for the discrimination and detection of feed or food adulteration due to the stable properties of DNA (Song et al., 2019). (Taboada et al., 2014) reported that the presence and characteristics of proteins depend on tissue type, and at the same time, he also clarified that DNA is present in all cells and is almost identical and allows differentiation in closely related species by its unique variability and diversity. In the category of DNA based techniques, PCR is the most utilized, straightforward, efficient, sensitive, and specific method that can identify the species of origin presented to various processing circumstances (Manso uri et al., 2020).
Both mitochondrial, as well as nuclear DNA, have been used for meat differentiation; but mitochondrial analysis of DNA has been more frequently used method, because of its highly conserved sequences in various organism species (Hsieh et al., 2016). Mitochondria are the powerhouse of the cell, which have small filamentous and granular intracellular bodies. In comparison to other molecular markers like nuclear DNA, sequences of mtDNA (mitochondrial DNA) provide a lot of advantages. Due to the low number copy of DNA sequences, identification of nucleus DNA might be low scoring. In comparison to nucleus DNA, mitochondrial genes evolve a lot quicker, and, in this manner, mt DNA holds more sequence diversity variety, encouraging the distinguishing proof of phylogenetically related meat species (Qin et al., 2019).
For this study, genomic DNA was isolated from the meat samples by Phenol-chloroform method as described by Sambrook and Russel (2001) with slight modifications. In order to know the quality and quantity of DNA from agarose gel (Figure 7) and spectrophotometer, DNA was eluted in equal amounts, and the same amount of DNA was loaded into DNA gel. On the basis of DNA gel electrophoresis result and spectrophotometer reading, maximum DNA yield was obtained with the Sambrook and Russel standard method. For this work, two different sets of primers with similar annealing temperatures were designed using Primer3. Both sets contained a common forward primer that could amplify the genes of all species used in this work, and different special reverse primers were designed for each of the species.
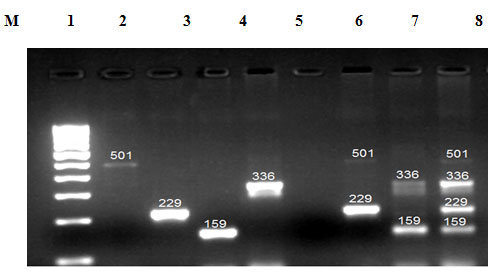
The first set was specific for cyt b gene (goat and sheep), and the second set was for 12S rRNA genes (cow and buffalo). Mitochondrial sequences used to design the primer were downloaded from the NCBI database and aligned using M-Coffee alignment program. The NCBI accession numbers of cow (Bos Taurus), buffalo (Bubalus bubalis), goat (Capra hircus), and sheep (Ovisaries) were respectively AF492351.1, KX758401.1, KY662383.1, and KP229295.1. The specific primers were verified in-silico by SnapGene software. In the present study, to identify meat species, mt DNA of cyt b gene fragments were amplified by conventional PCR from genomic DNA of meat sample using a common forward primer, a species-specific primer for goat and sheep. All autonomous PCRs were successfully amplified and displayed the expected size fragment. Amplicons of size 159 bp (goat) and 336 bp (sheep) were observed when amplified products were run on agarose gel and size of amplicons was confirmed by running parallel a 100 bp DNA marker (Figure 8).
In the present study, to identify meat species, mt DNA of 12S rRNA gene fragments were amplified by conventional PCR from the genomic DNA of meat sample using a common forward primer, species-specific primer for cow and buffalo. All autonomous PCRs were successfully amplified and displayed the expected size fragment. Amplicons of size 501 bp (cow) and 229 bp (buffalo) were observed when amplified products were run on an agarose gel, and size of amplicons was confirmed by running parallel a 100 bp DNA marker (Figure 8). Multiplex PCR is a technique that includes the concurrent detection of various species. Both mitochondrial and genomic genes have been targeted for species detection by utilizing a multiplex PCR technique (Qin et al., 2019). Among the benefits of this technique are its efficiency, reliability, and sensitivity for mixed meat samples. For identification of poultry residuals (Song et al., 2019) utilized this technique by using mitochondrial 16S rRNA and 12S rRNA as a molecular marker.
In the present study after confirming the species specificity of each primer independently by conventional PCR, a multiplex PCR was prepared by mixing all primers in a single microcentrifuge tube. In multiplex PCR, a primer cocktail containing the designed common primers and species-specific primer was used with DNA extracted from the meat samples. After successful completion of the multiplex PCR reaction, when the amplified product was run on agarose gel and size of amplicons was confirmed by running parallel a 100 bp DNA marker, the same expected size bands were seen as shown in conventional PCR for specific species, no difference was found between them.
After studying the electrophoretic pattern, it became clear that there was a complete absence of any cross-reaction in this work, and in fact, only species-specific bands were clearly visible. To differentiate the meat mixture and show the applicability of multiplex PCR on mixed meat, we adapted multiplex PCR under the same conditions in which simplex PCR was run. The size of the PCR products of the target species was expected, and no additional fragments were displayed. Studying this result made it clear that species-specific primers amplified only a particular size fragment from a target species, and amplification of this primer was not possible with any other species. The PCR product showed species-specific DNA fragments of 159, 336, 501, and 229 bp from goat, sheep, cow and buffalo meat respectively (Figure 8). All PCR amplicons resulting from these particular reverse primers had a size of between 159–501bp, thus using this technology, the species of all animals used in this task can be detected very easily by running their PCR amplicons in the same agarose gel.
Figure 7: Agarose gel Electrophoresis of genomic DNA isolated from meat samples, where Lane M, 1, 2, 3 4 and 5 represent Marker (1kb), cow, buffalo, goat, and sheep respectively.
Figure 8: Agarose gel electrophoresis of PCR products amplified with Multiplex-PCR for the fragments of 12S r RNA (Cow and Buffalo) and Cyt b (Goat and Sheep) gene. Lane 1 represents product from Cow (501bp), Lane2 represent product from Buffalo (229bp), lane 3represent product from Goat (159bp), lane 4 represent the product from Sheep (336bp) , Lane 5 represents the Negative control ,Lane 6 represents the cow buffalo duplex ,Lane 7 represents Goat and Sheep Duplex, Lane 8 represents the Multiplex PCR products of Cow, Buffalo, Goat and Sheep. M – 100bp DNA ladder.
CONCLUSION
Meat positions among one of the most nutritious and vitality rich sustenance item, used by the people to satisfy their standard body necessities. It is considered significant in keeping up a better and balanced eating routine, which is basic in achieving ideal human development and advancement. In the present time, notably, meat and meat-related products are high in demand, consequent to the rise in the human population and their disposable income. In the present study, after confirming the species specificity of each primer independently by conventional PCR, a multiplex PCR was prepared by mixing all primers in a single microcentrifuge tube. In multiplex PCR, a primer cocktail containing the designed common primers and species-specific primer was used with DNA extracted from the meat samples. After successful completion of the multiplex PCR reaction, when the amplified product was run on an agarose gel and the size of amplicons was confirmed by running parallel a 100 bp DNA marker, the same expected size bands were seen as shown in conventional PCR for specific species, no difference was found between them.
After studying the electrophoretic pattern, it became clear that there was a complete absence of any cross-reaction in this work. In fact, only species-specific bands were clearly visible. In order to differentiate the meat mixture and show the applicability of multiplex PCR on mixed meat, we optimized multiplex PCR in the same conditions as for simplex PCR. The size of the PCR products of the target species was expected, and no additional fragments were displayed. This result showed that, as species-specific primers amplified only one size fragment from a target species, amplification of this primer was not possible with any other species. The PCR product showed species-specific DNA fragments of 159, 336, 501, and 229 bp from goat, sheep, cow and buffalo meat respectively. In conclusion, it was observed that all PCR amplicons resulting from these particular reverse primers had a size of between 159-501bp, thus using this technology, the species of all animals used in this task can be detected very easily by running their PCR amplicons in the same agarose gel.
Conflict of Interests: There is no conflict of interests between the authors.
REFRENCES
Ali, M. E., Hamid, S. B. A., Hossain, M. M., Mustafa, S., Kader, M. A., and Zaidul, I. S. M. (2016). Lab-on-a-Chip-based PCR-RFLP assay for the detection of Malayan box turtle (Cuora amboinensis) in the food chain and traditional Chinese medicines. PloS one, 11(10), e0163436.
Arihara, K. (2006). Strategies for designing novel functional meat products. Meat science, 74(1), 219-229.
Arslan, A., Ilhak, O. I., and Calicioglu, M. (2006). Effect of method of cooking on identification of heat processed beef using polymerase chain reaction (PCR) technique. Meat Science, 72(2), 326-330.
Ashoor, S. H., Monte, W. C., and Stiles, P. G. (1988). Liquid chromatographic identification of meats. Journal-Association of Official Analytical Chemists, 71(2), 397-403.
Azain, M. J. (2003). Conjugated linoleic acid and its effects on animal products and health in single-stomached animals. Proceedings of the Nutrition Society, 62(2), 319-328.
Ballin, N. Z. (2010). Authentication of meat and meat products. Meat science, 86(3), 577-587.
Cahyadi, M., Taufik, I. M., Pramono, A., and Abdurrahman, Z. H. (2019). Development of mitochondrial 12S rRNA gene for identification of dog and rat in beef using multiplex PCR. Journal of the Indonesian Tropical Animal Agriculture, 44(1), 10-18.
Chen, F. C., and Hsieh, Y. H. (2000). Detection of pork in heat-processed meat products by monoclonal antibody-based ELISA. Journal of AOAC International, 83(1), 79-85.
Chikuni, K., Tabata, T., Kosugiyama, M., Monma, M., and Saito, M. (1994). Polymerase chain reaction assay for detection of sheep and goat meats. Meat Science, 37(3), 337-345.
Dalvit, C., De Marchi, M., and Cassandro, M. (2007). Genetic traceability of livestock products: A review. Meat Science, 77(4), 437-449.
Devi, S. M., Balachandar, V., Lee, S. I., and Kim, I. H. (2014). An outline of meat consumption in the Indian population-A pilot review. Korean journal for food science of animal resources, 34(4), 507.
Ghovvati, S., Nassiri, M. R., Mirhoseini, S. Z., Moussavi, A. H., and Javadmanesh, A. (2009). Fraud identification in industrial meat products by multiplex PCR assay. Food control, 20(8), 696-699.
Guo, L., Ya, M., Hai, X., Guo, Y. S., Li, C. D., Xu, W. L., and Cai, Q. (2019). A simultaneous triplex TaqMan real-time PCR approach for authentication of caprine and bovine meat, milk and cheese. International Dairy Journal, 95, 58-64.
Hajmeer, M., Cliver, D. O., and Provost, R. (2003). Spinal cord tissue detection in comminuted beef: comparison of two immunological methods. Meat Science, 65(2), 757-763.
Hsieh, H. M., Chiang, H. L., Tsai, L. C., Lai, S. Y., Huang, N. E., Linacre, A., and Lee, J. C. I. (2001). Cytochrome b gene for species identification of the conservation animals. Forensic Science International, 122(1), 7-18.
Hsieh, Y. W., and Hwang, D. F. (2004). Molecular phylogenetic relationships of puffer fish inferred from partial sequences of cytochrome b gene and restriction fragment length polymorphism analysis. Journal of agricultural and food chemistry, 52(13), 4159-4165.
Ilhak, O. I., and Arslan, A. (2007). Identification of meat species by polymerase chain reaction (PCR) technique. Turkish Journal of Veterinary and Animal Sciences, 31(3), 159-163.
Islam, M. M., Anjum, S., Modi, R. J., and Wadhwani, K. N. (2016). Scenario of livestock and poultry in india and their contribution to national economy. International Journal of Science, Environment and Technology, 5(3), 956-65.
Jain, S., Brahmbhatt, M. N., Rank, D. N., Joshi, C. G., and Solanki, J. V. (2007). Use of cytochrome b gene variability in detecting meat species by multiplex PCR assay. Indian Journal of Animal Sciences, 77(9), 880.
Kumar, D., Singh, S. P., Singh, R., and Karabasanavar, N. S. (2011). A highly specific PCR assay for identification of goat (Capra hircus) meat. Small ruminant research, 97(1-3), 76-78.
Lee, C. S., Yoo, Y. B., Chung, T. Y., Rye, J. C., and Oh, S. J. (1994). Meat identification of Korean native cattle (Hanwoo) and cattle breeds from abroad by DNA polymorphic analysis. RDA Journal of Agricultural Science (Korea Republic).
Li, T. T., Jalbani, Y. M., Zhang, G. L., Zhao, Z. Y., Wang, Z. Y., Zhao, X. Y., and Chen, A. L. (2019). Detection of goat meat adulteration by real-time PCR based on a reference primer. Food chemistry, 277, 554-557.
Lin, C. C., Tang, P. C., and Chiang, H. I. (2019). Development of RAPD-PCR assay for identifying Holstein, Angus, and Taiwan Yellow Cattle for meat adulteration detection. Food Science and Biotechnology, 28(6), 1769-1777.
Liu, W., Tao, J., Xue, M., Ji, J., Zhang, Y., Zhang, L., and Sun, W. (2019). A multiplex PCR method mediated by universal primers for the identification of eight meat ingredients in food products. European Food Research and Technology, 245(11), 2385-2392.
Lockley, A. K., and Bardsley, R. G. (2000). DNA-based methods for food authentication. Trends in Food Science and Technology, 11(2), 67-77.
Mansouri, M., Fathi, F., Jalili, R., Shoeibie, S., Dastmalchi, S., Khataee, A., and Rashidi, M. R. (2020). SPR Enhanced DNA Biosensor for Sensitive Detection of Donkey Meat Adulteration. Food Chemistry, 127163.
Matsunaga, T., Chikuni, K., Tanabe, R., Muroya, S., Shibata, K., Yamada, J., and Shinmura, Y. (1999). A quick and simple method for the identification of meat species and meat products by PCR assay. Meat science, 51(2), 143-148.
Mitani, T., Akane, A., Tokiyasu, T., Yoshimura, S., Okii, Y., and Yoshida, M. (2009). Identification of animal species using the partial sequences in the mitochondrial 16S rRNA gene. Legal medicine, 11, S449-S450.
Murugaiah, C., Noor, Z. M., Mastakim, M., Bilung, L. M., Selamat, J., and Radu, S. (2009). Meat species identification and Halal authentication analysis using mitochondrial DNA. Meat science, 83(1), 57-61.
Qin, P., Qu, W., Xu, J., Qiao, D., Yao, L., Xue, F., and Chen, W. (2019). A sensitive multiplex PCR protocol for simultaneous detection of chicken, duck, and pork in beef samples. Journal of food science and technology, 56(3), 1266-1274.
Rashid, N. R. A., Ali, M. E., Hamid, S. B. A., Rahman, M. M., Razzak, M. A., Asing, and Amin, M. A. (2015). A suitable method for the detection of a potential fraud of bringing macaque monkey meat into the food chain. Food Additives and Contaminants: Part A, 32(7), 1013-1022.
Renon, P., Bernardi, C., Scocca, S., Cantoni, C., and Gridavilla, G. (2003). IEF (isoelectric focusing) and gas chromatography to identify wild ruminant species [game meat]. Industrie Alimentari (Italy).
Sentandreu, M. Á., and Sentandreu, E. (2014). Authenticity of meat products: Tools against fraud. Food Research International, 60, 19-29.
Shi, Z., Yin, B., Li, Y., Zhou, G., Li, C., Xu, X., and Liu, L. (2019). N-glycan profile as a tool in qualitative and quantitative analysis of meat adulteration. Journal of agricultural and food chemistry, 67(37), 10543-10551.
Song, Q., Chen, Y., Zhao, L., Ouyang, H., and Song, J. (2019). Monitoring of sausage products sold in Sichuan Province, China: A first comprehensive report on meat species’ authenticity determination. Scientific Reports, 9(1), 1-9.
Taboada, L., Sánchez, A., Velasco, A., Santaclara, F. J., Pérez-Martín, R. I., and Sotelo, C. G. (2014). Identification of Atlantic cod (Gadus morhua), ling (Molva molva), and Alaska pollock (Gadus chalcogrammus) by PCR–ELISA using duplex PCR. Journal of agricultural and food chemistry, 62(24), 5699-5706.
Unseld, M., Beyermann, B., Brandt, P., and Hiesel, R. (1995). Identification of the species origin of highly processed meat products by mitochondrial DNA sequences. Genome Research, 4(4), 241-243.
Wang, L., Hang, X., and Geng, R. (2019). Molecular detection of adulteration in commercial buffalo meat products by multiplex PCR assay. Food Science and Technology, 39(2), 344-348.
Williams, P. G., Droulez, V., Levy, G., and Stobaus, T. (2006). Composition of Australian red meat 2002. 1. Gross composition.
Zhang, C. L., Fowler, M. R., Scott, N. W., Lawson, G., and Slater, A. (2007). A TaqMan real-time PCR system for the identification and quantification of bovine DNA in meats, milks and cheeses. Food control, 18(9), 1149-1158.