Department of Biotechnology, Muthayammal Engineering
College, Rasipuram, Namakkal, Tamil.
Corresponding Author email: gandepallipratap@gmail.com
Article Publishing History
Received: 15/10/2022
Accepted After Revision: 25/11/2022
Over the past few decades, extensive research in the field of carcinogenesis has been the toughest challenge in finding newer drugs. One of the leading causes of death in women worldwide is breast cancer. Cucurbitacin is one such compound identified to suppress the oncogenic signalling pathways for survival. JAK/STAT pathways were identified for tumour growth as one of the key targets for cucurbitacin. Mainly, the compound cucurbitacin Q against estrogen receptors could be a target of concern among researchers around the globe. The structured review of cucurbitacin was documented by retrieving the data from various literature reports, review articles and research papers published on the PMC platform.
In context with the fascinating role of cucurbitacin Q against estrogen receptors, it inhibits the tumour progression by blocking the STAT3 pathway. Cucurbitacin Q induces apoptosis in the tumour that activates the STAT3 gene when compared to other genes, which were found to be susceptible to breast cancer cell lines. Therefore, Cuc Q finds itself a new way of intervening with the JAK/STAT3 pathway by suppressing the progression of the tumour. Increased production of Cuc Q if proved to be active against oncogenes by blocking the STAT3 pathway. This article discusses the background, chemical structure and biological mechanism of cucurbitacin Q compound against estrogen receptors for breast cancer treatment.
Carcinogenesis, Cucurbitacin, Estrogen Receptor, Mechanism and Oncogene
Kumar G. P, Saravanan N. Cucurbitacin Compounds Against Estrogen Receptor: Literature Review. Biosc.Biotech.Res.Comm. 2022;15(4).
Kumar G. P, Saravanan N. Cucurbitacin Compounds Against Estrogen Receptor: Literature Review. Biosc.Biotech.Res.Comm. 2022;15(4). Available from: <a href=”https://bit.ly/3NBAhLD“>https://bit.ly/3NBAhLD</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Medicinal plants are found all over the globe for the benefit of mankind. The plant consists of various secondary metabolites found with different compositions in various parts of the plant. Mainly potential secondary metabolites from the traditional medicinal plant are responsible for different disorders, ailments and other treatments. Natural products are familiar for exerting anti-tumour activities partly based on their ability to lessen ROS (Reactive Oxygen Species) and to defend critical cellular components like DNA, proteins and lipids from oxidative damage (Rafter 2002). The pharmaceutical market needs to be updated with newer drugs for developing effective treatments against deadly diseases (Gupta and Kohli 2019). Developing treatment plans for cancer is an unending struggle, but relapses and treatment-related complications continue as the main impediment (Miladiyah et al. 2020).
To eliminate the possible failures in the drug development stage several approaches such as in silico, in vitro, in vivo, and cell lines are being practised (Umar et al. 2020). Estrogen receptor alpha (ERα) and progesterone receptor (PR) are found in cancer cells of the breast. If the breast cancer cells have estrogen receptors, the cancer is called ER-positive breast cancer (Altwegg and Vadlamudi 2021). ER, signalling is a key driver of ER + breast carcinogenesis and inhibition of ER signalling is the mainstay of ER + BC therapy and has enhanced the patient’s survival rate (Scabia et al. 2022).
This review focuses on E2 binding to membrane-bound ERα and ERβ receptors that swiftly stimulate nuclear transcription factors via the MAPK pathway and other pathways involved. The rationale of the study includes molecular targets, especially like JAK2/STAT3 pathway for tumorigenesis where such cucurbitacin compounds could prove to inhibit these pathways (Scabia et al. 2022).
Cancer: Cancer is a disease resulting in abnormal growth of cells and uncontrolled multiplication of cells within the body (Pushpalatha et al. 2017). According to World Health Organization (WHO), cancer is one of the second main reasons of mortality around the globe with about 9.6 million death in 2018 (Bray et al. 2018). Mostly the cancers are treated through chemotherapy. Drugs from herbal sources are low in toxicity, low cost and bioavailable. The most common cancers are lung, liver, colorectal, stomach and breast cancer (WHO 2017; Scabia et al. 2022).
The International Agency for Research on Cancer (IARC), a unit of WHO reported that 28 types of cancer are found in 184 countries and this is alarmingly increasing (Kumar et al. 2020). Breast cancer (BC) is the most common sort of tumour mostly in females, yet metastases are the key reason for deaths (Cava and Castiglioni 2020). Breast cancer chemotherapy is marked by pointing to the role of receptors such as ERα (Estrogen Receptor alpha), PR (Progesterone Receptor), EGFR (Epidermal Growth Factor Receptor) (Acharya et al. 2019). Sahayarayan et al. (2021) reported that over 60% of breast cancer cases are diagnosed as estrogen receptor alpha positive (ERα) cancers mainly in Asian countries. In humans, both alpha and beta estrogen receptors are revealed and many studies are focused on these ER receptors (McDonnell et al. 2015).
Regarding the issue, many researchers have focused on finding highly sensitive and specific markers for the initial detection of breast cancer (Yan et al. 2015). Moreover, the anticancer effects of cucurbitacin compound on different tumour types like neuroblastoma, breast cancer, lung cancer, endometrial cancer and hepatocellular carcinoma have been well studied and documented (Si et al. 2019). We provide a framework of the main estrogen receptor and various oncogenic pathways which regulates the processes of human tumorigenesis (Scabia et al. 2022).
Estrogen Receptor (ER): In humans, alpha and beta estrogen receptors were described, and many studies focused on these receptors (McDonnell et al. 2015). The increased production of estrogen is one of the main foremost causes of breast cancer (Sahayarayan et al. 2021). In women, ER (Figure 1) plays a vital part in apoptosis, inflammation, homeostasis, differentiation, maturation, metabolism and proliferation in breast cancer (Bai and Gust 2009). Several studies have reported that estrogen, in specific 17 β-estradiol, has been reported to up-regulate the expression. Also, the purpose of c-Myc and cyclin D1 genes is to lead the promotion of the cell cycle from G1 phase to S phase in the epithelial cells of mammary glands (Acharya et al. 2019; Scabia et al. 2022).
Figure 1: Three-dimensional (3D) structure of Estrogen receptor protein (PDB ID: 3ERT)
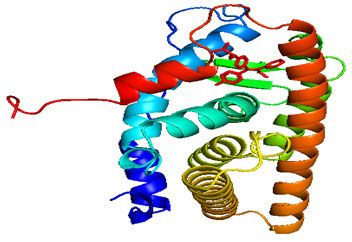
The hyperactivity of ER-α in the mammalian cells leads to the conservation and growth of types of breast cancers and also holds many molecular targets for the study of cancer drugs (Sahayarayan et al. 2021). Peng et al. (2009) reported that nearly 60% of pre-menopausal women and about 75% of post-menopausal women have suffered from estrogen-dependent breast cancer, and ER-α activity was efficiently inhibited using cancer therapy. Even though a lot of anticancer drugs and potential inhibitors against various targets are available, the effective surge in resistance along with side effects indicates that there is an urgent need for novel tumour therapy (Sahayarayan et al. 2021).
Women with breast cancer who took tamoxifen treatment are at higher risk. They also have an increased occurrence of endometrial cancer but a reduced amount of certain bone fractures and a dramatic 45% diminution in the incidence of breast cancer (Shaiau et al. 1998). Still, a lot more research is to be done, and is tremendous progress toward finding a cure for ER-α of breast cancer. Bernards (2012) reported that the vital issue is the lesser dependability on cell lines that predicts the efficacy of drugs since cell lines didn’t confirm to be a perfect model. Therefore, computational methods are in demand to expand the potential role of a drug in the context of the pathway (Cava et al. 2018; Scabia et al. 2022).
Molecular Mechanism of Cucurbitacin and Cucurbitacin Q: There are many oncogenic signaling pathways that are frequently included in cancer cell proliferation and survival. Recent studies have uncovered that several molecular targets of cucurbitacin such as the JAK2/STAT3 pathway, cofilin, cyclins, cdc2, COX-2, TYR and EcR among which actin cytoskeleton appears to be a prime target (Blaskovich et al. 2003; Chen et al. 2012). The JAK/STAT (Signal Transducers and Activators of Transcription) pathway (Figure 2), Akt-PKB pathway and MAPK Pathway are significant pathways in cancer cells and are also targets of the Cucurbitaceae family (Lee et al. 2010). In many cancer cells, activation of STAT3 and STAT5 has been known to play key roles in tumorigenesis (Yu and Jove 2004). During the initial findings, reports revealed that Cuc I is a dual inhibitor of STAT3 and JAK2 pathways but didn’t affect any other oncogenic signaling pathways such as Akt-PKB or MAPK (Blaskovich et al. 2003; Scabia et al. 2022).
In cancer cells, cucurbitacin compounds labour as STAT3 inhibitors and make cells prone to the attack of reactive oxygen species (ROS) and free radicals during inflammation (Jayaprakasam et al. 2003). Inhibition of the IKK/NF-kB pathway by cucurbitacin relies on the inhibition of key inflammatory enzymes, like cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) whose overproduction leads to tumorigenesis (Jayaprakasam et al. 2003; Park et al. 2004; Escandell et al. 2007). Although other mechanisms like activation of the MAPK pathway leading to cancer cell proliferation and survival, another study revealed that only STAT3, but not the MAPK pathway which was impaired in breast cancer cells when treated with cucurbitacin E compound (Lan et al. 2013; Alsayari et al. 2018).
Figure 2: The mechanistic inhibitory activity of cucurbitacin in pathway (Alsayari et al. 2018)
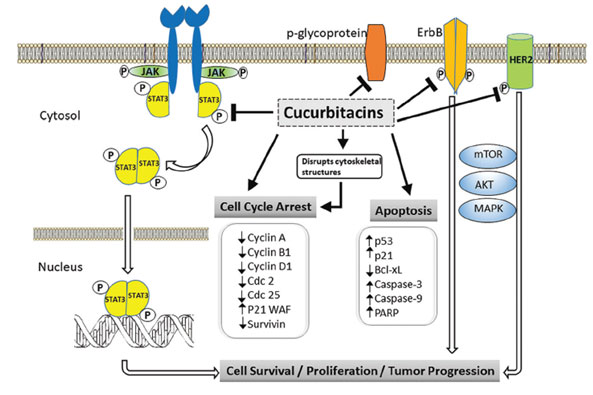
However, cucurbitacin F, O, P, and Q and their derivatives were identified to have the finest anticancer activity (Chen et al. 2005). Alghasham (2013) reported that cucurbitacin Q induces apoptosis more effectively in human and murine tumours. It selectively blocks the activation of STAT3 and induces apoptosis without inhibiting JAK2, Src, Akt, Erk or JNK. A study showed that in nude mouse tumour xenograft model, Cuc Q, but not Cuc A suppresses tumour growth signifying that JAK2 blockage alone is not enough but suggests the competence of Cuc Q to impede tumour growth that is linked to antiSTAT3 activity (Sun et al. 2005). Among the two, Cuc A was shown to be an inhibitor of the JAK2 pathway, whereas Cuc Q induces apoptosis and inhibits tumour growth that contains activated STAT3 (Sun et al. 2005; Scabia et al. 2022).
Bernard et al. (2010) reported that conversion of the C3 carbonyl of the cucurbitacin to a hydroxyl result in loss of anti-JAK2 activity, whereas the addition of a hydroxyl group to C11 of cucurbitacin results in loss of anti-STAT3 activity. Also, Cuc Q persuades cell death more potently in human and murine tumours which constitutively activates STAT3 (A549, MDA-MB-435 and v-Src/NIH3T3) when compared to other (H-Ras/NIH 3T3, MDA-MB-453 and NIH 3T3 cells) (Bernard et al. 2010). Therefore, suppression of oncogene STAT3 seems to be related to blocking the tumour which doesn’t eliminate alternate mechanisms (Zhang et al. 2004; Chan et al. 2010b; Yasuda et al. 2010; Scabia et al. 2022).
Cucurbitacin Q: Cucurbitacin (Figure 3) are one synthesized chemically by tetracyclic cucurbitane (triterpene hydrocarbon) nucleus skeleton 19-(10à9β)-abeo-5α-lanostane base, altered by the positional replacement of oxygen atom (Sharpless et al. 2002; Kaushik et al. 2015).
Figure 3: The chemical structure skeleton of main cucurbitacin (Cai et al. 2015)
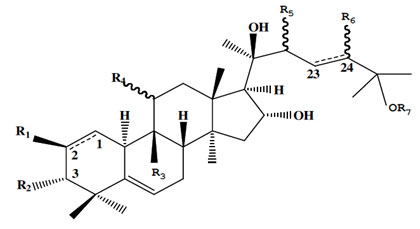
Overall, there are notably 40 identified species of cucurbitacin and their derivatives, that are classified into 12 groups namely A, B, C, D, E, I, H, Q, R and dihydrocucurbitacin B (Alghasham 2013). Other variations of cucurbitacin are now under exploration for their potential as anticancer drugs (Cai et al. 2015; Garg et al. 2017).
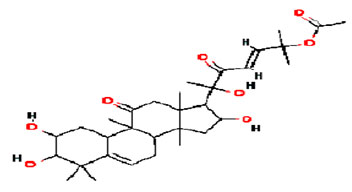
Different studies have revealed by examining the effects of these compounds in several cell lines including in vitro and in vivo against diverse malignant subtypes. Figure 4 shows the compound cucurbitacin Q (formula: C32H48O8, molecular weight: 560.7 g/mol) identified in the plants of Cucurbitaceae and other families with peculiar biological properties (Garg et al. 2017).
Figure 4: Structure of cucurbitacin Q compound
Jayaprakasam and co-researchers revealed the cytotoxic properties of cucurbitacin B, D, E and I identified from the fruits of Cucurbita andreana against colon, breast, lung and CNS cancer cell lines (Jayaprakasam et al. 2003). Interests in cucurbitacin have developed in recent years and countless studies have demonstrated that analogues of Cus have a wide variety of therapeutic activity that includes, hepatoprotective, anti-cancer and anti-inflammatory activities (Rios et al. 2012). Yet, thorough molecular mechanisms underlying their biological activity remain indescribable (Zhong et al. 2019).
A recent study confirmed that Cissampelos pareira contains a substantial amount of Cuc Q compound with antiproliferative activity (Amresh et al. 2007; Thavamain et al. 2014). Ali et al. (2019) reported that it doesn’t signify that the anticancer potential of the C. pareira plant is only due to the existence of a large amount of cucurbitacin Q compound as it requires further research (Ali et al. 2019).
Cucurbitacin Q – Sources: In general, other families of Scrophulariaceae, Begoniaceae, Primulaceae, Liliaceae, Tropaeolaceae and Rosaceae contain cucurbitacin apart from the Cucurbitaceae family (Ali et al. 2019). The seeds of certain cruciferous plants such as Iberis species and Lepidium sativum to comprise the cucurbitacin compound (Teuscher and Lindequist 1994; Ali et al. 2019). Cucurbitacin Q has been isolated from plants of different families and genera around the globe for research findings (Table 1). The bioactivity of cucurbitacin Q showed its activity on cancer cells in lung A549 human and murine cancer A549, MDA-MB-435 and v-SRV/NIH 3T3 isolated from Cayaponia tayuya (Hernandez et al. 2015). Sun et al. (2005) reported that cucurbitacin Q is susceptible to breast cancer cell lines: MDA-MB-435, MDA-MB-453 (Sun et al. 2005; Ali et al. 2019).
Table 1. Potency of cucurbitacin Q against cancer
| S. No | Plant sources | Presenting condition | Activity explored | References |
| 1. | Helicteres isora | anticancer | Anti-tumour/STAT3 pathway | Cai et al. 2015 |
| 2. | Anagallis arvensis | |||
| 3. | Gurania Subumbellata | |||
| 4. | Picrorhiza Kurrooa | |||
| 5. | Cissampelos pareira | anticancer | Anti-tumour | Bala et al. 2015, Bala et al. 2019 |
| 6. | Citrulllus colocynthis | anticancer | Anti-tumour | Al-Snafi 2016, Hussain et al. 2014 |
| 7. | Wilbrandia species | anticancer | Anti-tumour | Matos et al. 1991 |
| 8. | Ecballium elaterium | anticancer | Anti-tumour | Chen et al. 2005 |
Cucurbitacin Q – Toxicity: Cucurbitacin belongs to the terpenoids class of various compounds found in the plants of the Cucurbitaceae family which has both medicinal and toxic properties (Ali et al. 2019). Gry et al. (2006) reported that the main reason for cucurbitacin being cytotoxic in vitro might be due to the compounds influencing cell adhesion to culture vessels. In addition, giving rise to the cytotoxicity of the compound and receptor interaction, isolated cucurbitacin or extracts comprising cucurbitacin have proved to contain biological effects in vitro (Gry et al. 2006; Ali et al. 2019).
Especially, very minute concentrations of Cuc E and Cuc B, less than 1 µM was shown to inhibit the adhesion of transformed B cells (Musza et al. 1994; Ali et al. 2019). As Raikhlin-Eisenkraft and Bentur (2000) point that various factors can affect the toxicity of the cucurbitacin compounds. Most of the cucurbitacin-containing plants have been documented where the plants or extracts were screened for cytotoxicity in a battery of human tumour cell lines (Gry et al. 2006; Ali et al. 2019).
Cucurbitacin Q – Biological Supply Chain and Future Scope: Due to the extreme bitterness of cucurbitacin, plants comprising these compounds would usually not be consumed. The biological activity of cucurbitacin including its pharmacological effect has been analysed from traditional medicinal plants as an active principle (Gry et al. 2006; Ali et al. 2019). The existence and production of cucurbitacin Q have to be extensively studied in future and also about the efficiency of the drug. Over the last few decades, the Cuc Q compound has been shown to block the STAT3 pathway which inhibits tumour progression. Another prominent modification was that cucurbitacin researchers began to examine the biological mechanism of action of cucurbitacin at the molecular level (Lee et al. 2010; Ali et al. 2019).
In silico Analysis of Compounds on Breast Cancer Cell Receptors: A study on molecular docking of compounds that reported its presence in fungal endophytes of Chaetomium sp as one of the cytotoxic agents against breast cancer protein (HER α – 1G50). The results revealed that 2 compounds bearing xanthone and benzonaphtyridinedione scaffolds as hit ligands (Hariono and Rollando 2016).
Tamoxifen is an antagonist of ER-α and commercially available as a medicine to inhibit the growth of breast cancer (Jordan 1992). It binds with ARG 394 and blocks the role of ER (Desai et al. 2012). Recently, screening of bioactive compounds from Phyllanthus emblica namely quercetin, kaempferol, kaempferol 3-bita-D-glucopyranoside, isocorilagin and 1,1-diphenyl-2-picrylhydrazyl which showed good binding affinity of -7.57 kJ/mol, -7.66 kJ/mol, -6.77 kJ/mol, -7.90 kJ/mol and -5.06 kJ/mol respectively against ER (3ERT) protein (Afrin et al. 2018).
A recent study showed that among the phytochemicals like anthocyanins, isoflavone and carnosol with ER, carnosol compound revealed to inhibit with higher binding affinity of -12.1 kJ/mol (Pandian et al. 2014). Among the compounds, SANDB_11243993 has the highest binding affinity of -14.253 kcal/mol against 3ERT protein (Sahayarayan et al. 2021). Paclitaxel, a compound showed good binding interactions with the target proteins in the order ER > PARP 1 > AKT 2 > CDK 6 > HER 2 (Kumar et al. 2020).
In our current study, molecular docking of protein with ligands were studied to analyze the interactions and among the cucurbitacin compounds, cucurbitacin Q has shown to inhibit the ER-α protein (3ERT) which showed maximum docking score of -9.3 kcal/mol. Among the compounds, cucurbitacin paved a way much into the development of drug in near future. Predicting the drug candidates for pharmacokinetic and dynamic profile early in the drug development preparation which is the key aspect of ADME was tested using SWISS software (Daina et al. 2017). Molecular dynamic simulation studies of the protein-ligand complex are under process using the NAMD software (Phillips et al. 2005) to predict the stability of the molecule or compound (Ali et al. 2019).
Cucurbitacin Q – In Vitro Production Studies: To increase the biomass and yield of cucurbitacin Q, in vitro production studies have to be implemented from traditional medicinal plants using plant tissue culture technology as an alternative source. Enhanced accumulation of total cucurbitacin content was shown to be higher than the parent plant. This in vitro secondary metabolite production from medicinal plants was considered a suitable alternative method compared to whole plant extraction (Devendra et al. 2012). Till now up to date, there is no report on increased production of cucurbitacin Q compound from medicinal plants. Further, investigation of the effect of plant growth regulators and elicitors plays a vital role in the biosynthesis of cucurbitacin Q and their intermediates (Ali et al. 2019).
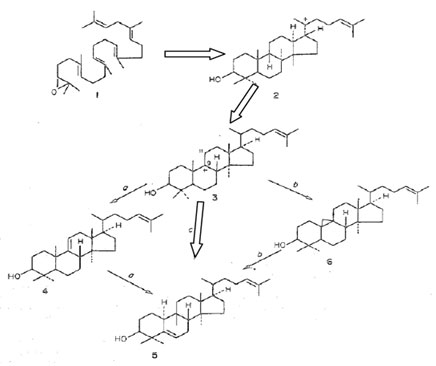
Biosynthetic Pathway of Cucurbitacin: Balliano et al. (1983) have explored the biosynthesis of cucurbitacin glycosides from squalene-2,3-epoxide to the final cucurbitacin, aiming at the possible routes for biosynthesis (Figure 5). The occurrence of 10α-cucurbita-5,24-dien-3β-ol in many seeds of food plants has been revealed. This compound is now considered a primary intermediate in the biogenesis of cucurbitacin (Akihisa et al. 1986; Ali et al. 2019). The most probable change of lanostane C-9 carbonium ion (3) to cucurbita-5,24-dienol (5) was examined as the precursor. The fact that two pentacyclic compounds, glutinol and simiarenol are frequently found together with cucurbita-5,24-dienol is taken as support for the biosynthetic route of triterpenoid compounds (Balliano et al. 1983; Ali et al. 2019).
Figure 5: Biosynthesis of cucurbitacin compounds (Balliano et al. 1983)
CONCLUSION
The findings of the present reiew has shown that over several decades, this neglected compound is gaining attention as a potential anticancer drug. Cucurbitacin Q is an under-explored compound which can be verified and worth due to its cytotoxic potential activity against cancer and other activities. Through this study, the suppression of STAT3 and JAK2 in the JAK/STAT pathway can be deactivated by inhibiting its process using Cuc Q and Cuc A and is an excellent candidate for clinical investigation. Moreover, studies related to the genetic mice tumour model should be considered to assess the active anticancer activities of cucurbitacin Q in the STAT3 pathway which inhibits tumour progression. Subsequently, it caused growth arrest, apoptosis, cellular differentiation and blockage of proliferation in cancer cells. Finally, clinical trials for Cuc Q as the targeted compound for the anticancer agent as an independent effector.
Conflicts of Interests: Authors declare no conflict of interests to disclose.
Data Availability Statement: The authors declare that the information provided in this paper is available and can be shared when required based on the request made to the corresponding author.
ACKNOWLEDGEMENTS
Required support to complete this study was provided by students and Dr. Saravanan N / Head, Department of Biotechnology, Muthayammal Engineering College, Rasipuram.
Funding: This research work did not have any particular funding.
REFERENCES
Acharya R, Chacko S, Bose P et al. (2019). Structure based multitargeted molecular docking analysis of selected Furanocoumarins against breast cancer, Scientific Reports, 9(1), 15743-15761.
Afreen S, Uddin N, Mehjabin KZ et al. (2018). In silico docking approach of some selected isolated phytochemicals from Phyllanthus emblica against breast cancer, Biomedical Journal of Scientific and Technical Research, 10(2), 7661-7664.
Akihisa T, Ghosh P, Thakur S et al. (1986). Widespread occurrence of cucurbita-5,24-dienol in Cucurbitaceaes, Journal of Japan Oil Chemists Society, 35(12), 1036-1040.
Alghasham AA (2013). Cucurbitacin – A Promising Target for Cancer Therapy, International Journal of Health Sciences, 7(1), 67-79.
Ali MS, Mukherjee S, Makar S et al. (2019). Cucurbitacin a vibrant triterpenoid: A review on it’s anticancer property, Pharma Tutor, 7(2), 43-54.
Alsayari A, Halaweish FT and Gurusamy N (2008). The role of Cucurbitacin in combating cancers: A mechanistic review, Pharmacognosy Reviews, 12(24), 157-165.
Al-Snafi AE (2016). Chemical constituents and pharmacological effects of Citrullus colocynthis –A review, IOSR Journal of Pharmacy, 6(3), 57-67.
Altwegg KA and Vadlamudi RK (2021). Role of estrogen receptor coregulators in endocrine resistant breast cancer, Exploration of Targeted Anti-tumor Therapy, 2(10), 385-400.
Amresh G, Kant R, Rao V et al. (2007). Chemomodulatory influence of Cissampelos pareira (L.) Hirsuta on gastric cancer and antioxidant system in experimental animal, Acta Pharmaceutica Sciencia, 49(1), 71-83.
Bai Z and Gust R (2009). Breast cancer, Estrogen receptor and Ligands, Archiv der Pharmazie – Chemistry in Life Sciences, 342(3), 133-149.
Bala M, Kumar S, Pratap K et al. (2019). Bioactive isoquinoline alkaloids from Cissampelos pareira, Nat Prod Res, 33(5), 622-627.
Bala M, Pratap K, Verma PK et al. (2015). Cytotoxic agents for KB and SiHa cells from n-hexane fraction of Cissampelos pareira and it’s chemical composition, Natural Products Research, 29(7), 686-691.
Balliano G, Caputo O, Viola F et al. (1983). The transformation of 10a-cucurbita-5,24-dien-3ß-ol into cucurbitacin C by seedlings of Cucumis sativus, Phytochemistry, 2(4), 909-913.
Bernard SA and Olayinka OA (2010). Search for a novel antioxidant, anti-inflammatory/analgesic or anti-proliferative drug: Cucurbitacin hold the ace, Journal of Medicinal Plants Research, 425(25), 2821-2826.
Bernards R (2012). A missing link in genotype-directed cancer therapy, Cell, 151(3), 465-468.
Blaskovich MA, Sun J, Cantor A et al. (2003). Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice, Cancer Research, 63(6), 1270-1279.
Bray F, Ferlay J, Soerjomataram I et al. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA, A Cancer Journal of Clinicians, 68(6), 394-424.
Cai Y, Fang X, He C et al. (2015). Cucurbitacin: A systematic review of the phytochemistry and anticancer activity, The American Journal of Chinese Medicine, 43(7), 1-20.
Cava C and Castiglioni I (2020). Integration of molecular docking and in vitro studies: A powerful approach for drug discovery in breast cancer, Applied Sciences, 10(10), 6981-6999.
Cava C, Bertoli G and Castiglioni I (2018). In silico identification of drug target pathways in breast cancer subtypes using pathway cross-talk inhibition, Journal of Translational Medicine, 16(154), 154-171.
Chan KT, Meng FY, Li Q et al. (2010). Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration, Cancer Letters, 294(1), 118-124.
Chen JC, Chiu MH, Nie RL et al. (2005). Cucurbitacin and cucurbitane glycosides: structures and biological activities, Natural Product Reports, 22(3), 386-399.
Chen X, Bao J, Guo J et al. (2012). Biological activities and potential molecular targets of cucurbitacin: A focus on cancer, Anti-Cancer Drugs, 23(8), 777-787.
Daina A, Michielin O and Zoete V (2017). Swiss ADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Scientific Reports, 7(3), 42717.
Desai N, Mahto MK, Alekhya B et al. (2012). Comparative docking studies of estrogen receptor inhibitors and their binding interaction analysis, International Journal of Pharmaceutical Sciences Review and Research, 16(1), 91-95.
Devendra NK, Attard EG, Raghunandan D et al. (2012). In vitro production of cucurbitacin from Trichosanthes cucumerina L. var cucumerina, Advances in Life Sciences, 2(4), 108-111.
Escandell JM, Recio MC, Manez S et al. (2007). Cucurbitacin R reduces the inflammation and bone damage associated with adjuvant arthritis in lewis rats by suppression of tumor necrosis factor-alpha in T lymphocytes and macrophages, Journal of Pharmacology and Experimental Therapeutics, 320(2), 581-590.
Garg S, Kaul SC and Wadhwa R (2018). Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms (Review), International Journal of Oncology, 52(1), 19-37.
Gry J, Soborg I and Andersson HC (2006). Cucurbitacin in plant food, TemaNord, 556(12), 1-68.
Gupta D and Kohli P (2019). In silico target identification and molecular docking studies of natural cytotoxic compound Borivilianoside H, Current Biotechnology, 8(10), 127-137.
Hariono M and Rollando M (2016). Molecular docking of compounds from chaetomium sp. against human estrogen receptor alpha in searching anti breast cancer, Journal of Pharmaceutical Sciences and Community, 13(1), 35-43.
Hernandez MS, Iniguez JC, Galarza LCA et al. (2015). Lead compounds from cucurbitaceae for the treatment of cancer Phytochemicals – Isolation, Characterisation and Role in Human Health, 1(10), 289-304.
Hussain AI, Rathore H, Abdur-Sattar MZ et al. (2014). Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential, Journal of Ethnopharmacology, 155(1), 54-66.
Jayaprakasam B, Seeram NP and Nair MG (2003). Anticancer and anti-inflammatory activities of cucurbitacin from Cucurbita andreana, Cancer Letters, 189(1), 11-16.
Jordan VC (1992). The role of tamoxifen in the treatment and prevention of breast cancer, Current Problems in Cancer, 16(3), 134-176.
Kaushik U, Aeri V and Mir SR (2015). Cucurbitacin – An insight into medicinal leads from nature, Pharmacognosy Reviews, 9(17) 12-18.
Kumar S V, Kumar TV and Parthasarathy V (2020). Assessing the specificity of Paclitaxel towards the marker proteins of breast cancer using in silico molecular docking study, Journal of Pharmaceutical Research International, 32(10), 64-73.
Lan T, Wang L, Xu Q et al. (2013). Growth inhibitory effect of cucurbitacin E on breast cancer cells, International Journal of Clinical & Experimental Pathology, 6(9), 1799-1805.
Lee DH, Iwanski GB and Thoennissen NH (2010). Cucurbitacin: Ancient compound shedding new light on cancer treatment, The Scientific World Journal, 10(5), 413-418.
Matos MEO, Machado MIL, Craveiro AA et al. (1991). Nor-cucurbitacin glucosides from Wilbrandia species, Phytochemistry, 30(3), 1020-1023.
McDonnell DP, Wardell SE and Norris JD (2015). Oral selective estrogen receptor down regulators (SERDs) a break through endocrine therapy for breast cancer, Journal of Medicinal Chemistry, 58(12), 4883-4887.
Miladiyah I, Yuanita E, Nuryadi S et al. (2020). Synergistic effect of 1,3,6-Trihydroxy-4,5,7-Trichloroxanthone in combination with Doxorubicin on B-Cell Lymphoma cells and its mechanism of action through Molecular docking, Current Therapy Research, 92(30), 100576-100586.
Musza LL, Speight P, McElhiney S et al. (1994). Cucurbitacin, cell adhesion inhibitors from Conobea scoparioides, Journal of Natural Products, 57(11), 1498-1502.
Pandian CJ, Jayaraj V, James S et al. (2015). Docking exploration of human estrogen receptor to decipher phytochemicals as tumor suppressors, International Journal of Engineering Research Technology, 3(33), 1-4.
Park CS, Lim KJ, Baek SH et al. (2004). Inhibition of nitric oxide generation by 23,24-dihydrocucurbitacin D in mouse peritoneal macrophages, Journal of Pharmacology and Experimental Therapeutics, 309(2), 705-710.
Peng J, Sengupta S and Jordan VC (2009). Potential of selective estrogen receptor modulators as treatments and preventives of breast cancer, Anticancer Agents in Medicinal Chemistry, 9(5), 481-499.
Phillips JC, Braun R, Wang W et al. (2005). Scalable molecular dynamics with NAMD, Journal of Computational Chemistry, 26(16), 1781-1802.
Pushpalatha R, Selvamuthukumar S and Kilimozhi D (2017). Comparative in silico docking analysis of Curcumin and Resveratrol on Breast Cancer proteins and their synergistic effect on MCF-7 cell line, Journal of Young Pharmacists, 9(4), 480-485.
Rafter JJ (2002). Scientific basis of biomarkers and benefits of functional foods for reduction of disease risk: Cancer, British Journal of Nutrition, 88(2), 219-224.
Raikhlin-Eisenkraft B and Bentur Y (2000). Ecballium elaterium (squirting cucumber) – remedy or poison?, Journal of Clinical Toxicology, 38(3), 305-308.
Rios JL, Andujar I, Escandell JM et al. (2012). Cucurbitacin as inducers of cell death and a rich source of potential anticancer compounds, Current Pharmaceutical Design, 18(12), 1663–1676.
Sahayarayan JJ, Rajan KS, Vidhyavathi R et al. (2021). In silico protein-ligand docking studies against the estrogen protein of breast cancer using pharmacophore based virtual screening approaches, Saudi Journal of Biological Sciences, 28(1), 400-407.
Scabia V, Ayyanan A, De Martino F et al. (2022). Estrogen receptor positive breast cancers have patient specific hormone sensitivities and rely on progesterone receptor, Nature Communications, 13(10), 3127 3137.
Sharpless NE, Alson S, Chan S et al. (2002). p16 INK4a and p53 deficiency cooperate in tumorigenesis, Cancer Research, 62(10), 2761-2765.
Shiau AK, Barstad D, Loria PM et al. (1998). The structural basis of estrogen receptor/Coactivator recognition and the antagonism of this interaction by tamoxifen, Cell, 95(7), 927-937.
Si DP, Wardell SE and Norris JD (2019). Oral selective estrogen receptor down regulators (SERDs) a break through endocrine therapy for breast cancer, Journal of Medicinal Chemistry, 58(12), 4883-4887.
Sun J, Blaskovich MA, Jove R et al. (2005). Cucurbitacin Q: A selective STAT3 activation inhibitor with potent antitumor activity, Oncogene, 24(20), 3236-3245.
Teuscher E and Lindequist U (1994) Triterpene. New York 159-175.
Thavamani BS, Mathew M and Dhanabal SP (2014). Anticancer activity of Cissampelos pareira against Dalton’s lymphoma ascites bearing mice, Pharmacognosy Magazine, 10(39), 200-206.
Umar AB, Uzairu A, Shallangwa GA et al. (2020). QSAR modelling and molecular docking studies for anti-cancer compounds against melanoma cell line SK-MEL-2, Heliyon, 6(3), 1-11.
WHO. (2017). Media Center: Fact Sheet Cancer, World Health Organization, http://www.who.int/mediacentre/factsheets/fs297/en/ accessed on Oct 15, 2017.
Yan X, Lin Y, Liu S et al. (2015). Fucosyltransferase IV (FUT4) as an effective biomarker for the diagnosis of breast cancer, Biomedicine & Pharmacotherapy, 70(10), 299-304.
Yasuda S, Yogosawa S, Izutani Y et al. (2010). Cucurbitacin B induces G2 arrest and apoptosis via a reactive oxygen species-dependent mechanism in human colon adenocarcinoma SW480 cells, Molecular Nutrition & Food Research, 54(4), 559-565.
Yu H and Jove R (2004). The stats of cancer –new molecular targets come to age, Nature Reviews Cancer, 4(2), 97-105.
Zhang X, Yue P, Page BDG et al. (2004). Orally bioavailable small molecule inhibitor of transcription factor stat 3 regresses human breast and lung cancer xenografts, Proceedings of the National Academy of Sciences, 109(24), 9623-9628.
Zhong Y, Xu H, Zhong Y et al. (2019). Identification and characterization of the Cucurbitacin, a novel class of small molecule inhibitors of Tropomyosin receptor kinase, A BMC Complementary Alternative Medicine, 19(295), 295-304.


