1Rajiv Gandhi Technical University, Bhopal, India
2Madhav Institute of Technology & Science, Gwalior, India
Corresponding author email: kirtirajbhatele8@gmail.com
Article Publishing History
Received: 21/10/2020
Accepted After Revision: 12/12/2020
This paper presents an automated system for the classification of two commonly diagnosed neurodegenerative diseases i.e. Alzheimer and Parkinsons, with the aid of deep transfer learning model Visual Geometry Group 19 (VGG19). As deep learning architectures are already very much used for the classification of various brain tumors and other types of cancers but not used much for the classification of Neurodegenerative diseases. There is an imperative need to have an automated system that can perform the classification among the Neurodegenerative diseases as the number of patients suffering from these disease are increasing. This system can even help the radiologists to enhance their diagnostic efficiency and accuracy of these Neurodegenerative diseases. In this paper, VGG19 deep transfer learning model is implemented, hyper tuned and trained over the Alzheimer and Parkinson disease (ADPP) dataset, which is developed using Alzheimer’s disease Neuroimaging Initiative (ADNI) and Parkinson’s Progression Markers Initiative (PPMI) databases for the multiclass classification of Alzheimer, healthy control and Parkinson disease.
As there is no such system proposed yet to perform this degree of multiclass classification. So other popular deep transfer learning models like ResNet 50, Inception Net and VGG 16 are also implemented over the same ADPP dataset and their performance are compared with the proposed system based on VGG19 architecture. The proposed system based on VGG 19 outperforms the other three popular deep transfer learning models and delivers an average accuracy of 90% with 70/30 (training/validation split) and 93% with 80/20 (training/validation split) for the multiclass classification as Alzheimer disease, Healthy Control ADNI, Healthy Control PPMI and Parkinson disease. This work can be extended in future in order to propose an automated system for the multiclass brain structural disorders classification.
Alzheimer, Inception Net, MPRAGE, Parkinson, ResNet 50, VGG 16, VGG 19 etc.
Bhatele K. R, Bhadauria S. S. Classification of Neurodegenerative Diseases Based on VGG 19 Deep Transfer Learning Architecture: A Deep Learning Approach. Biosc.Biotech.Res.Comm. 2020;13(4).
Bhatele K. R, Bhadauria S. S. Classification of Neurodegenerative Diseases Based on VGG 19 Deep Transfer Learning Architecture: A Deep Learning Approach. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/3eC5Rrg
Copyright © Bhatele and Bhadauria This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The two most common diagnosed neurodegenerative diseases are the Alzheimer’s disease (AD) and Parkinsons disease (PD), which are diagnosed in old age people. Alzheimer disease is responsible for the dementia (Alzheimer’s Association, 2017). A person suffering from Alzheimers, normally showcases the symptoms like difficulty in completing normal tasks, challenges in planning, memory loss etc. This disease is a result of depletion of some particular nerve cells along with the existence of plaques (neuritic) just outside the neuron and aggregation of neurofibrillary tangles in the interior of neuron (McKhann et al., 1984). The neuritic and neurofibrillary proteins are whole sole responsible for the depletion of brain cells and destructing the functionality of brain cells. Thus the brain of people suffering from the advance stage of Alzheimer exhibits swelling and high shrinkage from cell loss.
As AD is still incurable disease and its early diagnosis will be very useful for the good care of patients. There are many ways adopted by the physician in order to diagnose the Alzheimer disease which involves problem solving, memory test, attention and the most effective is to take the brain scans of the patient in the form of either magnetic resonance imaging (MRI) or positron emission tomography (PET) or computed tomography (CT) scans. Mostly MRI scans are used by the physicians to diagnose Alzheimer disease. A number of deep learning-based approaches are proposed recently utilizing the MRI scans in order to automate the task of diagnosis and early prediction of Alzheimer disease (El-Sappagh et al., 2020; Feng et al., 2020; Rehman et al., 2020).
The Parkinson’s disease was initially explained by James Parkinson, diagnosed in the older adults just like Alzheimer disease. The major symptoms of PD are muscle rigidity, tremor and slow movements (Borek et al., 2006). The various other indications of this disease are very low speech, face with no expressions, shaky handwriting, difficulty in standing from the chair etc. The PD is caused due to the deficiency of dopamine which acts as a neurotransmitter in the human brain. The loss of dopamine in the human brain is related to the depletion of neurons, which is a phenomenon occurs in old age people. The nature of this neurodegenerative disease in progressive which is similar to Alzheimer and hence early stage diagnosis of this disease will play an important role in the handling as well as caring of the patients (Ferreri et al., 2006). As the population of old age or senior citizens are increasing at a very rapid rate (Rehman et al., 2020).
So, there is a need of a systems that can diagnose these two neurodegenerative diseases at an early stage. As the MRI imaging exhibits accurate Neuro-anatomic biomarkers which plays an important role in the diagnosis of PD, so MRI scans are mostly used for the diagnosis of PD. Indeed MRI scans presents minute anatomical details related to the subcortical structures of the human brain whose analysis can be done in order to diagnosis this PD at an early stage. As these MRI scans are bound to be very difficult to analyze and observe the heterogeneous attributes as well as intrinsic details of subcortical structures captured in these MRI scans with the help of human eye because of the three dimensional nature. Hence there is a need of an intelligent system to perform this high data computing and processing task (Bakator et al., 2018; Lundervold et al., 2019). Recently a number of deep learning based classification approaches are proposed which have delivered high classification accuracy for the Parkinson disease prediction and classification utilizing MRI scans (Gautam and Sharma, 2020; Chakraborty et al., 2020).
Machine learning is very successful especially in the automated classification of different objects and various types of brain as well as human disorders, which is already proved by the number of papers published each year. In the context of brain disorders, machine learning and deep learning are widely used for the classification of brain tumors, glioma classification etc. utilizing electronic medical records and medical imaging data. Computer aided diagnosis (CAD) systems proposed over the years for the classification and diagnosis of breast cancer, lung cancers, liver cancer etc. are the application of machine learning in the domain of medical imaging. The huge amount of research is already been carried out in order to propose various CAD systems, which could be used to automate the task of diagnosis of various types of disease utilizing the PET, CT, fMRI and Structural MRI scans etc.
Now a days deep convolutional neural networks (CNN) are very popular and widely used by the researchers due to the high performance delivered by these models. As in deep learning models, there is no need of manual segmentation and feature extraction unlike conventional machine learning models, so these deep learning models are more popular and hence completely automate the task of either binary or multi class classification (Russakovsky et al., 2015). The deep transfer learning models like VGG 16, Inception Net etc. are widely used especially in the developments of CAD systems for the classification of various types of cancers and brain tumors (Mehrotra et al., 2020; Kaur and Gandhi 2020).
The literature review section given below simply highlights the very fact that there is no such unified approach proposed yet for the classification of both Alzheimer and Parkinson diseases. Although there are various deep learning based approaches proposed over the years for the individual classification of either Alzheimer or Parkinson disease. Another important fact proved by the literature review section that almost all these approaches based on machine learning and deep learning had used either the ADNI or the PPMI dataset for training and validation. The major contributions of this paper are as follows: A VGG-19 architecture based framework for the classification of two most common neurodegenerative disease used for the first time.
The ADPP dataset, which is developed using the global datasets like ADNI and PPMI are used for training and validation purpose in order to showcase the applicability of this model in real time. As there is no such unified framework or system for the classification of Alzheimer and Parkinson disease yet, so other popular deep transfer learning models like VGG-16, Res Net 50 and Inception Net are also implemented and trained over the same ADPP dataset in order to perform a genuine comparison with the proposed system based on VGG-19.
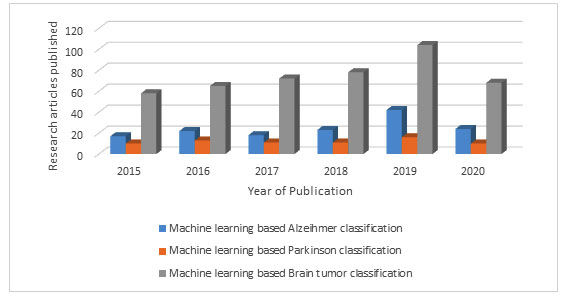
There is not much research carried out in the field of Alzheimer and Parkinson disease classification using machine learning as compare to the amount of research carried out for the brain tumor classification. But still a number of state of the art approaches based on traditional machine learning and deep learning models are presented in this section for both the neurodegenerative diseases. This fact is very well proved with the help of graph 1 given below which illustrates a comparison among the number of research papers published in the field of Alzheimer, Parkinson and Brain tumor classification based on machine learning.
Graph 1: The result of search for yearly rise in number of research papers related to the application of machine learning for the Alzheimer, Parkinson and Brain tumor classification as per PubMed from 2015 to 2020.
Initially Li et al. (2014) proposed an approach based on local binary pattern (LBP) and Support vector machine (SVM) classifier for the classification of Alzheimer. This machine learning based approach offers an accuracy of 83% on the MRI dataset prepared from the Alzheimer’s disease Neuroimaging Initiative (ADNI) database. Then Dyrba et al. (2015) proposed another method based on Multi kernel SVM is proposed for the differentiation of Alzheimer patients from the normal ones. This approach uses graph-theoretical measures ‘local clustering coefficient’ methods for accurate segmentation from the diffusion tensor imaging (DTI) in order to calculate the grey matter (GM) volume. This approach offers an accuracy of 85% on the local dataset composed of MRI images of around 28 AD patients collected from the German Centre for Neurodegenerative Diseases (DZNE) Rostock database.
A SVM and multiple kernel learning (MKL) based approach was proposed for the correct classification of AD patients by Ni et al. (2016). The two above mentioned classifiers are trained with the help of linear, multi-fractal and mono fractal features extracted from the Resting estate functional MRI (rs-fMRI) scans. This approach offers an accuracy of 76% on the dataset prepared with the help of ADNI database. Another approach based on shape and volume based features along with the SVM classifier was proposed by Glozman et al. (2017) for the accurate classification of AD patients. This approach achieves an accuracy of 88% on the large size MRI dataset prepared with the help of ADNI database.
Valliani et al. (2017) used a deep residual convolutional neural networks (CNNs) pre-trained model known as the Resnet-18 for the classification and differentiation of Alzheimer patients from the normal ones. This Resnet-18 architecture performs learning of cross-domain features in order to optimize the interpretations of MRI AD images for the correct classification. This architecture achieves an accuracy of 81.3% on the dataset constructed with the help of ADNI database. In the same year Hon et al. (2017) came with an approach in which VGG-16 and Inception V4 transfer learning models are used for the classification of Alzheimer disease and delivers an accuracy of over 90% on the small size dataset prepared with the help of Open Access Series of Imaging Studies (OASIS) database. Another VGG-16 transfer learning based architecture is used by the Jain et al. (2018) for the correct classification of Alzheimer disease. This architecture delivers an accuracy of 95 % on the ADNI database based dataset.
Fulton et al. (2019) have proposed a Resnet-50 based approach, which delivers an accuracy of 98% on the dataset consist of cross-sectional and longitudinal MRI scans obtained from the OASIS database. A Resnet-34 deep transfer learning model based approach was also proposed by Talo et al. (2019), which performs multiclass classification for the Alzheimer disease. In this paper, data augmentation is also done in order to enhance the dataset for training. This approach based on Resnet-34 delivers an accuracy of 99% on a large size augmented MRI dataset. Similarly there are number of approaches based on the deep transfer learning models like VGG-16, Resnet 50, Alex net etc. which are used for the early classification of Alzheimer disease from the healthy ones and delivers accuracies in the range of 90-95%( Raghu et al., 2019; El-Sappagh et al., 2020; Feng et al., 2020; Rehman et al. 2020).
For the Parkinson disease detection and classification even less amount of research is carried out. Initially researchers are using traditional machine learning classifiers then employed deep learning models and recently deep transfer learning based approaches are used, which even delivering good results. A multi-kernel SVM based approach was proposed for the classification of PD utilizing T1 weighted MRI scans all obtained from the Parkinson’s Progression Markers Initiative (PPMI) repository and delivers an accuracy of 86% (Peng et al., 2017). Similarly another approach based on the SVM and random forest (RF) classifier was proposed which delivers an accuracy of 93% on the PPMI dataset (Amoroso et al., 2018). Then a transfer learned pre-trained AlexNet model based approach was proposed by Sivaranjini et al. (2019) in order to perform differentiation of PD patients from the normal ones. This Alexnet model is based on the proposed approach and delivers an accuracy of 88% on the dataset prepared from the PPMI repository.
Then Yagis et al. (2019) proposed another approach based on the two deep transfer learning models like VGG 16 and Resnet-50 for the correct classification of PD patients from the healthy controls. Although VGG 16 and Resnet-50 delivers accuracy of more than 82 % on the large size dataset prepared with the aid of PPMI repository. Various papers for the automated classification of Parkinson disease have been published in the last two years primarily based on the 3D convolutional neural network (CNN) architecture (Chakraborty et al., 2020), Alex net architecture (Krizhevsky et al., 2012; Ortiz et al., 2019), conventional CNN model (Shah et al., 2018; Gautam and Sharma, 2020) providing accuracies in the range of 85-91% on the PPMI dataset.
The following points can be concluded from the brief literature review, which are as follows:1. There is a need of a unified automated system in order to classify both the Alzheimer and Parkinson disease as both are neurodegenerative diseases in real time, as there is no such system proposed yet for diagnosing the same.2. As ADNI and PPMI databases are the mostly used repositories consist of MRI images of both the neurodegenerative diseases. So these two databases are used to develop a common ADPP datasets consisting of Alzheimer and Parkinson diseases MRI scans.
MATERIAL AND METHODS
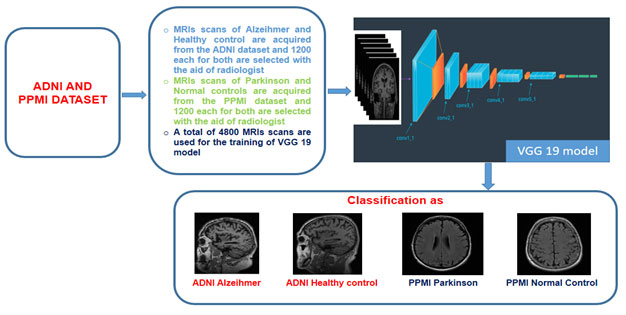
The proposed system based on VGG-19 deep transfer learning architecture consist of two major stages, first stage is all about downloading the MRI scans of Alzheimer and Parkinson’s diseases from the ADNI and PPMI databases. Then conversion of MRI scans present in DICOM (Digital Imaging and Communications in Medicine) format into the PNG (Portable Network Graphics) format as this format offers lossless compression. Selection of informative MRI scans are carried out manually under the supervision of a radiologist, so that only the informative and correct MRI scans are present in the training and validation datasets.
The second stage is all about customizing and training the VGG-19 with the help ADPP dataset. In the deep transfer learning VGG 19 architecture, the last three layers are modified in order to adapt to our problem domain and to perform correct multiclass classification into the Alzheimer, healthy control ADNI, Parkinson and healthy control PPMI classes. The proposed classification system is illustrated with the help of figure 1 given below:
Figure 1: Proposed automated system based on the VGG 19 architecture for the classification of Alzheimer and Parkinson neurodegenerative diseases.
ADPP Dataset description: ADPP is a balance dataset consisting a total of 4800 MRI scans of Alzheimer patients, healthy control scans from ADNI, Parkinson patients and healthy controls from PPMI is used in this paper for the training and validation/testing purpose. Around 1200 Magnetization Prepared Rapid Gradient Echo (MPRAGE) MRI scans each of Alzheimer patients and healthy control are taken from the ADNI database. Whereas 1200 axial T2 weighted MRI scans each of Parkinson’s and healthy controls are taken from the PPMI database. Both these ADNI (www.adni.loni.usc.edu) and PPMI (Marek et al., 2011) datasets are publicly available datasets constantly used by the researchers working in the field of classification of different neurodegenerative diseases using machine learning. From the ADNI database around 50 Alzheimer and 50 healthy control cases are downloaded in Dicom format. Similarly from the PPMI dataset around 50 Parkinson and 50 healthy control cases are downloaded. As the MRI scans are present in the Dicom format hence converted into the PNG format. The table 1 below simply illustrates the demographic information of the ADPP dataset used in this paper.
Table 1. Demographic information of the ADPP dataset
| Database | Groups | Number of objects | Sex | Age group | MRI Modality | Number of MRI slices taken |
| ADNI | AD | 50 | 24 females and 26 males | 65-90 | MP-RAGE | 1200 |
| HC | 50 | 35 females and 15 males | 60-93 | MP-RAGE | 1200 | |
| PPMI | PD | 50 | 20 females and 30 males | 39-80 | T2 weighted | 1200 |
| HC | 50 | 26 females and 24 males | 31-80 | T2 weighted | 1200 |
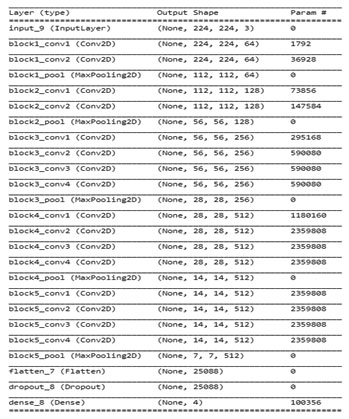
VGG 19 architecture: The VGG-19 architecture used in this paper simply belongs to the class of the visual geometry group network (VGGNet) developed by the Oxford university (Tajbakhsh et al., 2016). The VGGNet is one of the popular models of deep transfer learning used for the image classification task. This VGGNet is trained on the ImageNet database (Yosinski et al., 2014; Russakovsky et al., 2015). The main reason behind using a VGG-19 architecture as it offers high accuracy, efficiency and adaptability to other classification problems.
The architecture of VGG-19 used in this paper consist of total 19 layers which is divided into five blocks (Simonyan et al., 2014). Five max pooling functions are used to join these blocks. The input size is kept 224*224*3 and the size of the filter is kept very tiny i.e. 3*3 in each and every layers in order to handle the trainable parameters (Lee et al., 2018). The last three layers like Flatten, dropout and dense are added in our architecture in order to perform the multiclass classification and delivers the output. The architecture of the VGG-19 model used in this paper is illustrated with the help of figure 2 below.
Figure 2: The VGG 19 architecture description along with the total number of parameters, trainable and non-trainable parameters information.
| Trainable parameters | 4819972 |
| Non trainable parameters | 15304768 |
| Total parameters | 20124740 |
RESULTS AND DISCUSSION
The simulation and experimentation of the proposed system based on VGG19 architecture and its comparison with the other three i.e. VGG 16, ResNet50 and Inception Net deep transfer learning models is performed using the Google Colaboratory (colab) platform powered by the NVidia Tesla T4 GPU. Whereas, Python 3.6 is used as an implementation programming language. The performance of the proposed system is evaluated and presented using the following statistical parameters or classification rates like Accuracy, Sensitivity, Specificity and F1-score, which are defined as:
![]()
Where FP= False positive, FN= False Negative, TP= True positive and TN= True Negative.
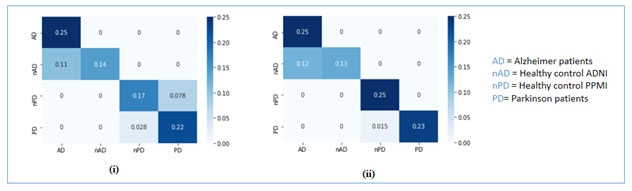
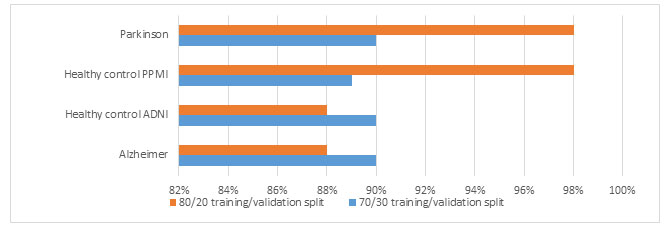
The proposed system is evaluated in terms of above mentioned classification rates using two types of mostly used training/validation splits of the ADPP dataset as 70/30 and 80/20, which means 70, 80 % ADPP dataset is used for training and 30, 20 % for validation. The proposed system gives best results with 80/20 splits on the ADPP dataset. The classification rates are summarized in the table 2. The confusion matrices of the proposed system is illustrated with the help of figure 3. The figure 4 simply presents the accuracy comparison of the proposed automated system with both 70/30 and 80/20 splits.
Table 2. Performance of the proposed VGG 19 based system on the ADPP dataset with 70/30 and 80/20 splits
| Training and validation split percentage | Neurodegenerative diseases type classification | Accuracy | Precision | Sensitivity | F1 score |
| 70/30 split | Alzheimer | 90% | 100% | 70% | 83% |
| Healthy control ADNI | 90% | 58% | 100% | 73% | |
| Healthy control PPMI | 89% | 69% | 86% | 76% | |
| Parkinson | 90% | 89% | 74% | 81% | |
| Average Accuracy, Precision, sensitivity and F1 score | 90% | 80% | 83% | 79% | |
| 80/20 split | Alzheimer | 88% | 100% | 67% | 81% |
| Healthy control ADNI | 88% | 52% | 100% | 68% | |
| Healthy control PPMI | 98% | 100% | 94% | 97% | |
| Parkinson | 98% | 94% | 100% | 97% | |
| Average Accuracy, Precision, sensitivity and F1 score | 93% | 86% | 91% | 86% | |
Figure 3: Confusion matrices of the proposed system based on VGG19 (i) with 70/30 validation split; (ii) with 80/20 validation split
Figure 4: Accuracy comparison of the proposed automated system on the ADPP dataset with both 70/30 and 80/20 training/validation splits
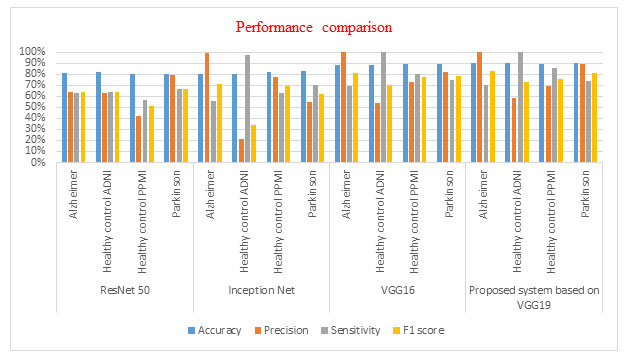
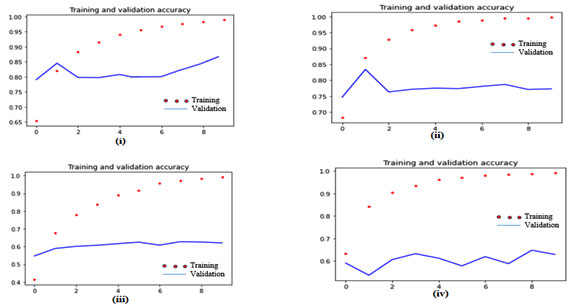
The proposed automated system based on VGG 19 is compared with some of the existing recent deep transfer learning models like VGG16, ResNet50 and Inception Net on the same dataset. The training and validation progress graph of VGG19, VGG16, ResNet 50 and Inception Net Model with split 70/30 are shown with the help of figures 6. All these models are implemented using the same programming language and same computing environment as of the proposed system based on VGG 19. Their brief comparison is illustrated with the help of table 3 and figure 5.
Table 3. Performance comparison of the VGG19 based proposed system with the VGG16, ResNet50 and Inception Net based system on the ADPP dataset with 70/30 split.
| Deep transfer learning model | Neurodegenerative diseases type classification | Accuracy | Precision | Sensitivity | F1 score |
| ResNet 50 | Alzheimer | 81% | 64% | 63% | 64% |
| Healthy control ADNI | 82% | 63% | 64% | 64% | |
| Healthy control PPMI | 80% | 42% | 57% | 51% | |
| Parkinson | 80% | 79% | 67% | 67% | |
| Inception Net | Alzheimer | 80% | 99% | 56% | 71% |
| Healthy control ADNI | 80% | 21% | 97% | 34% | |
| Healthy control PPMI | 82% | 77% | 63% | 69% | |
| Parkinson | 83% | 55% | 70% | 62% | |
| VGG16 | Alzheimer | 88% | 100% | 69% | 81% |
| Healthy control ADNI | 88% | 54% | 100% | 70% | |
| Healthy control PPMI | 89% | 73% | 80% | 77% | |
| Parkinson | 89% | 82% | 75% | 78% | |
| Proposed system based on VGG19 | Alzheimer | 90% | 100% | 70% | 83% |
| Healthy control ADNI | 90% | 58% | 100% | 73% | |
| Healthy control PPMI | 89% | 69% | 86% | 76% | |
| Parkinson | 90% | 89% | 74% | 81% |
Figure 5: Performance comparison graph for comparing the performance of VGG19 based proposed system with the VGG16, ResNet50 and Inception Net based system on the ADPP dataset with 70/30 split.
Figure 6: The training and validation progress graph of (i) proposed system based on VGG 19; (ii) VGG 16; (iii) ResNet 50; (iv) Inception Net on the ADPP dataset with 70/30 split.
The proposed VGG19 based system is also compared with some of the state of the art approaches proposed in the recent years for either performing the Alzheimer classification or Parkinson classification on the ADNI or PPMI datasets. Their comparison is presented with the help of table 4 and 5.
Table 4. Performance comparison of the proposed VGG19 based system with the existing approaches for the Alzheimer classification on the ADNI dataset.
| Author and year | Machine learning classifier or deep learning model used | Accuracy | Precision | Sensitivity | F1 score |
| Li et al. and 2014 | Support vector machine | 83% | – | 80.4% | – |
| Glozman et al. and 2017 | Support vector machine | 80.54% | – | 70.59% | – |
| Valliani et al. and 2017 | Residual Network 18 (ResNet18) | 81.3% | – | – | – |
| Proposed one | VGG19 | 90% | 80% | 83% | 79% |
Table 5. Performance comparison of the proposed VGG19 based system with the existing approaches for the Parkinson’s disease classification on the PPMI dataset.
| Author and year | Machine learning classifier or deep learning model used | Accuracy | Precision | Sensitivity | F1 score |
| Peng et al. and 2017 | Multi-kernel support vector machine (SVM) | 85.78% | – | 87.64% | – |
| Sivaranjini et al. and 2019 | Alexnet | 88.9% | – | 89.3% | – |
| Yagis et al. and 2019 | VGG16 and ResNet 50 | 82% | – | – | – |
| Proposed one | VGG19 | 90% | 80% | 83% | 79% |
CONCLUSION
The proposed automated system is a unified framework based on VGG19 for the classification of the two mostly diagnosed neurodegenerative diseases like Alzheimer and Parkinson. There are deep learning based systems or approaches for the classification of Alzheimer or Parkinson diseases, but there is not any system proposed yet which can perform the multiclass classification of these two neurodegenerative diseases. The proposed system is tested on the ADPP dataset which is developed using the global datasets like ADNI for Alzheimer and PPMI for Parkinson with the assistance of radiologist, so that only the informative MRI scans are used for training and validation. This systems delivers an encouraging average accuracy of above 90% for the multiclass classification, as this type of unified systems are proposed for the first time.
In order to present a genuine comparison, three mostly used deep transfer learning architectures like VGG16, ResNet50 and Inception Net are implemented and evaluated on the same ADPP dataset. The training and validation graph given in the result section, moreover proves that the proposed system based on VGG19 outperforms the VGG16, ResNet50 and Inception Net models in terms of accuracy and performance. In future, such unified systems can be enhanced and used in real time in order to assist the radiologist. More and more multiclass classification systems can be developed for the classification of other brain structural disorders like brain tumors, schizophrenia, dementia etc.
ACKNOWLEDGMENTS
We like to thank Dr. Pushraj Bhatele (Chief Radiologist) of MP MRI and CT Scan Centre at Netaji Subhash Chandra Bose Medical College, Jabalpur for providing his valuable support in terms of medical knowledge and validate this proposed work.
Conflict of Interest:The authors have declared no conflict of interest.
REFERENCES
Alzheimer’s Association (2017) Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, Vol 13 No 4 Pages 325-373.
Amoroso N, La Rocca M, Monaco A, Bellotti R, and Tangaro S (2018) Complex networks reveal early MRI markers of Parkinson’s disease. Medical Image Analysis, Vol 48 Pages 12–24. https://doi.org/10.1016/j.media.2018.05.004.
Bakator M, and Radosav D (2018) Deep learning and medical diagnosis: A review of literature. Multimodal Technol. Interact., Vol 2 Pages 47.
Borek LL, Amick MM, and Friedman JH (2006) Non-motor aspects of Parkinson’s disease. CNS Spectr, Vol 11 No 7 Pages 541-54.
Chakraborty S, Aich S, and Kim HC (2020) Detection of Parkinson’s disease from 3T T1 Weighted MRI Scans Using 3D Convolutional Neural Network. Diagnostics Vol 10 No 6 Pages 402-419.
Dyrba M, Grothe M, Kirste T, and Teipel SJ (2015) Multimodal analysis of functional and structural disconnection in Alzheimer’s disease using multiple kernels SVM. Hum Brain Mapping, Vol 36 No 6 Pages 2118–31.
El-Sappagh S, Abuhmed T, Riazul Islam SM, and Kwak KS (2020) Multimodal Multitask Deep Learning Model for Alzheimer’s Disease Progression Detection Based on Time Series Data. Neurocomputing, Vol 418 Pages 197-215.
Feng W, Halm-Lutterodt NV, Tang H, Mecum A, Mesregah MK, Ma Y, and Guo X (2020) Automated MRI-Based Deep Learning Model for Detection of Alzheimer’s Disease Process. International Journal of Neural Systems, Vol 30.
Ferreri F, Agbokou C, and Gauthier S (2006) Recognition and management of neuropsychiatric complications in Parkinson’s disease. CMAJ, Vol 175 Pages 1545-52.
Fulton LV, Dolezel D, Harrop J, Yan Y, and Fulton CP (2019) Classification of Alzheimer’s disease with and without Imagery using Gradient Boosted Machines and ResNet-50. Brain Sciences, Vol 9 No 9 Pages 212. https://doi.org/10.3390/brainsci9090212.
Gautam R, and Sharma M (2020) Prevalence and Diagnosis of Neurological Disorders Using Different Deep Learning Techniques: A Meta-Analysis. Journal of Medical System, Vol 44 pages 1-24.
Glozman T, Solomon J, Pestilli F, and Guibas L (2017) Alzheimer’s disease Neuroimaging I. Shape-attributes of brain structures as biomarkers for Alzheimer’s disease. J. Alzheimer’s Dis., Vol 56 No 1 Pages 287–95.
Hon M, and Khan NM (2017) Towards Alzheimer’s disease classification through transfer learning. 2017 IEEE International Conference on Bioinformatics and Biomedicine (BIBM). doi:10.1109/bibm.2017.8217822.
Jain R, Jain N, Aggarwal A, and Hemanth DJ (2019) Convolutional Neural Network based Alzheimer’s disease Classification from Magnetic Resonance Brain Images. Cognitive Systems Research, Vol 57 pages 147-159. doi:10.1016/j.cogsys.2018.12.015.
Kaur T, and Gandhi TK (2020) Deep convolutional neural networks with transfer learning for automated brain image classification. Machine Vision and Applications, Vol 31 No 3.
Krizhevsky A, Sutskever I, and Hinton GE (2012) Imagenet classification with deep convolutional neural networks. In Advances in Neural Information Processing Systems; Neural Information Processing Systems Foundation, Inc.: La Jolla, CA, USA, Pages 1097–1105.
Lee JH, Kim DH, and Jeong SN (2018) Diagnosis and prediction of periodontally compromised teeth using a deep learning based convolutional neural network algorithm. J. Periodontal Implant Sci., Vol 48 No 2 Pages 114-123.
Li M, Oishi K, He X, Qin Y, Gao F, and Mori S (2014) An efficient approach for differentiating Alzheimer’s disease from normal elderly based on multicentre MRI using gray-level invariant features. PLoS One, Vol 9 No 8 Pages 105563.
Lundervold AS, and Lundervold A (2019) An overview of deep learning in medical imaging focusing on MRI. Zeitschrift für Medizinische Physik, Vol 29 Pages 102–127.
Marek K, Jennings D, and Taylor P (2011) The Parkinson progression marker initiative (PPMI), Progress in neurobiology, Vol 95 Pages 629–635.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, and Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the nincds-adrda work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology, Vol 34 No 7 Pages 939.
Mehrotra R, Ansari MA, Agrawal R, and Anand RS (2020) A Transfer Learning approach for AI-based classification of brain tumors. Machine Learning with Applications, Vol 2.
Ni H, Zhou L, Ning X, and Wang L (2016) Alzheimer’s Disease Neuroimaging. Exploring multifractal-based features for mild Alzheimer’s disease classification. Magnetic Resonance in Medicine, Vol 76 No 1 Pages 259–69.
Ortiz A, Munilla J, and Martínez M (2019) Parkinson’s Disease Detection using isosurfaces-based features and Convolutional Neural Networks. Front. Neuroinform. Vol 13 Pages 48.
Peng B, Wang S, Zhou Z, Liu Y, Tong B, Zhang T, and Dai Y (2017) A multilevel-ROI-features-based machine learning method for detection of morph metric biomarkers in Parkinson’s disease. Neuroscience Letters, Elsevier Vol 651 Pages 88–94.
Raghu M, Zhang C, Kleinberg J, and Bengio S (2019) Transfusion: Understanding transfer learning with applications to medical imaging. arXiv preprint arXiv:1902.07208.
Ramzan F, Khan MUG, Rehmat A, Iqbal S, Saba T, Rehman A, and Mehmood Z (2020) A Deep Learning Approach for Automated Diagnosis and Multi-Class Classification of Alzheimer’s Disease Stages Using Resting-State fMRI and Residual Neural Networks. Journal of Medical System, Vol 44 pages 37.
Russakovsky O, Deng J, Su H, Krause J, Satheesh S, Ma S, Huang Z, Karpathy A, Khosla A, Bernstein M, Berg AC, and Fei-Fei L (2015) ImageNet large scale visual recognition challenge. International Journal of Computer Vision, Vol 115 No 3 Pages 211–252.
Shah PM, Zeb A, Shafi U, Zaidi SFA, and Shah MA (2018) Detection of Parkinson Disease in Brain MRI using Convolutional Neural Network. In Proceedings of the 2018 24th International Conference on Automation and Computing (ICAC), Newcastle upon Tyne, UK, 6–7 September 2018 Pages 1–6.
Simonyan K, and Zisserman A (2014) Very deep convolutional networks for large-scale image recognition. arXiv:1409.1556. https://arxiv.org/abs/1409.1556
Sivaranjini S, and Sujatha CM (2019) Deep learning based diagnosis of Parkinson’s disease using convolutional neural network. Multimedia Tools and Applications. https://doi.org/10.1007/s11042-019-7469-8.
Talo M, Baloglu UB, Yıldırım Ö, and Acharya R (2019) Application of deep transfer learning for automated brain abnormality classification using MR images. Cognitive Systems Research, Vol 54 Pages 176–188. https://doi.org/10.1016/j.cogsys.2018.12.007.
Tajbakhsh N, Shin JY, Gurudu SR, Todd Hurst R, Kendall CB, Gotway MB, and Liang J (2016) Convolutional neural networks for medical image analysis: Full training or ne tuning? IEEE Transactions of Medical Imaging, Vol 35 No 5 Pages 1299-1312.
Valliani A, and Soni A (2017) Deep Residual Nets for Improved Alzheimer’s Diagnosis. Proceedings of the 8th ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics – ACM-BCB ’17. doi:10.1145/3107411.3108224.
Yagis E, De Herrera AGS, and Citi L (2019) Generalization Performance of Deep Learning Models in Neurodegenerative Disease Classification. 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 2019, Pages 1692-1698. https://doi.org/ 10.1109/BIBM47256.2019.8983088.
Yosinski J, Clune J, Bengio Y, and Lipson H (2014) How transferable are features in deep neural networks? In Proceedings of Advance Neural Information Processing System, Pages 3320-3328.