Department of Computational Biology, Indraprastha Institute of Information Technology, New-Delhi, India.
Corresponding author email : hdgen31@gmail.com
Article Publishing History
Received: 28/12/2020
Accepted After Revision: 23/03/2021
Corona viruses COVID-19 is a novel and highly infectious virus emerged in December 2019, (Wuhan) China and affected a wide range of global population with deaths worldwide till date. Lack of knowledge regarding the clinical management and treatment of infected patient has augmented this epidemic of China to get transformed into a global pandemic. This review outlines briefly about the novel coronavirus, its taxonomic classification, associated symptoms, origin, life cycle and the treatment strategy which includes diagnosis as well as on-going trials related to antiviral drugs and vaccine with its pros and cons to combat SARS-CoV 2 infection. Presently, to deal with the infection therapeutic strategies are only supportive and prevention is the best weapon to abate transmission in the community. Efforts have been made to develop vaccines against human coronavirus (CoV) infections such as MERS and SARS in the past decades. However, to date, no licensed antiviral treatment or vaccine exists for MERS and SARS. Researchers are searching for effective and suitable vaccine candidates and therapeutics for controlling the deadly COVID-19. There are no effective vaccines or specific antiviral drugs for COVID-19. Hence, we have to rely exclusively on enforcing strict preventive and control measures that minimize the risk of possible disease transmission. There is an urgent requirement of vaccine to stop the spreading of SARS-CoV2 infection. Since there is no antiviral drug or vaccine so it is important to enhance the host immune response against the infection. We except our analysis to become a milestone for future studies helping with acting as a credible groundwork for their results.
Infectitious, Anti-Viral, Therapeutics, Exclusively, Vaccine, Immune.
Kashyap D. Brief Analysis on the Advantages and Disadvantages of Novel Severe Acute Respiratory Syndrome Corona Virus Disease 19 Ongoing Treatment. Biosc.Biotech.Res.Comm. 2021;14(1).
Kashyap D. Brief Analysis on the Advantages and Disadvantages of Novel Severe Acute Respiratory Syndrome Corona Virus Disease 19 Ongoing Treatment. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/30dv1WX“>https://bit.ly/30dv1WX</a>
Copyright © Kashyap This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Corona viruses are characterised by their largest genome with single positive stranded RNA (27-32Kbs) that is packaged in nucleo-capsid and the membrane protein as well as has been named as ‘Corona’ or crown like morphological appearance with the spike proteins. These enveloped viruses recently became associated with humans and animals respiratory and gastrointestinal infections. In the year 2002 to 2003, viral epidemic severe acute respiratory syndrome (SARS-CoV), HINI in 2009 and in 2012 Middle East respiratory syndrome coronavirus in Saudi Arabia have been recorded by World Health Organization. Among coronaviruses severe acute respiratory syndrome (SARS- CoV) and MERS-CoV are found zoonotic and highly pathogenic causing global outbreaks (Luk et al. 2020).
Based on nucleotide sequence SARS-CoV 2 are new human infecting betacoronavirus 2019-nCoV originated probably from bats to humans after passing in one or more intermediate hosts as evidenced by the molecular data and experiments (Chen et al. 2015; Luk et al. 2020). However, the original source and association with the animal host still remains indefinable. Additionally, Xiong et al. (2020) on the basis of molecular evolution data suggested that two different viral strains of SARS-CoV 2 might be involved in the outbreak (Xiong et al. 2020).
This mini review describes briefly the novel corona viruses COVID 19 and summarises its historical background, probable source of origin, life cycle and the treatment strategies describing the ongoing trials to combat SARSCoV2 infection. Therefore, this review focussed to assemble updated information and evidences to understand the evolving virus.
A brief history and origin: In December (2019) pneumonia cases were recorded in Wuhan, China and the cause identified was due to novel Beta-coronavirus. WHO named this virus initially as the 2019-novel coronavirus on 12 January, 2020 and Corona Study Group (CSG) of the International committee proposed the name SARS-CoV-2 on 11 February, 2020. On 7thJanuary 2020, Chinese Scientists isolated SARS CoV-2 and sequenced the virus genome. After virus genome and evolutionary history analysis, bat has been considered as the possible origin of corona virus infection initiation. 96.2% genome sequence identity showed that Bat CoV Ratg13 and human COVID 19 genomes probably shared the same ancestors and is also in a way identical to 79.5% with SARS-CoV.
However, it is clear now to infect humans, SARS-CoV-2 could use angiotensin-converting enzyme 2 (ACE2), the same receptor as SARS-CoV (Zhou et al. 2020). Interestingly, on the basis of similar residue of receptors apart from protein homology and phylogenetic analysis, few studies also reported alternative intermediate hosts such as pangolin, turtles and snakes as the possible reservoir hosts of corona virus. However, the origination of Corona virus still remains uncertain.
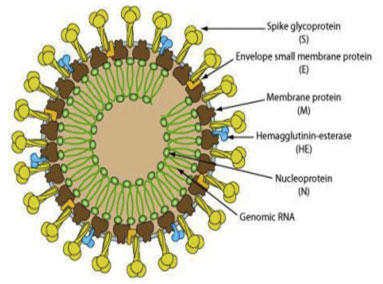
Model of life cycle and transmission: The regions that are encoded for viral replication, nucleocapsid and spike formation is present in ORF 1a/b downstream region in all coronaviruses. The spike of viruses (Figure 1) that is made of glycoprotein is meant for entry and attachment of the virus with the host cell (Cui et al. 2019; Perlman, 2020). Among viruses loosely attached receptor binding domain is present due to which it can attack multiple hosts. A typical corona virus structure consists of spike proteins as well as other polyproteins, nucleoproteins and membrane proteins that include RNA Polymerase, papain like protease, chymotrypsin like protease, helicase, glycoproteins and accessory proteins (Raj et al. 2013; Elfiky et al. 2017).
The three-dimensional structure in the receptor binding domain in SARS-CoV 2 is recognised by human ACE2 receptor that facilitates viral envelop fusion with the host cell. The RNA of SARS-CoV2 is released in the host cell. Viral gRNA by viral replicase is translated into polyproteins and cleaved by viral proteases. Thus, viral genome and proteins are assembled in virions in ER and Golgi and by vesicle trafficking release out of the cell (Wong et al. 2015). The virus spread from one person to another via droplets or aerosols or by fomite transmission. Doremanlan et al. (2020) in his study have shown that virus survive in different surfaces for days and in aerosols for hours. Incubation period of virus has been reported to be of 5.1days and the initiation of symptoms up to 14 days after incubation (period of self-isolation/ quarantine) (Doremanlan et al. 2020; Lauer et al. 2020).
Figure:.Novel Coronavirus structure HCoVs: structural proteins, such as Spike (S) marking all coronaviruses, Nucleocapsid (N), Matrix (M), and Envelope (E) (From Biowiki : http://ruleof6ix.fieldofscience.com/2012/09/a-new-coronavirus-should-you-care.html).
SARS CoV and COVID 19: Outbreak of Severe Acute Respiratory Syndrome (SARS-CoV) was in the year 2003, MERS –CoV in 2012 and COVID 19 in 2019. Bat is thought to be the source of origin of SARS-CoV and spread by an intermediate host before jumping to humans. Compared to SARS-CoV, COVID 19 transmits more easily probably because viral load appears initially to be higher in nose and throat before the symptom develop. Some researchers from Centre of Disease control suggested that COVID 19 might spread without the carrier showing any symptom of the virus.
Additionally, the transmission of SARS-CoV and MERS-CoV has been testified to occur mostly through nosocomial transmission (Gao et al. 2020). Infections of healthcare workers in 33–42% of SARS cases and transmission between patients (62–79%) were the most common route of infection in MERS-CoV cases (Chowell et al. 2015). Direct contact with intermediate host animals or consumption of wild animals was also supposed to be the main route of SARS-CoV-2 transmission. Caution should be, however, exercised to promptly identify asymptomatic viral carriers. Additionally, fomite transmission of SARS-CoV-2 might have predisposed to the rapid spread globally (Cornman et al. 2020).
Treatment strategies: Diagnostic techniques: Fever, cough and shortness of breath are the specific noticeable symptoms to start the diagnosis. Patient with epidemiological link includes basically the travel history of an individual in an outbreak area, or contact with an infected individual being asymptomatic within 14 days as estimated incubation time of viruses and close contact with an individual having infection. SARS-CoV2 has been detected in urine, gastric mucosa, saliva, stool (Xie et al. 2019; To et al. 2019; Guan et al. 2019) and accordingly different types of corona virus test involved: swab test, nasal and tracheal aspirate test, sputum, blood test as well as rapid test that ensure speedy and rapid diagnosis.
Rapid Diagnostic test detects viral protein from the samples taken from respiratory tract of the COVID 19 infected person. If the target antigen is present in the sample, it will bind to the antibodies fixed in a paper strip generating a visual detectable signal. However, this test is not 100% accurate as this test may show false positive results (antibodies on the strip recognise other viral antigens) also concentration of the virus in the specimen, reagents in the test kit might give a false result. Apart from this rapid diagnostic test based on host antibody detection has been widely exploited, (Cornman et al. 2020).
During recovery stage of the corona virus infected individual antibodies are produced in the blood in response to viral infection and therefore, target the COVID 19 virus. PCR testing has been recommended by WHO for identification and laboratory confirmation of COVID 19 virus. Along with, RT-PCR (Real Time Reverse Transcription Polymerase Chain reaction) has been a reliable diagnostic tool in terms of sensitivity and specificity for COVID 19 virus detection (Cornman et al. 2020).
On-going trials: SARS and MERS outbreak in 2003 and 2012 has been guidance for doctors and researchers to treat the present COVID 19 infected patients. For treating corona virus infection initially HIV drugs such as lopinavir/ritonavir that is a protease has been administered and inhibit corona virus replication but this drug combination caused diarrhoea and jaundice like situation and in some COVID 19 infected patients no beneficial effect was observed (Cao et al. 2020). Nucleotide analog like ribavirin (Table 1) that has been used to treat SARS might also have antiviral effect by inhibiting nucleotide biosynthesis. Additionally, combination of ribavirin and interferons (IFN-α) have been used to treat SARS.
Ramdesvir is a novel nucleotide analog is presently under clinical trials. It has been recognised as a promising anti -viral drug for SARS/ MERS-CoV and its activity inhibit RNA dependent RNA polymerase from MERS-CoV (Gordon et al. 2020; de Wit et al. 2020). For inhibiting viral infection from SARS-CoV, it is essential to block the binding of S-protein to ACE2 (acetyl-converting enzyme 2) and an anti-malarial drug Chloroquine /Hydroxychloroquine have been highly commercialized as potent inhibitor of SARS-CoV and a study reported by Wang et al. chloroquine has been found to be highly effective against COVID 19 (Wang et al.
2020; Gao et al. 2020). Chloroquine and hydroxychloroquine block channels on heart muscle cells that control the flow of ions, which governs the heart’s electrical recharging between beats. Immune system modulating agents have also been proposed to combat virus COVID 19 infection. The entry of virus in the host cell activates an immune response to increase in cytokines which is responsible for disease severe condition. Individuals with low number of lymphocytes CD4+ T cells, those having some chronic illness in the history are badly affected by SARS-CoV 2 (Beigel et al. 2019; Chen et al. 2020).
Convalescent Plasma Therapy: In the year 1890, Emil Von Behring discovered Convalescent plasma therapy for which he got Nobel Prize in medicine. This therapy presently has been widely used to treat COVID 19 patients by taking out the plasma of an individual who has recovered from COVID 19 infection and thus has sufficient antibodies and thus transferring plasma to freshly infected individual. This therapy has been used to treat MERS and SARS and EBOLA virus diseases but this therapy renders passive immunization and is impermanent as it cannot provide lifelong immunity (Burnouf et al. 2016; Chen et al. 2020).
Table 1. Therapeutic targets in clinical trials against SARS-CoV 2 infection
| Drugs | Mode of action against SARS-CoV2 | Probable pros and cons |
|
Ribavirin Remdesvir Sofosbuvir Galidesvir Tenofovir
|
Act against RNA dependent RNA polymerase (Elfiky, 2020) |
Yet to establish Safety and efficiency. |
|
Lianhuaqingwen
|
Inhibit virus replication (Runfeng et al. 2020)
|
Antiviral and anti-inflammatory. Might have potential to inhibit cytokine storm
|
|
Oseltamivir |
Neuraminidase inhibitor |
No exact evidence regarding its effectiveness against COVID 19
|
|
Lopinavir Atazanavir Darcenavir Nelfinavir Sequinavir Tipranavir
|
HIV protease inhibitor |
Invitro activity against SARS-CoV2 in Vero E6 cells but no data to support COVID 19 |
|
Favilavir (Avigan)
|
Inhibit RNA dependent RNA polymerase (Wang et al. 2020) |
Used in Japan and China for treating influenza. Showed invitro activity against SARS-CoV 2 in Vero E6 cells but its use contradicted in pregnant women. |
BCG vaccine and COVID 19: BCG vaccine has been given to children to prevent tuberculosis and being heterologous also provides protection against other non-related infections. BCG vaccination primes histone modifications and epigenetic reprogramming of human monocytes resulting in a more active innate immune response in terms of trained immunity. Professor Netea pointed out those studies that were done among adults, also showed lower incidence of respiratory tract infections in those who were given the BCG vaccine (Kleinnijenhuis et al. 2012; Netea et al. 2016). BCG (Bacillus Calmette Guerin) an attenuated strain of Mycobacterium bovis studies are in progress in order to investigate this vaccine against COVID 19 infections. Redelman (2020) explained why BCG could be a candidate vaccine for treating life threatening SARS-CoV2 infections are as follows:
- Molecular similarity in BCG antigens and viral antigens
- Long term activation and reprogramming of innate cells
- Developing B and T cell memory for recognizing BCG antigen and another respiratory antigen
- BCG vaccination increases the expression of surface markers producing high cytokines such as IL-1B, IFNY, TNF.
Need of the hour: Viruses, an obligate intracellular parasite evolved to hijack the host cell machinery. In the same manner the emergence of SARS-CoV 2 resulted in high mortality all over the world by overtaking the host cell function thus threatening the global public health. So, it is very important to gain more insights in understanding pathogenesis of human infecting corona viruses for developing targeted therapeutics and vaccines. At present everyone should strictly follow the preventive measures recommended by WHO and other health organizations to protect oneself from SARS-CoV2 infection, (Chen et al. 2020). Frequent hand washing, use of portable sanitizers, avoid public gathering, maintaining hygiene, and use of N95 mask, gloves, etc by the health- workers to prevent pathogen transmission. Convincingly, ‘each one treats one for one’ is the only strategy to overcome the corona virus global pandemic, (Chen et al. 2020).
ACKNOWLEDGEMENTS
The author would like to express with deep gratitude for all health workers and staffs that are supporting and putting lot of efforts to combat COVID-19 infection.
Conflict of Interest: There was no conflict in the interests of the associated people.
REFERENCES
Beigel JH, Nam HH, and Adams PL (2019). Advances in respiratory virus therapeutics—A meeting report from the 6th isirv Antiviral Group conference. Antiviral Res. 167: 45–67.
Burnouf T, Conton B, and Dye JM (2016). Convalescent plasma for Ebola virus disease. N Engl J Med. 374: 25: 2498–2500.
Cao B, Wang Y, and Wen D (2020). A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. https://doi.org/10.1056/ NEJMoa2001282.
Chan JF, Lau SK, To KK, Cheng VC, Woo PC, and Yuen KY (2015). Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clinical microbiology reviews. 28:465522.
Chan JF, To KK, Tse H, Jin DY, and Yuen KY (2013). Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 21:10: 544-555.
Chen N, Zhou M, and Dong X (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 395: 10223: 507–13.
Chen Y, and Guo D (2015). Molecular mechanisms of coronavirus RNA capping and methylation. Virol Sin. 3:11:3‐11.
Cheng Y, Wong R, and Soo YO (2005). Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 24: 1: 44–46.
Chowell G, Abdirizak F, Lee S, Lee J, Jung E, and Nishiura H (2015). Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 13: 210.
Cornman VM, Landt O, and Kaiser M (2020). Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 25.
Cui J, Li F, and Shi ZL (2019). Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 17: 3; 181–192.
de Wit E, Feldmann F, and Cronin J, (2020). Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERSCoV infection. Proc Natl Acad Sci USA. https ://doi.org/10.1073/ pnas.19220 83117.
Elfiky AA, Mahdy SM, and Elshemey WM (2017). Quantitative structure-activity relationship and molecular docking revealed a potency of anti-hepatitis C virus drugs against human corona viruses. Journ. of Medical Virol. 89:6: 1040–1047. 10.1002/jmv.24736
Elfiky AA. (2020). Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study, Life Sciences, https://doi.org/10.1016/j.lfs.117592
Gao J, Tian Z, and Yang X (2020). Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 14: 1: 72–73.
Gordon CJ, Tchesnokov EP, and Feng JY (2020). The antiviral compound Ramdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. https ://doi. org/10.1074/jbc.AC120 .01305 6.
Graham RL, Donaldson EF, and Baric RS (2013). A decade after SARS: strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 11: 836–848.
Guan WJ, Ni ZY, and Hu Y (2020). Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. doi:10.1056/NEJMoa2002032.
Kleinnijenhuis J, Quintin F, Preijers LA, Joosten DC, Ifrim S, Saeed C, Jacobs J, van Loenhout D, de Jong HG, and Stunnenberg (2012). Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA. 109: 17537-17542.
Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, and Meredith HR (2020). The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. https://doi.org/10.7326/M20-0504.
Lu R, Zhao X, and Li J (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. pii: S0140-6736(20)30251-30258. doi: 10.1016/S0140-6736(20)30251-30258
Luk HKH, Li X, Fung J, Lau SKP, and Woo PCY. (2020). Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect Genet Evol. 71: 21–30.
Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, and Xavier RJ (2016). Trained immunity: A program of innate immune memory in health and disease. Scie. 352: aaf1098.
Perlman S and Netland J (2009). Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 7: 6: 439-450.
Perlman S. (2020). Another Decade, Another Coronavirus2020. N Engl J Med. 382: 760-762 Mass medical Soc DOI: 10.1056/NEJMe2001126
Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, and Dijkman R. (2013). Dipeptidylpeptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 495: 7440: 251-254. doi: 10.1038/nature12005.
Redelman-Sidi G (2020). Could BCG be used to protect against COVID-19? Nat Rev Urol. https://doi.org/10.1038/s41585-020-0325-9.
Runfeng L, Yunlong H, and Jicheng H (2020). Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 156.
To KK, Tsang OT, and Chik-Yan Yip C (2020). Consistent detection of 2019 novel corona virus in saliva. Clin Infect Dis. ciaa149.
Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, and Williamson BN (2020). Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. https://doi.org/10.1056/NEJMc2004973.
Wang M, Cao R, and Zhang L (2020). Ramdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV). Cell Res. https ://doi.org/10.1038/s4142 2-020-0282.
WHO (2016). Middle East respiratory syndrome coronavirus (MERS-CoV).
WHO (2020). Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situationreports.
Wong HH, Kumar P, Tay FPL, Moreau D, Liu DX, and Bard F (2015). Genome-Wide Screen Reveals Valosin-Containing Protein Requirement for Coronavirus Exit from Endosomes. J. Virol. 89: 11116–11128. doi: 10.1128/JVI.01360-15.
Xie C, Jiang L, and Huang G (2020). Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis. pii: S1201-9712(20)30108-9.
Xiong C, Jiang L, and Chen Y (2020). Evolution and variation of 2019-novelcoronavirus. .https://www.biorxiv.org/content/10.1101/2020.01.30.926477v1
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, and Zhang W (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature.


