Department of Biotechnology, U.I.E.T, Kurukshetra University Kurukshetra, Kurukshetra, Haryana, India-136119
Corresponding author Email: deepmolbio@rediffmail.com
Article Publishing History
Received: 18/01/2019
Accepted After Revision: 20/03/2019
The use, formation and manipulation of materials at nanoscale level is known as Nanotechnology, which change their properties at nanoscale. The nanoscale materials can be synthesized by using physical and chemical processes. Size, shape, morphology, stability and properties of nanoparticles can have direct impact on their potential applications. The design of an eco-friendly, time effective and economic synthesis method with easy control over their essential properties has become a leading area of research. Now a days, the biological entities are employed to improve nanoparticles production without the use of any harsh chemicals. The biological synthesis of nanoparticles is an eco-friendly method. Nanoparticles have broad range of applications such as optical, electronics, electrical, medical, environment, textile, energy science, optoelectronics, catalysis, single electron transistors, light emitters, nonlinear optical devices, photo-electrochemical applications, cosmetics and food industries owing to their unique physiochemical properties. The aim of this critical review is to provide an insight into the ecofriendly synthesis of nanoparticles with significant applications in various fields.
Biological eco-friendly, materials, Nanotechnology, manipulation
Singh V, Bhatia D, Khatak S, Kumar T, Malik D. K. Biological Eco-Friendly Synthesis of Nanoparticles and their Applications. Biosc.Biotech.Res.Comm. 2019;12 (1).
Singh V, Bhatia D, Khatak S, Kumar T, Malik D. K. Biological Eco-Friendly Synthesis of Nanoparticles and their Applications. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2MgHHYF
Copyright © Singh, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
The mechanical, magnetic, electrical and optical properties of materials having particle size between 0.1 and 100 nm can be different from the same materials at larger size. Now scientists are changing the form and size of materials at nano scale level to recognize the uncommon properties of the material. Metal nanoparticles like gold, titanium, titanium oxide, zinc oxide, iron, copper, silver and platinum are widely used in various domestic and commercial products. Silver nanoparticles are of interest owing to the distinctive properties which may be assimilated into antimicrobial applications, cosmetic products, biosensor materials, refrigerant super-conducting materials, composite fibres and electronic components. Gold nanoparticles are presently under intensive study for applications in ultrasensitive chemical, optoelectronic devices and biological sensors and as catalysts, (Perez et al., 2012 Khoei et al., 2017 Xu et al., 2018 ).
Evaporation-condensation and optical device ablation are the leading physical approaches for the synthesis of nanoparticles. However, Physical-chemical techniques of synthesis have many difficulty in scaling-up the process, separation and purification of nanoparticles. Microorganisms have been utilized in biotechnology implementations like bioremediation and bioleaching (Stephen et al., 1999). Microorganisms are able to carry out a range of oxidoreduction mechanisms and promote biochemical translation (Sastry et al., 2004). The biological methods are responsible for the synthesis of mono-dispersed silver nanoparticles starting from 5 to 15 nm in size (Karthik et al., 2014, Hassan et al., 2018). Now a days, biological methods are used for the synthesis of nanoparticles, to construct the sustainable processes, which do not produce any harmful chemicals in to the environment. The plants and microorganisms used for the synthesis of nanoparticles are listed in Table 1.
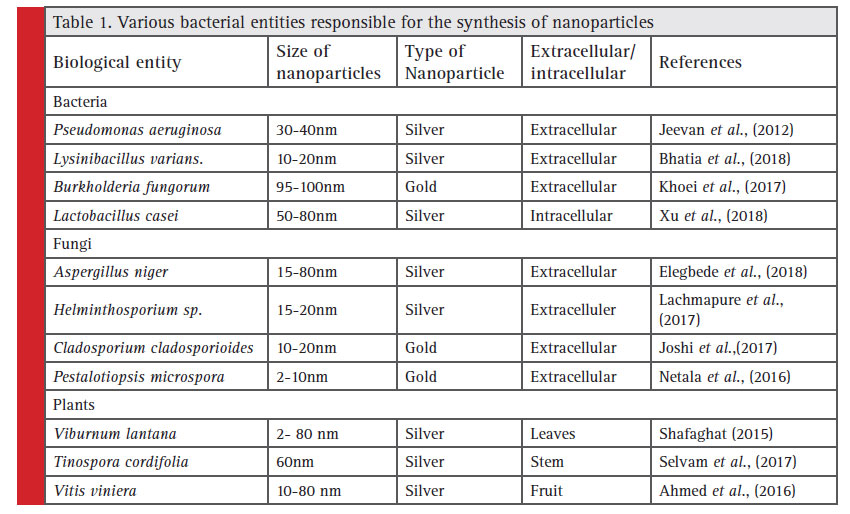 |
Table 1: Various bacterial entities responsible for the synthesis of nanoparticles |
Actinomycetes and Algae
Biogenesis of metal nanoparticles by using actinomycetes and algae is seeking attention since decade due to their unique property of intra and extra cellular synthesis of nanoparticles (Abdeen et al., 2014). Reduction of metal ions may be due to interacting enzymes being released from the cell membrane and cell wall. Actinomycetes mediated synthesis of copper oxide nanoparticles and study for its antibacterial activity against selected human and fi sh pathogens was reported by Nabila et al., (2018). In algae mediated synthesized nanoparticles decahedral, polyhedron and tetrahedral structure were observed (Luangpipat et al., 2011). Rajasulochana et al. (2010) reported the synthesis of silver nanoparticles by using Kappaphycus alvarezii. Senapati et al. (2012) reported the intracellular synthesis of silver nanoparticles via Tetraselmis kochinensis. Castro et al. (2013) reported the use of red Chondrus crispus and green algae Spirogyra insignis for the synthesis of gold and silver nanoparticles.
Bacteria and Fungi
Ability of bacteria to survive in diverse and sometimes extreme environmental situations renders them a suitable candidate for nanoparticles synthesis. Survival in these harsh conditions ultimately depends on their ability to resist the effects of environmental stresses. Bacteria are able to synthesize metallic nanoparticles by either intracellular or extracellular mechanisms. The various bacterial species were reported to synthesize the nanoparticles such as Actinobacter sp., Escherichia coli, Klebsiella pneumonia, Lactobacillus sp., Bacillus cereus, Corynebacterium sp. and Pseudomonas sp. (Mohanpuria et al., 2008; Iravani et al., 2014; Sunkar et al., 2014; Tollamadugu et al., 2011). The silver nanoparticles were synthesized by Pseudomonas stutzeri AG259 by reduction of Ag ions (Ahmad et al., 2003). Husseiny et al. (2007) reported extracellular synthesis of silver nanoparticles by using Pseudomonas. Sneha et al. (2010) reported that Corynebacterium sp. shows a non-enzymatic reduction mechanism in nanoparticle synthesis. The dried biomass of Lactobacillus sp. and Bacillus megaterium reduced silver ions using interaction of molecules present on the cytomembrane to produce silver nanoparticles (Fu et al., 2000).
Fungi being able to secrete large amounts of enzymes and proteins per unit of biomass can be utilized for synthesis of huge amounts of nanoparticles (Narayanan et al., 2010). The Aspergillus sp., Fusarium sp. and Penicillium sp. have been reported for their biosynthetic ability of silver and gold nanoparticles (Vigneshwaran et al., 2007; Shankar et al., 2003; Philip et al., 2009). The extracellular synthesis of silver nanoparticles by using Aspergillus fumigatus has been reported (Bhainsa and D’Souza, 2006). The biological synthesis of Au nanoparticles was carried out by reduction of Au ions by using Trichothecium sp. biomass (Ahmad et al., 2005). The extracellular synthesis of nanoparticles due to its proteins discharged by fungal biomass was reported (Macdonald et al., 1996). Bansal et al. (2007) reported that Fusarium oxysporum synthesized silica and titanium nanoparticles from binary compound solutions of SiO62- and TiF62- respectively.
Viruses, Yeast and Plants
The virus capsid proteins make an extremely reactive surface able to interact with metallic ions (Makarov et al., 2014). The selection of proteins will act as attachment area for the deposition of materials (Kobayashi et al., 2012). In virus mediated synthesis, size was significantly reduced along with increase in their numbers as compared to non viral mediated synthesis (Makarov et al., 2014). The low concentrations of TMV’s were supplementary to silver or gold salts before adding plant extracts of Nicotiana benthamiana or Hordeum vulgare.
Yeasts can absorb and accumulate significant amounts of toxic metals from their surrounding environment (Mandel et al., 2006). Due to this property, yeast has been exploited for synthesis of metal nanoparticlesn. Yeasts use different mechanism for the synthesis and stability of nanoparticles which leads to variation in particle size, location and properties (Hulkoti et al., 2014). Dameron et al. (1989) reported Candida glabrata mediated intracellular synthesis of CdS quantum dots. Schizosaccharomyces pombe cells were used for the synthesis of CdS quantum dots (Reese et al., 1998). The intracellular synthesis of PbS quantum dots was carried out by Torulopsis sp. when exposed to Pb2- ions (Kowshik et al., 2002).
Plants mediated synthesis is a relatively simple, less time consuming, cost effective and an ecofriendly process. The process begins by mixing a sample of plant extract with a metal salt solution. In plants mediated synthesis of nanoparticles, metal ions changed from mono or divalent oxidation states to zero-valent states. As growth progresses nanoparticles combine to form different morphologies (Akhtar et al., 2013; Malik et al., 2014). The plants extract properties and incubation temperature significantly influence the synthesis of nanoparticles (Dwivedi et al., 2010). The plant mediated nanoparticles synthesis are to be safe, less synthesis time and lesser cultivation value as compare to biological systems (Mittal et al., 2013).
Applications of nanoparticles One of the major applications of nanotechnology is in biology and medicine. Nanoparticles are used for detection of pathogens (Edelstein et al., 2000), detection of proteins (Nam et al., 2003), analysis of DNA structure (Mahtab et al., 1995), Tissue engineering (Ma et al., 20039, de la isla et al., 2003). Now days, superbugs with multi drug resistance are major threat to human being. Nanotechnology can be a solution to combat antimicrobial drug resistance. Nanotechnology provides synthesis and design strategies to develop antimicrobial nanotherapeutics to fi ght the trouble of antimicrobial resistance. The large surface area-to-volume ratio at nanoscale may be the reason of antimicrobial mechanism against a broad spectrum of microorganisms. Nanoparticles can also be manipulated for effective and targeted delivery of drugs and imaging labels by overcoming the many biological, biophysical, and biomedical barriers. Nanoparticles has been also employed for targeted drug delivery at the tumor site or a certain group of cells without affecting non target cells (Huang, et al., 2015 Shen et al., 2016, Escarcega et al., 2018).
The silver nanoparticles accumulate in tumours and their distinctive optical and chemical properties may be utilized in thermal treatment procedures (Hirsch et al., 2003). Nanoparticles have been used in anti-odour clothes, furnishings textiles, kitchen cloths, sponges, towels, antibacterial drugs, patient dresses, reusable surgical gloves, protecting face masks, suits against biohazards, cosmetic products, toothbrushes, ultra hydrophobic materials with potential applications within the production of extremely water repellent materials, bactericide material to coat hospital instrumentation, antitumor medication, active wear, food packaging and waste water treatments (Pissuwan et al., 2009, Cheng et al., 2010, Lee et al. 2007, Ramaratnam et al. 2008, Hassan et al., 2012). TiO2 nanoparticles, as a result of their antibacterial activity, are utilized in antibacterial coatings and effluent disinfection processes (Miller et al., 2012).
References
Abdeen, S.; Geo, S.; Sukanya, S.; Praseetha, P.K.; Dhanya, R.P. Biosynthesis of Silver nanoparticles from Actinomycetes for therapeutic applications. Int. J. Nano Dimens. 2014, 5, 155– 162.
Ahmad A., Senapati S., Khan M. I., Kumar R., Ramani R., Srinivas V., and Sastry M., (2003) Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete Rhodococcus species. Nanotechnology. Vol. 14: Pages 824–828.
Edelstein R. L., Tamanaha C .R., Sheehan P. E., Miller M. M., Baselt D. R., Whit- man L. J., Colton R. J. (2000) The BARC biosensor applied to the detection of biological warfare agents. Biosensors Bioelectron Vol.14: Pages 805-813.
Nam J. M., Thaxton C. C., Mirkin C. A. (2003) Nanoparticlesbased bio-bar codes for the ultrasensitive detection of proteins. Science Vol. 301: Pages 1884-1886.
ahtab R., Rogers J. P., Murphy C. J. (1995) Protein-sized quantum dot luminescence can distinguish between “straight”, “bent”, and “kinked” oligonucleotides. J. Am. Chem. Soc. Vol. 117: Pages9099-9100.
Ma J., Wong H., Kong L. B., Peng K. W. (2003) Biomimetic processing of nanocrystallite bioactive ap atite coating on titanium. Nanotechnol. Vol. 14: Pages 619-623.
e la Isla A., Brostow W., Bujard B., Estevez M., Rodriguez J. R., Vargas S., Castano V.M. (2003) Nanohybrid scratch resistant coating for teeth and bone viscoelasticity manifested in tribology. Mat. Resr. Innovat. Vol. 7: Pages107-114
Ahmad A., Senapati S., Khan M. I., Kumar R., and Sastry M., (2005) Extra-/intracellular, biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J. Biomed. Nanotechnol. Vol. 1: Pages 47–53.
Ahmed S. M., Ahmad B. L., Swami., and Ikram S., (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise, J. Adv. Res. Vol. 7: Pages 17–28.
Akhtar M. S., Panwar J., and Yun Y. S. (2013) Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. Vol. 1: Pages 591–602.
ansal V., Syed A., Bhargava S. K., Ahmad A., and Sastry M. (2007). Zirconia enrichment in zircon sand by selective fungus- mediated bioleaching of silica. Langmuir. Vol. 23(9): Pages 4993-4998.
Bhainsa C.K. and D’Souza, F. S., (2006). Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus, Colloids Surfaces B; Biointerfaces. Vol. 47: Pages 160-164.
Shen B., Ma Y., (2016). Smart multifunctional magnetic nanoparticle- based drug delivery system for cancer thermo-chemotherapy and intracellular imaging. (2016) ACS Appl. Mater Interf. Vol. 8(37): Pages 24502–24508.
Huang B., Abraham W. D., et al., (2015) Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci. Trans. Med. 7, 291ra94
Bhatia D., Mittal A., and Malik D. K., (2016). Antimicrobial activity of PVP coated silver nanoparticles synthesized by Lysinibacillus varians. 3 Biotech. Vol. 6(2): Pages 196.
Castro L., Blazquez M. L., Munoz J. A., Gonzalez F., and Ballester A., (2013) Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. Vol. 7: Pages 109–116.
Cheng, Y., Samia A. C., Li J., Kenney M E., Resnick A., and Burda C., (2010) Delivery and efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir.Vol. 26: Pages 2248–2255.
Dwivedi A. D., and Gopal K., (2010) Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A. Vol. 369: Pages 27–33.
legbede J. A., Lateef A., Azeez, M. A., Asafa T. B., Yekeen T. A., Oladipo I. C., and Gueguim-Kana E. B. (2018) Fungal xylanases-mediated synthesis of silver nanoparticles for catalytic and biomedical applications. IET Nanobiotechnology. Vol.12(6): Pages 857-863.
Escarcega-Gonzaez C. E., Garza-Cervantes J. A., Vazquez-Rodriguez A., Montelongo-Peralta L. Z., Trevino-Gonzalez M. T., Castro E. D. B., and Rosales J. C. (2018). In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. International journal of nanomedicine. Vol. 13: Pages 2349.
Fu J. K., Liu Y. Y., Gu P. Y., Liang S. D., Yu L. Z., Xin Y. B., and Zhou W.S., (2000) Spectroscopic characterization on the biosorption and bioreduction of Ag(I) by Lactobacillus sp A09. Acta Phys. Chim. Sin. Vol. 16: Pages 779–782.
Hassan H. S., Elkady M. F., El-Sayed E. M., Hamed A. M., Hussein A. M., and Mahmoud I. M., (2018). Synthesis and Characterization of Zinc Oxide Nanoparticles using Green and Chemical Synthesis Techniques for phenol decontamination. International Journal of Nanoelectronics and Materials. Vol. 11(2): Pages 179-194.
Hassan M.S., Amna T., Yang O.B., El-Newehy M. H., Al-Deyab S. S., and Khil M. S., (2012) Smart copper oxide nanocrystals: Synthesis, characterization, electrochemical and potent antibacterial activity. Colloids Surf. B Biointerfaces. Vol. 97: Pages 201–206.
Hirsch L. R., Stafford R. J., Bankson J. A., Sershen S. R., Rivera B., Price R. E., and West J. L., (2003). Nanoshell-mediated nearinfrared thermal therapy of tumours under magnetic resonance guidance. Proceedings of the National Academy of Sciences. Vol. 100 (23): Pages 13549-13554.
Hulkoti N. I., and Taranath T. C., (2014). Biosynthesis of nanoparticles using microbes—a review. Colloids and Surfaces B: Biointerfaces. Vol. 121: Pages 474-483.
Husseiny M.I., El-Aziz M.A., Badr Y., and Mahmoud, M. A., (2007) Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta A. Vol. 67: Pages 1003–1006.
Iravani S., (2014) Bacteria in nanoparticle synthesis: Current status and future prospects. Int. Sch. Res. Not. Vol. 2014: Pages 359-316.
Jeevan P., Ramya K., and Rena A. E., (2012). Extracellular biosynthesis of silver nanoparticles by culture supernatant of Pseudomonas aeruginosa. Indian Journal of Biotehnology. Vol. 11: Pages 72-76.
Joshi C. G., Danagoudar A., Poyya J., Kudva A. K., and Dhananjaya B. L. (2017) Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides iso lated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochemistry. Vol. 63: Pages 137-144.
Karthik L., Kumar G., Vishnu-Kirthi A., Rahuman A. A., Rao V. B., (2014) Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess. Biosyst. Eng. Vol. 37: Pages 261–267.
Khoei N. S., Lampis S., Zonaro E., Yrjala K., Bernardi P., and Vallini G., (2017). Insights into selenite reduction and biogenesis of elemental selenium nanoparticles by two environmental isolates of Burkholderia fungorum. New biotechnology. Vol. 34: Pages 1-11.
Kobayashi M., Tomita S., Sawada K., Shiba K., Yanagi H., Yamashita I., and Uraoka Y., (2012) Chiralmeta-molecules consisting of gold nanoparticles and genetically engineered tobacco mosaic virus. Opt. Express. Vol. 20: Pages 24856–24863.
Kowshik M., Vogel W., Urban J., Kulkarni S. K., and Paknikar K. M., (2002) Microbial synthesis of semiconductor PbS nanocrystallites. Adv. Mater. Vol. 14: Pages 815–818.
Lachmapure M., Paralikar P., Palanisamy M., Alves M., and Rai, M., (2017). Effi cacy of biogenic silver nanoparticles against clinical isolates of fungi causing mycotic keratitis in humans. IET Nanobiotechnology. Vol. 11(7): Pages 809-814.
Lee H. Y., Park Y. K., Lee Y. M, Kim K., and Park S.B., (2007) A practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applications. Chem Commun. Pages 2959–2961.
Luangpipat T., Beattie I.R., Chisti Y., and Haverkamp R.G., (2011) Gold nanoparticles produced in a microalga. J. Nano part. Res. Vol. 13: Pages 6439–6445.
Macdonald I.D.G., and Smith W.E., (1996) Orientation of cytochrome c adsorbed on a citrate-reduced silver colloid surface. Langmuir. Vol. 12: Pages 706–713.
Makarov V. V., Love A.J., Sinitsyna O. V., Makarova S. S., Yaminsky I. V., Taliansky M. E., Kalinina, N. O. (2014) Green nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae. Vol. 6: Pages 35–44.
Malik P., Shankar R., Malik V., Sharma N., and Mukherjee T. K, (2014) Green chemistry based benign routes for nanoparticle synthesis. J. Nanopart. Vol. 2014: Pages 302-429.
Miller R. J., Bennett S., Keller, A. A., and Pease, S., Lenihan, H. S., (2012) TiO2 nanoparticles are phototoxic to marine phytoplankton. PLOS Biol. Vol. 7: Pages 1–7.
Mittal A. K., Chisti Y., and Banerjee U. C., (2013) Synthesis of metallic nanoparticles using plants. Biotechnol. Adv. Vol. 31: Pages 346–356.
Mohanpuria P., Rana N. K., and Yadav S. K., (2008) Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. Vol. 10: Pages 507–517.
Mandel, C. R., Gebauer, D., Zhang, H., & Tong, L. (2006). A serendipitous discovery that in situ proteolysis is essential for the crystallization of yeast CPSF-100 (Ydh1p). Acta Crystallographica Section F: Structural Biology and Crystallization Communications, 62(10), 1041-1045.
Nabila M. I., and Kannabiran K., (2018). Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatalysis and Agricultural Biotechnology. Vol. 15: Pages 56-62.
Narayanan K. B., and Sakthivel N., (2010) Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. Vol. 156: Pages 1–13.
Netala V. R., Bethu M. S., Pushpalatha B., Baki V. B., Aishwarya S., Rao J. V., and Tartte V., (2016). Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. International journal of nanomedicine. Vol. 11: Pages 56-83.
Perez-Espitia P.J., Ferreira-Soares N. F., Dos Reis Coimbra J. S., De Andrade N. J., Cruz, R. S., and Medeiros, E. A. A., (2012) Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. Vol. 5: Pages 1447–1464.
Philip, D., (2009) Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A. Vol. 73: Pages 374–381.
Pissuwan D., Cortie C. H. Valenzuela S. M., and Cortie M. B., (2009) Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol. Vol. 28: Pages 207–213.
Rajasulochana P., Dhamotharan R., Murugakoothan P., Murugesan S., and Krishnamoorthy P., (2010) Biosynthesis and characterization of gold nanoparticles using the alga Kappaphycus alvarezii. Int. J. Nanosci. Vol. 9: Pages 511–516.
Ramaratnam K., Iyer S. K., Kinnan M. K., Chumanov G., Brown P. J., and Luzinov I., (2008) Ultra hydrophobic textiles using nanoparticles: lotus approach. J Eng Fibers Fabrics. Vol. 3: Pages 1–14.
Reese R. N., and Winge D. R., (1998) Sulfide stabilization of the cadmium--glutamyl peptide complex of Schizosaccharomyces pombe. J. Biol. Chem. Vol. 263: Pages 12832–12835.
Sastry M., Ahmad A., Khan M. I., and Kumar R., (2004) Microbial nanoparticle production. Nanotechnology, (Wiley-VCH, 2004).
Selvam K., (2017) Eco-friendly biosynthesis and characterization of silver nanoparticles using Tinospora cordifolia (Thunb.) Miers and evaluate its antibacterial, antioxidant potential, J.Radiat. Res. Appl. Sci. Vol. 10: Pages 6–12.
Senapati S., Syed A., Moeez S., Kumar A., and Ahmad, A. (2012) Intracellular synthesis of gold nanoparticles using alga Tetraselmis ochinensis. Mater. Lett. Vol. 79: Pages 116–118.
Shafaghat A., (2015) Synthesis and Characterization of Silver Nanoparticles by Phytosynthesis Method and Their Biological Activity, Synth. React. Inorganic, Met. Nano-Metal Chem. Vol. 45(3): Pages 381–387.
Shankar S. S., Ahmad A., Pasricha R., and Sastry M., (2003) Bio reduction of chloroaurate ions by Geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. Vol.13: Pages 1822–1826.
Sneha K., Sathish K. M., Mao J., Kwak I. S., and Yun Y. S., (2010) Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem. Eng. J. Vol. 162: Pages 989–996.
Stephen J. R., and Macnaughton S. J., (1999) Developments in terrestrial bacterial remediation of metals. Curr. Opin. Biotechnol. Vol. 10: Pages 230–233.
Sunkar S., and Nachiyar C. V., (2014) Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymu. Asian Pac. J. Trop. Biomed. Vol. 12: Pages 953–959.
Tollamadugu N. V. K. V., Prasad T., Kambala V. S. R., and Naidu R., (2011) A critical review on biogenic silver nanoparticles and their antimicrobial activity. Curr. Nanosci. Vol. 7: Pages 531–544.
Vigneshwaran N., Ashtaputre N. M., Varadarajan P.V., Nachane R.P., Paralikar K.M., and Balasubramanya R. H., (2007) Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. Vol. 61: Pages 1413–1418.
Xu C., Guo Y., Qiao L., Ma L., Cheng Y., and Roman, A., (2018). Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by Enterotoxigenic E. coli K88. Frontiers in microbiology. Vol. 9: Pages 11-29.


