1School of Biological Engineering and Life Sciences, Shobhit Institute of Engineering & Technology, Deemed-to-be-University, Meerut, Uttar Pradesh, India
2Associate Professor & Head (CIRD), Dr. B. Lal Institute of Biotechnology, Jaipur, Rajasthan, India
3 Vice Chancellor, Shobhit Institute of Engineering & Technology, Deemed-to-be-University, Modipuram, Meerut, Uttar Pradesh, India.
Corresponding author email: vicechancellor@shobhituniversity.ac.in
Article Publishing History
Received: 19/11/2020
Accepted After Revision: 23/03/2021
Lactic acid bacteria (LAB) play an important role in digestion of food material in the gut. Recent researches reveal that the signals from gut are sent to the brain which controls all body functions. LAB acts as probiotics and plays significant role in health of man and animals. LAB found in human and animal milk influence the health of its consumers and the taste of milk also depends upon the type of LAB found in the milk. Deficiency of probiotics is very commonly reported in the people of developing nations. Demand of naturally occurring good probiotic strains with ideal characteristics meeting the eligibility criteria framed by WHO is the necessity of time. Therefore, In vitro study on biochemical characterization of Lactic Acid Bacteria (LAB) was performed using 12 different camel milk samples collected from the Bagru village of Jaipur district (Rajasthan).
The isolates were initially confirmed to be LAB using biochemical tests – catalase, oxidase, Gram’s staining, MR-VP and Sugar Fermentation tests. The isolates were also checked for their probiotic potential by examining their growth at low pH, different temperatures and different bile salt concentrations. The isolates which gave satisfactory results were further examined for their resistance against antibiotics as well as their antimicrobial activity against common human pathogens. In view of high rise in demand of good probiotic supplements throughout the world, there is a need of suitable probiotic local strains. Five best species of LAB isolated from camel milk were characterized phenotypically and were evaluated for their probiotic potentials. The present study was carried out with the aim of phenotypic characterization of Lactic Acid Bacteria from camel milk and to evaluate their probiotic potential.
Camel Milk, Probiotics, Lactic Acid Bacteria, Antimicrobial activity.
Hirani K. J, Shrivastava S. K, Garg A. P. Biochemical Characterization and Probiotic Potential of Lactic Acid Bacteria Isolated from Camel Milk. Biosc.Biotech.Res.Comm. 2021;14(1).
Hirani K. J, Shrivastava S. K, Garg A. P. Biochemical Characterization and Probiotic Potential of Lactic Acid Bacteria Isolated from Camel Milk. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3pOa4wQ”>https://bit.ly/3pOa4wQ</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
As per FAO report, the population of camels at global stage is estimated to be around 26.99 million which is spread over 47 different countries. It is also reported that about 83% of camel population is mainly found in Northern as well as Eastern part of Africa whereas the rest of the population inhabits in Middle East Asia and Indian subcontinent. Across the globe, Somalia is the only country having 7.10 million camels which is the highest amongst all. India ranks 10th in the world with a population of 0.38 million camels (FAOSTAT, 2015). Camels in India are mainly found in the states of Uttar Pradesh (2.0%), Bihar (2.2%), Haryana (4.7%), Gujarat (7.6%) and Rajasthan leading with 81.4% (DAHDF,2014). Camel is considered to be the ship of desert since years and the milk laid by camel is believed to be the white gold of desert. Camel milk has wide range of health benefits and believed to be a good source of probiotics (Seifu et al., 2012). Camel milk is reported to contain both Gram-positive as well as Gram-negative bacteria (Kumar et al., 2016). Past studies have mainly focused on the physiological adaptations, anatomic characteristics and bio-molecules present in camel milk (Benmechernene et al., 2014).
Large numbers of studies in the past have focused on the microbiology of cow, buffalo, goat and sheep milk, however, very little scientific information on the microbiology of camel milk is available till date. Very few researchers have tried to focus on the microbial population of camel milk and to find the differences in the comparison to milk of other animals (Fguiri et al., 2017). Camel itself is a unique animal having the ability of surviving in both the extreme heat as well as cold temperatures which may produce significant differences in microbial composition as well as its biological characteristics of milk (Vimont et al., 2017). Isolation, characterization and applications of Lactic acid bacteria in human colostrums, and from cow and buffalo milk have previously been studied by our group (Bisht 2019, 2020; Arya et al., 2020).Large number of lactic acid bacteria (LAB) found in raw camel milk have been proven to be of great technical relevance in diary industry. Among all LAB, species of Lactococcus have shown to be the best starter culture for cheese manufacturing as well as in production of several flavor compounds (Ruggirello et al., 2016). Lactobacillus is another important genus of LAB which is very commonly found in all raw milk and has wide range of dairy applications.
These have the ability of producing various aroma compounds and have been proven to be highly probiotic which contributes to the quality and nutrition of dairy products (Stefanovic et al., 2018). Besides these two, there are several other genera of LAB such as Streptococci, Bifidobacterium, Aerococcus, Pediococcus and Enterococcus are used in dairy. Camel milk is also known for health promoting effects such as aiding digestion, reducing the risk of asthma, atopic diseases and several other allergies (Zibaee et al., 2015). Camel milk has evidently proven to be a safer consumption even after storage of several days without chilling it in refrigerator. That could be only possible if biological active bacteria produce antimicrobials such as bacteriocins, antifungal agents and other organic acids (Kumari et al., 2008; Omer et al., 2009). The production of antimicrobials by microbial species may act as bio-preservative agents which could increase the shelf life of camel milk. This brought us great interest for carrying out such a study on camel milk. The entire study will highlight the biochemical characteristics of LAB which were isolated from camel milk.
MATERIAL AND METHODS
Sample collection: All the camel milk samples were collected from the Bagru village of Jaipur District (Rajasthan, India). Nipples of camel were washed with sterile distilled water and were carefully cleaned with cotton dipped in alcohol. The tubes used for sample collection were autoclaved using standard procedure before collection. The first 5 mL of milk sample was discarded to avoid contamination from skin flora. The mid flow of milk was carefully aseptically collected in the sterile tubes and these were sealed capped immediately. About 10 to 15 mL of milk sample was collected from each camel. The data regarding the diet, age, habitat and its other physiological activities were recorded by consulting the owner of camels.
The samples were immediately brought to the laboratory and processed as described earlier (Bisht 2019; Arya 2020).Isolation of Probiotic Bacteria: MRS (de Man Rogosa and Sharpe agar) [Hi-Media] was used for isolating probiotic bacteria from camel milk. The milk samples were serially diluted up to 10-6in sterile peptone water and 0.1mL of inoculum from each dilution was aseptically inoculated on MRS agar plates using spread plate technique. The inoculated plates were placed in inverted position in desiccators using standard protocol and were incubated at 37°C for 48h in incubator (Bisht 2019; Arya 2020). Characterization of Probiotic Bacteria: The colony characteristics (size, shape, texture, opacity, pigmentation, margins and color) of isolated bacteria were carefully noted down. The bacterial colonies were counted using digital colony counter and CFU/mL was calculated using standard protocol. The distinct colonies were further sub-cultured on MRS agar plates to obtain pure cultures for further studies. Gram’s staining: A single pure colony was picked from the surface of plate and was gently mixed with a single drop of sterile distilled water on the surface of cleaned glass slide to prepare a smear which was further heat fixed carefully using Bunsen burner.
The standard protocol of gram’s staining was performed and the slide was observed under oil immersion lens of microscope. All the bacteria which were Gram positive in nature were further tested for other biochemical activities (Hammes et al., 2009). Catalase test: Catalase is an enzyme which break down hydrogen peroxide (H2O2) into water and oxygen gas is liberated. On basis of this principle, all isolates were examined for their catalase activity. A drop of 3% hydrogen peroxide was gently mixed with a single distinct colony on the surface of clean glass slide, and the production of bubbles was indicator of catalase activity (Kumar and Kumar, 2015). The bacteria which did not show catalase activity were further screened for its oxidase activity.
Oxidase activity: Cytochrome c oxidase is a type of enzyme which is found in bacteria as an electron transport chain. Presence of this enzyme oxidizes a reagent called tetramethylphenilindiamine and gives indophenols as an end product in blue color. All catalase negative isolates were examined for their oxidase activity and all oxidase negative were further tested for their ability to ferment different sugars (Kumari et al., 2008; Bisht and Garg, 2019). Carbohydrate test: Bacteria ferment carbohydrates to form acid and gas or only acid as their end product. Different bacteria ferment different sugars or their combination which greatly help in the biochemical characterization of various species as described in Bergey’s Manual of Systematic Biology, 9th edition.
For this test, different sugars were prepared such as Glucose, Galactose, Maltose, Mannitol, Fructose, Sucrose, Xylose, Arabinose, Cellulose and Lactose using standard protocol [Hi-Media].Durham’s tube was added to each tube of sugar to which 0.1mL of pure overnight grown culture was inoculated and the tubes were allowed to incubate at 37°C for 48h. Results were recorded after incubation and were compared with Bergey’s Manual for identification (Bisht and Garg, 2019).
Assessment of probiotic activity: For determining the probiotic activities of isolated bacteria, all the isolates were tested for their survival at low pH, their growth at different temperatures, tolerance again different bile salt concentrations, antimicrobial activity and their ability to resist against different common antibiotics (Bisht 2019; Kang et al.,2019). Survival at low pH: It is believed that the food eaten by us stays in our stomach for at least 3 h and according to the literature the pH of human stomach is found in between 2-3 in healthy human (Arya et al., 2020). Therefore, all the isolates of this study were checked for their growth at acidic pH values. For this test, desired MRS broth was prepared of different pH (1, 2, 3, 4, 5 and 6) by adding 0.1N HCL to which 0.1mL of overnight grown culture was added aseptically separately in each tube prepared at different pH. These were incubated at 37°c for 3 h on shaking incubator. After incubation the absorbance was measured at 620 nm. The initial absorbance was measured at 0h. The survival rate of isolates was measured by plotting graph using standard protocol (Powthong and Suntornthiticharoen, 2015). Tolerance against different bile salt concentrations: To check the tolerance of isolates against different bile salt concentrations, desired MRS broth was prepared with concentration (0.3%) by adding Ox-bile.
Overnight grown culture of isolates was inoculated to MRS broth prepared of 0.3% bile salt concentration and was incubated at 37°c for 6 h in shaking incubator. The absorbance (620 nm) of broth was measured at the regular interval of 1 hour to measure the growth curve of isolates (Goswami et al., 2017; Bisht and Garg, 2019). Growth at different temperatures: All the isolates which were able to tolerate bile salt concentration, were further examined for the growth at different temperatures. The pure culture of isolates was inoculated on MRS agar plate and were incubated at different temperatures (10°,20°,30°,37°,45°and 50°) in incubator (Bisht 2019; Kang et al., 2019).
Antibiotic susceptibility test: The isolates which showed good probiotic potentials were checked for their resistance against common antibiotics using Kirby Buayer’s method. The overnight grown cultures were inoculated on Muller Hilton (MH) agar plates using spread plate technique. Wafers of different antibiotics (Penicillin G, Amoxicillin, Ciprofloxacin, Trimethoprime, Gentamycin, Erythromycin, Streptomycin and Tobramycin) of different concentrations were placed on the surface of agar and were gently pressed (Abdullah and Osman, 2010; Bisen et al., 2013; Bisht and Garg, 2019; Kang et al., 2019). The plates were allowed to incubate in upright condition at 37°C for 24-48h.
The zones of inhibition were observed and measured with scale after incubation. Antimicrobial activity against pathogens: The isolates which were found to be resistant against antibiotics were further tested for their antimicrobial activity against human pathogens. The pathogens used in the study were Escherichia coli (ATCC-35218), Staphylococcus aureus (ATCC-25923), Salmonella typhi (MTCC-733), Pseudomonas aeruginosa (ATCC-27853) and Proteus vulgaris (ATCC-33420) using Agar well diffusion method. The overnight grown cultures of pathogen were inoculated on MH plates using spread plate technique (Batdroj et al.,2006; Gaspar et al., 2018; Bisht 2019). A sterile cork borer of (diameter 6 mm) was used to puncture the agar surface to prepare wells. The overnight grown liquid cultures were filled in the wells using micropipette (Putra et al., 2017). The plates were allowed to incubate in upright position at 37°C for 24-48h. The zones of inhibition were observed and measured using scale (Bisht 2019; Kang et al., 2019).
RESULTS AND DISCUSSION
A total number of 12 camel milk samples were studied for the isolation and characterization of LAB and for the assessment of their probiotic potential. In the present study, 23 different species of LAB were isolated which were primarily identified on the basis of biochemical characterization as per description given in Bergey’s Manual of Systematic Bacteriology, 9th edition. All the isolates of the present study showed positive growth on MRS agar plates and were Gram’s positive but catalase and oxidase negative in nature (Table 1). On examination of their probiotic potential, 5 best isolates were screened on the basis of their ability to grow at low pH, bile salt tolerance, growth at different temperatures, their antibiotic and antimicrobial activities against human pathogens. The study found that camel milk can be one of the good sources of quality LAB. Through, this study out of 23 isolates of LAB, 12 possessed the ability to survive at pH 2 which constitute about 52.17%.
The ability of isolates to tolerate bile salt was found to be 39.13% which comes to 9 isolates. Seven of nine isolates had the potential to survive at both higher as well as lower temperatures which comes to about 30.43%. Seven isolates were checked for the resistance out of which 5 best were chosen for testing their antimicrobial activities against human pathogens. All the 5 best isolates were precisely identified on the basis of their biochemical characterization using Bergey’s manual of Systematic Bacteriology (Olmo et al. 2020).
Table 1. Some characteristics of presumptive LAB isolated from camel’s milk.
| Characteristic of isolates | RR | SP76 | LB005 | SP53 | DW2 |
| Gram stain | + | + | + | + | + |
| Morphology | Cocci | Bacilli | Cocci | Bacilli | Cocci |
| Presence of spore | – | – | – | – | – |
| Catalase test | – | – | – | – | – |
| Oxidase test | – | – | – | – | – |
| Indole test | – | – | – | – | – |
| MR | + | + | + | + | + |
| VP | – | – | – | – | – |
| Gas from glucose | – | – | – | – | – |
| Citrate utilization | – | – | – | – | – |
| Gelatin hydrolysis | – | + | – | – | – |
| Starch hydrolysis | – | – | – | – | – |
‘+’: Growth and ‘-’: No Growth
Antibiotic susceptibility pattern of LAB: Antibiotic susceptibility pattern of selected LAB isolates was observed using Kirby-Bauer disc diffusion method. The results are shown in Table 2. Isolate RR was sensitive to Trimethoprime (12mm), Streptomycin (20mm), Ciprofloxacin (22mm) but was resistant to Erythromycin, Penicillin-G, Gentamycin, Amoxicillin, Tobramycin. Isolate SP76 was only resistant to Penicillin-G, Ciprofloxacin but sensitive to Erythromycin (14mm), Trimethoprime (18mm), Gentamycin (20mm), Streptomycin (24mm), Amoxicillin (18mm), Tobramycin (25mm). Isolate LB005 was sensitive to Trimethoprime (20mm), Penicillin-G (16mm), Gentamycin (20mm), Amoxicillin (27mm), Ciprofloxacin (14mm) but resistant to Erythromycin.
Streptomycin, Tobramycin. Isolate SP53 was sensitive to Erythromycin (19mm), Penicillin-G (18mm), Gentamycin (14mm), Streptomycin (20mm), Ciprofloxacin (12mm) but resistant to Trimethoprime, Amoxicillin, Tobramycin. Isolate DW2 was resistant to Gentamycin, Ciprofloxacin but sensitive to Erythromycin (10mm), Trimethoprime (15mm), Penicillin-G (20mm), Streptomycin (16mm), Amoxicillin (15mm), Tobramycin (20mm) (Fig I and Fig II). Such resistance to a wide spectrum of antibiotics indicated that if isolated probiotics induced in patients treated with antibiotic therapy may be helpful in faster recovery of the patients due to rapid establishment of desirable microbial flora.
Resistance of the probiotic strains to some antibiotics could be used for both preventive and therapeutic purposes in controlling intestinal infections (EI-Naggar, 2004). Gad et al. (2014) isolated 244 LAB strains from dairy and pharmaceutical products and tested their antibiotic resistance against vancomycin, tetracycline, erythromycin and clindamycin and found that most LAB were within the normal range of susceptibility and 16 strains of Lactobacillus, 8 of Lactococcus and Streptococcus were resistant against tetracycline and/or erythromycin. PCR analysis showed that some strains harbor resistant genes. The antibiotic resistance of isolated LAB was assessed using antibiotic discs [Hi media] on MH agar plates against Erythromycin (5μg), Trimethoprime (30μg), Penicillin-G (1U), Gentamycin (120μg), Streptomycin (300μg), Amoxicillin (10μg), Tobramycin (30μg) and Ciprofloxacin (5μg) (EI-Naggar, 2004).
Table 2. Antibiotic Susceptibility test of Isolates (zone of inhibition mm diameter).
| Antibiotics | Symbol | 𝜇g/disc | Isolates | ||||
| RR | SP76 | LB005 | SP53 | DW2 | |||
| Erythromycin | E | 5 | R | 14 | R | 19 | 10 |
| Trimethoprime | TR | 30 | 12 | 18 | 20 | R | 15 |
| Penicillin-G | P | 1 | R | R | 16 | 18 | 20 |
| Gentamycin | HLG | 120 | R | 20 | 20 | 14 | R |
| Streptomycin | HLS | 300 | 20 | 24 | R | 20 | 16 |
| Amoxicillin | AMX | 10 | R | 18 | 27 | R | 15 |
| Tobramycin | CAZ | 30 | R | 25 | R | R | 20 |
| Ciprofloxacin | CIP | 5 | 22 | R | 14 | 12 | R |
Figure 1: Antibiotic Susceptibility
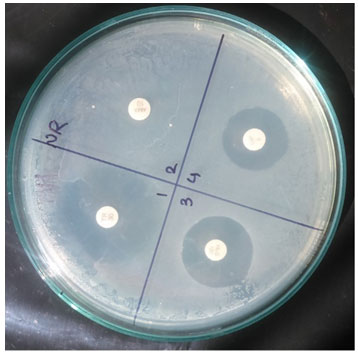
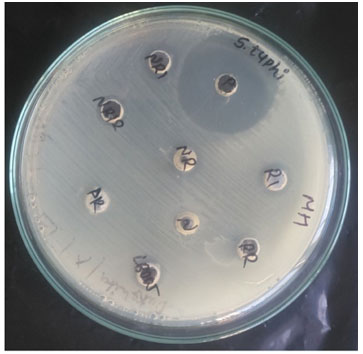
Figure 2: Antimicrobial Activity
Probiotic activity of selected isolates: A variety of acid levels has been found in different regions of gastrointestinal tract. Stomach and the other regions of gastrointestinal tract have the highest acidity and these areas may fall to as low as pH 2 – 3. In order to be used as beneficial effect, LAB must be able to survive under these harsh conditions and colonies in the gut. In present research, the selected LAB isolates were able to grow in pH 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0. The survival rate of RR LB005 was maximum at pH 1-6 and showed the highest viability and showed moderate growth even at pH 2 (Fig I) (Powthong and Suntornthiticharoen, 2015).
In general, the survival rate of three cultures RR, SP76 and DW2 during 3 h of incubation increased at all pH conditions. In present study, all the selected LAB isolates were able to survive at temperature10, 20, 30, 40 and 45°C. All the results are shown in table 3. Adamberg et al. (2003) have also evaluated the growth of LAB at various pH and temperature. Similar findings were shown by Powthong and Suntornthiticharoen, (2015). The change of pH and temperature is an effective method for determination of technological characteristics and comparative physiological study of LAB.
The temperature is an important factor which can dramatically affect the bacterial growth. The reason for choosing this temperature range was to detect whether the isolated cultures were able to grow within range of normal body temperature or not. As if the isolates were not able to survive within the selected temperature range then they would not have been able to survive in the human gut, which is an essential factor of probiotics to show their effectiveness. The results obtained were positive for growth at chosen temperature range (Powthong and Suntornthiticharoen, 2015).
Adamberg et al. (2003) have studied the effect of pH and temperature on selected LABs and found that these factors influence the growth of most lactic acid bacteria. Mu and Ohegbu (2018) correlated the effect of pH and temperature with bacteriocin production by LAB. Somashekaraiah et al. (2019) have also evaluated the probiotic activities 75 strains isolated from naturally fermented drink of coconut and found that 16 showed high probiotic activities in terms of antimicrobial and antibiotic resistance and concluded that they have good potential in functional fermented foods as bio-preservatives. Olmo et al.
(2020) have reported that storage of foods with LAB at lower temperature increases the shelf life of the food stuff while at room temperature, it is not so effective. Roger et al. (2015) has reported that LAB inhibit the growth of Aspergillus fumigatus and also reduces the production of aflatoxins. It suggests that addition of LABs in the food can act as good bio-preservative agents and can increase the shelf life and can protect against food spoilage microbes. Our results show that the strains showing high antibiotic resistance have great potential to be used as probiotic (Roger et al. 2015; Somashekaraiah et al. 2019; Olmo et al. 2020).
Figure 3
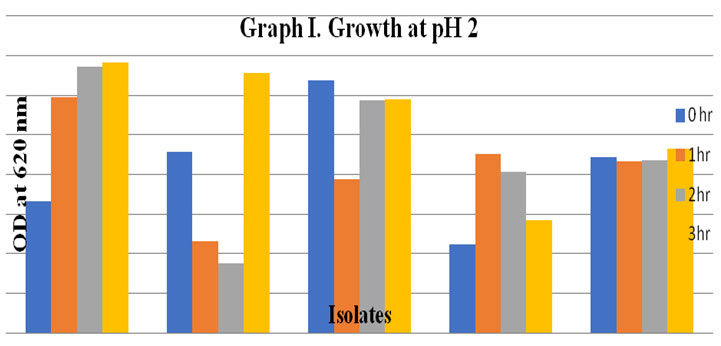
Table 3. Probiotic activity of Isolates in term of growth at different temperatures, pH and bile salt tolerance
| Isolates | Bile salt (%) | Growth at different temperature (°C) | Growth at different pH | |||||||
| 0.3 | 10 | 20 | 40 | 45 | 1 | 2 | 3 | 4 | 6 | |
| RR | + | + | + | + | + | + | + | + | + | + |
| SP76 | + | + | + | + | + | – | + | + | + | + |
| LB005 | + | + | + | + | + | + | + | + | + | + |
| SP53 | + | + | + | + | + | – | – | – | + | + |
| DW2 | + | + | + | + | + | – | + | + | + | + |
‘+’: Growth and ‘-’: No Growth
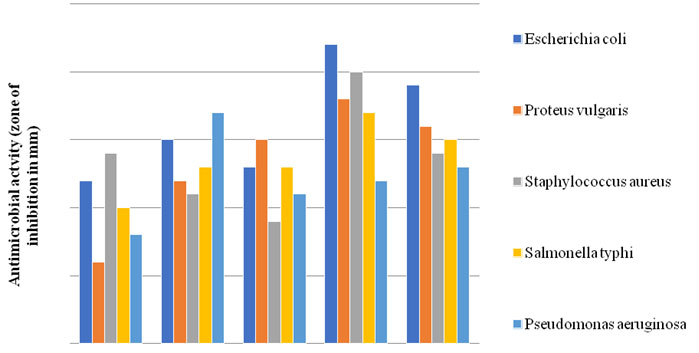
Antimicrobial Activity of Isolates: 5 isolates exhibited inhibitory activity against several pathogenic bacteria, including Escherichia coli (ATCC-35218), Proteus vulgaris (ATCC-33420), Staphylococcus aureus (ATCC-25923), Salmonella typhi (MTCC-733) and Pseudomonas aeruginosa (ATCC-27853) (fig III).
Figure 4: Antimicrobial activity of 5 isolates against pathogenic bacteria
CONCLUSION
Camel milk was found to be the good source of potential probiotic LAB. Large varieties of LAB were isolated in the present study. It was also found that few species of LAB are very difficult to sub-culture. On the basis of biochemical characterization, five good species of LAB with good probiotic potentials were identified. Further genomic studies are still required to be carried out for molecular characterization of these isolated species.
LAB’s have wide range of applications on human health and great potential to be used bio-preservatives. Therefore, further in vivo studies are required to be carried out for clear justification. The probiotic strains available and sold in the market have some or the other limitation with limited applications. A good strain of probiotic can be searched with wide range of applications which can solve the problem of deficiency of probiotics in humans. Even a mixture of two or more probiotic strains should be standardized and commercialized for human consumption.
ACKNOWLEDGEMENTS
The entire team is greatly thankful to the people of Bagru (Jaipur) for their contribution of camel in our current study. We are also thankful to the entire staff members of Dr. B. Lal Institute of Biotechnology for their support and help rendered to our study.
Conflict of Interest: None
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of Deemed-to-be-University, Meerut, Uttar Pradesh, India.
REFERENCES
Adamberg K, Signe Kask S, Laht TM and Paalme T (2003). The effect of temperature and pH on the growth of lactic acid bacteria: a pH-auxostat study. International Journal of Food Microbiology 85.171–183. doi:10.1016/S0168-1605(02)00537-8.
Araya M, Morelli L, Reid G, Sanders ME and Stanton C (2002). Guidelines for the evaluation of probiotics in foods. FAO/WHO report. http://www.who.int/foodsafety/fs_management/en/probiotic_ guidelines.pdf.
Arya R, Singh J and Garg A (2020). Isolation, Characterization and Evaluation of probiotic potentials of Lactic Acid Bacteria isolated from human colostrum Biosc.Biotech.Res.Comm…13(1):296-310.
Benmechernene Z, Fernández-No I, Quintela-Baluja M, Böhme K, Kihal M, and Calo-Mata P, (2014). Genomic and proteomic characterization of bacteriocin-producing Leuconostoc mesenteroides strains isolated from raw camel milk in two southwest Algerian arid zones. BioMed Research International :1-10. DOI: 10.1155/2014/853238.
Bisen SP, Sharma R, Sanodiya SB, Thakur SG, Jaiswal P, Pal S and Sharma A (2013). Characterization of Lactic acid bacteria from raw milk samples of goat, sheep, camel and buffalo with special elucidation to lactic acid production. Brit Micro Res J. 3(4):743-752.
Bisht N and Garg AP (2019). Isolation, Characterization and Probiotic Value of Lactic Acid Bacteria from Milk and Milk Products. Biotech Today. 9(2).54-63. 10.5958/2322-0996.2019.00022.X.
Bisht N and Garg AP (2019). Antagonistic Activity of Lactic Acid Bacteria against Common Enteric Pathogens Isolated from Milk and Milk Products and Evaluation of their Probiotic Attributes. Biosc.Biotech.Res.Comm.. 12 (4). http://dx.doi.org/10.21786/bbrc/12.4/42.
DAHDF (2014). Livestock census of India 19th edition. All India report, published by: Ministry of agriculture department of animal husbandry, dairying and fisheries, Krishi Bhawan, New Delhi, PP-26. http://dahd.nic.in/dahd/WriteReadData/Livestock.pdf.
Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blümer N, and von Mutius E, (2007). Acinetobacter lwoffiiand and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy protective properties. The Journal of Allergy and Clinical Immunology 119:1514-1521. DOI: 10.1016/j. jaci.2007.03.023.
FAOSTAT, (2015) Animal Production Yearbook, Food & Agricultural Organization, Rome, Italy. http://faostat3.fao.org/download/Q/QA/E (Accessed 31 July 2015)
Gad, Gamal Fadl M., Ahmed M. Abdel-Hamid and Zeinab Shawky H. Farag (2014). Antibiotic resistance in lactic acid bacteria isolated from some pharmaceutical and dairy products. Brazilian Journal of Microbiology 45(1):25-33. DOI10.1590/s1517-83822014000100005.
Gao ML, Hou HM, Teng XX, Zhu YL, Hao HS, and Zhang GL (2017). Microbial diversity in raw milk and traditional fermented dairy products (Hurood cheese and Jueke) from Inner Mongolia, China. Genetics and Molecular Research 16:16019451. DOI: 10.4238/gmr16019451.
Gaspar C, Donders GG, Oliveira De PR, Queiroz AJ, Tomaz C, Oliveira De MJ and Oliveira De PA (2018). Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Expr.8:153.
Goswami G, Bora S Tomas, Parveen A, Boro C R and Barooah M (2017). Identification and functional properties of dominant lactic acid bacteria isolated from Kahudi, a traditional rapeseed fermented food product of Assam, India. J. Ethn Foods.4, 187-197.
Kang HC, Han HS, Kim SJ, Kim GY, Jeong Y, Park M H and Paek SN (2019). Inhibition of Nitric Oxide Production, Oxidative Stress Prevention, and Probiotic Activity of Lactic Acid Bacteria Isolated from the Human Vagina and Fermented Food. Microorg.7,109. doi:10.3390/microorganisms7040109.
Kumar A and Kumar D (2015). Characterization of Lactobacillus isolated from dairy samples for probiotic properties. Anaerobe. 33: 117-123. https://doi.org/10.1016/j.anaerobe.2015.03.004.
Kumar D, Chatli MK, Raghvendar S, Mehta N, and Kumar P (2016a). Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Rumin Res 139:20–25.
Kumar D, Chatli MK, Raghvendar S, Mehta N, and Kumar P (2016b) Effects of incorporation of camel milk casein hydrolysate on quality, oxidative and microbial stability of goat meat emulsion during refrigerated (4 ± 1_C) storage. Small Rumin Res 144:149–157.
Kumari A, Garg AP, Makeen K and Lal M (2008). A bacteriocin production on soya nutri nuggets extract medium by lactococcus lactis subsp. Lactis CCSUB202. Int. J. dairy Sci. 3(1).49-54. DOI: 10.3923/ijds.2008.49.54.
Mu U and Ohaegbu CG (2018). Influence of Physical Parameters on Growth and Bacteriocin Activity by Species of Lactic Acid Bacteria Isolated from Fermenting Foods. J BiochemMicrobToxicol2: 104.
Olmo C M, Oneca M 1, Torre P 2, Díaz V J, Encio J I, Barajas M and Araña M (2020). Influence of Storage Temperature and Packaging on Bacteria and Yeast Viability in a Plant-Based Fermented Food. Foods 9, 302. doi:10.3390/foods9030302.
Omer RH, and Eltinay AH (2009). Changes in chemical composition of camel’s raw milk during storage. Pakistan Journal of Nutrition 8:607-610.
Powthong P and Suntornthiticharoen P (2015). Isolation, identification and analysis of probiotic properties of Lactic acid bacteria from selective various traditional thai fermented food and kefir. Pak. J. Nutr. 14(2):67-74.
Quigley L, O’Sullivan O, Stanton C, Beresford TP, Ross RP, and Fitzgerald GF (2013). The complex microbiota of raw milk. FEMS Microbiology Reviews.37:664-698. DOI: 10.1111/1574-6976.12030.
Roger T, Léopold TN and Carl M (2015). Effect of Selected Lactic Acid Bacteria on Growth of Aspergillus flavus and Aflatoxin B1 Production in Kutukutu. Journal of Microbiology Research. 5(3): 84-94.
Ruggirello M, Cocolin L, and Dolci P (2016). Fate of Lactococcus lactis starter cultures during late ripening in cheese models. Food Microbiology 59:112-118. DOI:10.1016/J. FM.2016.05.001
Somashekaraiah, Rakesh, Shruthi B, Deepthi BV and Sreenivasa, MY (2019). Probiotic properties of lactic acid bacteria isolated from Neera: A naturally fermenting coconut palm nectar. Frontiers in Microbiology 10:1-11 DOI:10.3389/fmicb.2019.01382.
Stefanovic E, Kilcawley KN, Roces C, Rea MC, O’Sullivan M, and Sheehan JJ, (2018). Evaluation of the potential of Lactobacillus paracasei adjuncts for flavor compounds development and diversification in short-aged cheddar cheese. Frontiers in Microbiology 9:1506. DOI: 10.3389/ fmicb.2018.01506.
Vimont A, Fernandez B, Hammami R, Ababsa A, Daba H, and Fliss I (2017). Bacteriocin-producing Enterococcus faecium LCW 44: A high potential probiotic candidate from raw camel milk. Frontiers in Microbiology 8:865. DOI: 10.3389/ fmicb.2017.00865.
Zibaee S, Hosseini SMA-R, Yousefi M, Taghipour A, Kiani MA, and Noras MR (2015). Nutritional and therapeutic characteristics of camel milk in children: A systematic review. Electron Physician 2015;7:1523-1528. DOI: 10.19082/1523.


