1Faculty of Biology, Thai Nguyen University of Education, Thai Nguyen 250000, Viet Nam.
2Faculty of Science and Technology, Tay Bac University, Son La 360000, Viet Nam.
Corresponding author email: quannh@tnue.edu.vn
Article Publishing History
Received: 09/07/2020
Accepted After Revision: 10/09/2020
Adinandra megaphylla Hu which belongs to Adinandra genus, Theaceae family, only narrowly distributed in Vietnam. Biological activities of these plant’s secondary compounds have been left open yet. In this study, the antibacterial, antioxidant abilities and inhibiting cancer cell lines activity of leaf extract of A. megaphylla collected in Lao Cai province, Vietnam were initially investigated. Poultice extracted from leaves of Adinandra megaphylla with three solvents of ethanol, ethyl acetate and dichloromethane were quantified and determined composition of the polyphenol, flavonoid and coumarin groups. The ethanol extract, ethyl acetate extract and dichloromethane extract have been shown to inhibit the growth of Staphylococcus aureus, Bacillus subtilis, Seratia marcessens, Sarcina lutea, Lactobacillus plantarum and Escherichia coli at concentration of 200 µg mL-1. The ethyl acetate extract and dichloromethane extract have DPPH free-radical activities; the EC50 value reached 30.3 and 33.2 µg mL-1, respectively. In particular, antimicrobial and free-radical activities of the dichloromethane extract were better than ethanol and ethyl acetate extracts. Extracts from A. megaphylla showed inhibition of gastric, lung and breast cancer cell lines with values of 67.76, 77.02 and 84.46 µg mL-1, respectively. Research results show that A. megaphylla is a potential plant containing many compounds with antibacterial, antioxidant abilities and inhibiting cancer cell lines.
Adinandria Megaphylla, Antioxidant, Antibacterial, Anti-Cancer, Poultice
Nguyen L. T. N, Nguyen Q. H, Nguyen N. T. T, Xuan Vi T. T., Sy T. D, Nguyen T. T, Chu M. H. Antibacterial, Antioxidant and Anti - Cancerous Activities of Adiandra megaphylla Hu Leaf Extracts. Biosc.Biotech.Res.Comm. 2020;13(3).
Nguyen L. T. N, Nguyen Q. H, Nguyen N. T. T, Xuan Vi T. T., Sy T. D, Nguyen T. T, Chu M. H. Antibacterial, Antioxidant and Anti – Cancerous Activities of Adiandra megaphylla Hu Leaf Extracts. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/3jUCiml
Copyright © Nguyen et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Vietnam has high potential for medicinal plants, which their chemical composition and pharmacological activities of some herbaceous species have been studied in previous studies (Hung et al., 2019; Minh et al., 2010; Vu et al., 2019), but there are still many species that have not been assessed their medicinal value, including Adinandra megaphylla of the genus Adinandra, the tea family (Theaceae). In the world, there are about 85 species in the Adinandra genus distributed in Bangladesh, Cambodia, China, India, Indonesia, Southern Japan, Laos, Malaysia, Myanmar, New Guinea, Philippines, Sri Lanka, Thailand, Vietnam and African rainforests. In China, the genus Adinandra has 22 species, of which up to 17 are endemic. In “An illustrated flora of Vietnam“, Pham Hoang Ho (1999) indicated that the genus Adinandra in Vietnam contains about 11 species which scattered throughout the country (Pham Hoang Ho, 1999 Min and Bruce 2007, Hung et al 2019 Vu et al 2019).
Species of the genus Adinandra consist of secondary compounds with antibacterial, anti-inflammatory, antioxidant, anti-free radical and anti-cancer activities Gao et al., 2010; Shi and Zhou, 2011, El-Haggar and Al-Wabli, 2015). However, studies on the genus Adinandra have been mainly focused on the species Adinandra nitida. In 2008, Liu et al. isolated camellianin A from A. nitida Merr by column chromatography and determined its content by HPLC. At the same time, their study demonstrated high antioxidant capacity from flavonoids extracted by DPPH and free radical cleaning method (Liu et al., 2008). In addition, Liu et al. (2013) optimized flavonoid extraction method and obtained camellianin A from A. nitida leaves; flavonoids were also reported to have antioxidant ability at the concentration of 0.02 mg mL-1 (Liu et al., 2013).
Thus, previous studies have shown that flavonoids like epicatechin, apigenin, quercitrin, camellianin A and camellianin B to have biological and antioxidant activities. Chemical composition of A. nitida has been isolated and determined by Wang et al. (2008) using column chromatography. The structure of saponins compounds comprise 6 types, including 2alpha, 3alpha, 19alpha- trihydroxy-olean-12-en-28-oic acid-28-O-beta-D-glucopyranoside; arjunetin; sericoside; glucosyl tormentate; nigaichigoside F1 and arjunglucoside I. Among of which, 2alpha, 3alpha, 19alpha-trihydroxy-olean-12-en-28-oic acid-28-O-beta-D-glucopyranoside is a new substance. The remaining substances were first discovered in A. nitida (Wang et al., 2008).
Moreover, in the Adinandra genus, Adinandra lienii was initially studied for its geographical distribution and matK sequence to help identify this species in Lao Cai province, Vietnam. Meanwhile, there have been not any studies on chemical composition and biological activity of total extracts from Adinandra megaphylla yet. In this study, we present results of qualitative analysis of chemical composition and evaluation of antibacterial, antioxidant, anti-cancer activities of the A. megaphylla Hu extracts.
MATERIAL AND METHODS
A. megaphylla samples: A. megaphylla samples was collected from Lao Cai province, Vietnam in the 1200-1800 m altitude at 21°59’15’’N; 104°19’28’’E. A. megaphylla Hu samples (branches with leaves and flowers) were collected to determine the scientific name in laboratory. A. megaphylla Hu leaves were used for poultice extraction with ethanol, ethyl acetate and dichloromethane. The scientific name of species is determined by comparative morphological methods according to monograph including “An Illustrate Flora of Vietnam” and “Flora of China” (Figure 1).
Figure 1: Morphological characteristics of A. megaphylla Hu collected in Lao Cai province, Vietnam. Life form (A), branches with buds (B, C), flowers (D)
Bacterial strains and cancer cell lines :Bacterial strains (Bacillus subtilis, Serratia marcescens, Escherichia coli, Sarcina lutea, and Lactobacillus plantanum) were selected for the antibacterial activity assay. They were grown in liquid Luria-Bertani (LB) medium (0.5% (w/v) yeast extract, 1.0% (w/v) peptone, 1.0% (w/v) NaCl, pH 7.0) overnight at 28°C, and the diluted bacterial suspension (106 mL-1) was ready for detection. Solid LB medium contained additionally 2.0% (w/v) agar. Cancer cell lines, including the breast cancer cell line (MDA-MB-231), the stomach cancer cell line (AGS) and the lung cancer cell line (A549) were used in cytotoxicity assays.
Materials and chemicals: Yeast extract and peptone were purchased from Bio Basic Inc. (USA); Ethanol, ethyl acetate and dichloromethane were from Fluka (China); TLC silica gel 60 F254 was from Merck (Germany).
Method of sample preparation: The leaves of A. megaphylla are washed thoroughly, then cut into pieces and dried at a temperature of 50°C to constant mass. The crushed sample is extracted twice with ethanol in an ultrasonic machine at room temperature. The crude extracts were collected by solvent removal under reduced pressure conditions, at 50°C and extracted with solvents of dichloromethane and ethyl acetate. The residue of ethanol, dichloromethane and ethyl acetate were cleaned solvent and dried at 50°C to collect ethanol extract, dichloromethane extract and ethyl acetate extract, respectively.
Polyphenols were detected by reaction with iron salts (III)/sulfuric acid:Reaction with iron salts (III): 5 mL of ethanol extract were added into two tubes, denoted by I and II, respectively. The tube II was supplemented with 0.5 mL iron salts (III), shake and observe color. Depending on the number and location of hydroxyl groups in polyphenol molecules, results are green, blue or brown.
Reaction with sulfuric acid: 2 mL of ethanol extract were added into two tubes, denoted by I and II, respectively. The tube II was supplemented with 1-2 drops of H2SO4, shake and observe color. Add H2SO4 concentrate to flavones and flavonols to give a deep yellow; to chalcones and aurones to produce a red, crimson red or bright red solution; to flavanones to give orange red.
Flavonoids were detected by reaction with hydrochloric acid and magnesium powder:The tube contained 0.05g ethanol extract and 10 mL CH3OH, which were shaken, heated to dissolve and filtered through filter paper. 2 mL of filtrate was added into two tubes, denoted by I and II, then was added a pinch of magnesium powder and shaken. The tube II was supplemented with five drops of HCl and boiled for 3 mins. The solution changed to yellow, red to green colors, as it contained flavonoids. Coumarin was detected by reaction with NaOH solution: 2 mL of ethanol extract were added into two tubes, denoted by I and II. The tube II was supplemented with 0.5 mL of 10% NaOH solution. Two tubes were boiled, cooled down to room temperature and added 4 mL distilled water. The tube II is more transparent or clear than tube I, indicates the presence of coumarin. When the two test tubes were added with a few drops of HCl, the solution turned a dull yellow, so the coumarin was determined following this method, (Nguyen and Hung, 2008).
Thin layer chromatographic method (TLC): Ethanol extract from A. megaphylla leaves were detected by TLC (3.5 × 10 cm layer of silica gel 60), performed with two mobile phases of n-hecxane/acetone (1:1, v/v) and dichlomethane/n-hexane (3:1, v/v). The products were visualized by spraying the TLC plate with 10% (v/v) sulfuric acid in ethanol and incubating at 100°C until color appeared.
Determination of antibacterial activity of extracts: Antibacterial activity of extracts was performed according to the method of Mahesh and Satish (2008). In order to determine antibacterial activity of the extracts, 70 mL of diluted bacterial suspension (106 mL-1) was brushed on 0.5-cm-thick LB plates. The LB plates were perforated with 0.5-cm-diameter holes, and each hole was supplemented with 100 mL of each ethanol, ethyl acetate and dichloromethane extract from A. megaphylla Hu leaves with different concentrations (20, 60 and 200 µg mL-1) or with DMSO for the control. The inhibition activity of extracts against bacterial growth was observed after incubation at 30°C for 18-40 hours. The antibacterial levels were determined by diameter of inhibition zones (in millimeters) around the holes. The diameter of antibacterial ring was determined by the formula: H = D-d (mm). In which: D is the diameter of the antibacterial ring from the center of perforations (mm); d is the diameter of perforated agar (mm).
Determination of oxidation activity of extracts: Antioxidant activity of A. megaphylla extracts was determined by Tabart et al. (2009) using DPPH radical scavenging. 100 µL of each extract at five concentrations, including 0.5, 2, 8, 32 and 64 µg mL-1 were added with 2.9 mL of 0.1 mM DPPH solution mixed in methanol solution, shook and left in the dark for 30 min at room temperature, the absorbance was measured at 517 nm. The inhibition of DPPH radical by the samples was calculated by following formula: DPPH activity (%) = 100 × (Ac – As)/Ac, in which Ac: the absorbance of control, As: the absorbance of sample. Antioxidant activity was determined based on EC50 values (the concentration of DPPH free radical scavenging samples is 50%) (Tabart et al., 2009).
Cytotoxic assays : Cancer cytotoxicity was determined using the method of Monks et al. (1991) (Monks et al., 1991). Poultice extracted from A. megaphylla leaves was prepared and tested at four concentrations, including 100, 20, 4 and 0.8 µg mL-1. Ellipticine was used as a reference at four concentrations: 10, 2, 0.4 and 0.08 µg mL-1. Dimethyl sulfoxide (DMSO) at 10% concentration was used as a negative control. Total protein content of a cell is determined based on the optical density (OD) when proteins of the cell are stained with sulforhodamine B (SRB). The OD results were read on a wave step of 515 nm in an ELISA Plate Reader. OD values are proportional to the amount of SRB, which is attached to protein molecules. The larger the OD value, higher the amount of protein and higher the amount of cells. Cytotoxicity was expressed as the concentration of drug that inhibited cell growth by 50%. Inhibitory concentration 50% (IC50) is the concentration of the sample at which it can inhibit 50% of cells. The substance is considered to have good activity when IC50 = 5 mM (Hughes et al., 2011).
RESULTS AND DISCUSSION
Results of thin layer chromatographic analysis show that, there were 7 marks in the ethanol extract and 6 marks in the ethyl acetate extract for the n-hexane/acetone at a 1:1 ratio (Figure 2A). There were 3 marks in the ethanol extract and 6 marks in the ethyl acetate extract for the dichloromethane/n-hexane solvent system at a 3:1 ratio (Figure 2B). Thus, on the thin layer chromatographic analysis with two different solvent systems showed that the extract from A. megaphylla Hu has many bands with different colors.
Figure 2: Results of thin layer chromatographic analysis of ethanol extract (A) and ethyl acetate extract (B) in the n-hexane/acetone at a 1:1 ratio (I) and dichloromethane/n- hexane solvent system at a 3:1 ratio (II)
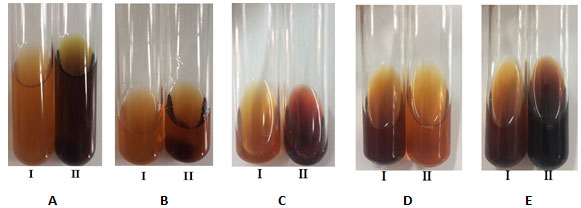
The extract of A. megaphylla was quantified polyphenols, flavonoids and coumarin by different reagents. The results were shown in Figure 3.
Figure 3: Color reactions for detection of polyphenols (A, B), flavonoids (C) and coumarin (D, E) in the ethanol extract from leaves of A. megaphylla. The ethanol extract before (I) and after (II) reacting with iron salts (III) (3A); with sulfuric acid (3B); with Mg in HCl solution (3C); with NaOH (3D); with HCl solution (3E).
Polyphenols in the ethanol extract was detected by reacting with iron salts (III), the solution in the tube II turned dark green due to reaction between polyphenols and iron salts (III). The solution in the tube II turned dark yellow (Figure 3B), if flavonoid reacted with sulfuric acid. According to flavonoids qualitative, the solution in the tube II changed color from yellow to dark red (Figure 3C). Coumarin was detected by reaction with 10% NaOH solution, the results showed in tube II is more transparent than tube I (Figure 3D). Then, when a few drops of HCl were added to both tubes, the tube II changed from dark opaque yellow to light transparent yellow color (Figure 3E). Therefore, it is clear from these results that the extract of A. megaphylla leaves contained polyphenols, flavonoids and coumarin. Previous studies have also demonstrated that leaves of Adinadra nitida contained total flavonoids, ( Gao et al., 2010 Chen et al 2017). Bioactive compounds in these plants have an important role in producing medicinal products as well as a basis for further research.
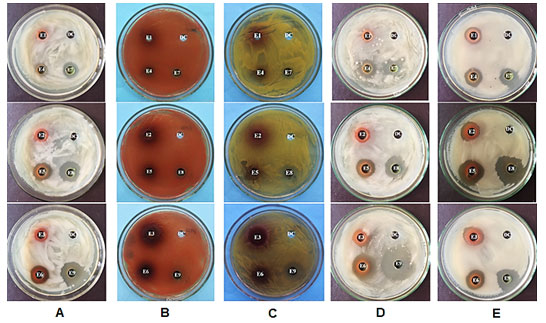
Antibacterial activity of ethanol, ethyl acetate and dichloromethane extracts from leaves of A. megaphylla Hu was tested at different concentrations using bacteria via the agar diffusion method (Table 1 and figure 4). The ethanol extract had no bactericidal effects at a concentration of 20 µg mL-1, and had low activity at 60 and 200 µg mL-1 concentrations on B. subtilis. Whereas, the ethyl acetate and dichloromethane extract showed antibacterial activity against B. subtilis at all concentrations tested, and the dichloromethane extract at 200 µg mL-1 had the strongest antibacterial activity (Figure 4A).
Table 1. Antibacterial activities of extracts from leaves of A. megaphylla Hu
| No. | Bacterial | Experimental concentrations of the extracts | ||||||||
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | ||
| 1 | B. subtilis | – | + | + | + | ++ | ++ | ++ | +++ | +++ |
| 2 | S. macescens | – | – | – | – | – | – | – | – | + |
| 3 | S. lutea | – | – | – | – | – | – | – | ++ | +++ |
| 4 | L. plantarum | + | ++ | ++ | ++ | ++ | + | +++ | +++ | +++ |
| 5 | E. coli | + | +++ | +++ | ++ | +++ | ++ | ++ | +++ | +++ |
| Note: (-) No inhibition (no antibacterial zones); (+) Weak inhibition (the diameters of inhibition zones are from 1 to 5 mm); (++) Inhibition (the diameters of inhibition zones are from 6-10 mm); (+++) Strong inhibition (the diameters of inhibition zones are >10mm).
The ethanol extract at concentration of 20 (E1); 60 (E2) and 200 (E3) µg mL-1; the ethyl acetate extract at concentration of 20 (E4); 60 (E5) and 200 (E6) µg mL-1; the dichloromethane extract at concentration of 20 (E7); 60 (E8) and 200 (E9) µg mL-1. |
||||||||||
The ethanol, ethyl acetate and dichloromethane extracts had no antibacterial activity against S. macescens at all concentrations (Figure 4B). For S. lutea bacteria, the ethanol extract, the ethyl acetate extracts at concentration of 20, 60 and 200 µg mL-1 and the dichloromethane extract at concentration of 20 µg mL-1 had no antibacterial activity. While, the dichloromethane extract at 60 and 200 µg mL-1 concentrations had strong antibacterial activity against S. lutea (Figure 4C). The ethyl acetate and dichloromethane extract had antibacterial activity against L. plantarum at all concentrations tested, and the strongest antibacterial activity was observed with the dichloromethane extract at 200 µg mL-1 (Figure 4D). The ethanol, ethyl acetate and dichloromethane extracts had antibacterial activity against E. coli at all concentrations; The strongest resistance activity was at 60 µg mL-1 for the dichloromethane extract, followed by the ethyl acetate (Figure 4E).
Antibacterial activities of extracts from leaves of A. megaphylla Hu on five bacterial strains showed that: (1) The ethanol and ethyl acetate extracts were able to inhibit
B. subtilis, L. plantarum, E. coli, and no inhibit S. lutea, S. macescens. (2) The dichloromethane extract had inhibited B. subtilis, S. marcessens, S. lutea, L. plantarum,
E. coli. (3) Antimicrobial activities of dichloromethane extract is better than the ethanol and ethyl acetate extracts.
Figure 4: Antibacterial activities against B. subtilis (A), S. marcescens (B), S. lutea (C), L. plantarum (D) and E. coli (E) of A. megaphylla Hu extracts; DC: Dimethyl sulfoxide (DMSO) served as a control.
Antioxidant activities of extracts from leaves of A. megaphylla Hu :The results showed that the DPPH free radical removal efficiency of the ethanol, dichloromethane and ethyl acetate extracts were directly proportional to the extract concentrations. The free radical removal efficiency increased from 0 to 75.7% with the increase of extract concentration from 0.5 to 128 µg mL-1. The ethyl acetate extract proved to have the strongest DPPH free radical activity with an EC50 value of 30.3 µg mL-1. The ethanol extract showed DPPH free radical activity with an EC50 value of 33.2 µg mL-1. By contrast, the DPPH free radical activity of dichloromethane extract was very weak with the EC50 value >128 µg mL-1 (Table 2).
Table 2. Antioxidant activities of extracts from leaves of A. megaphylla
| Concentrations (µg mL-1) | DPPH free radical scavenging activity (%) | ||
| Ethanol extract | Dichloromethane extract | Ethyl acetate extract | |
| 0.5 | 0 | 0 | 25.4 ± 0.54 |
| 2.0 | 0 | 0 | 28.6 ± 2.02 |
| 8.0 | 0 | 0 | 29.2 ± 4.04 |
| 32 | 48.2 ± 4.45 | 0 | 52.7 ± 5.52 |
| 128 | 71.8 ± 5.28 | 40.5 ± 0.35 | 75.7 ± 0.96 |
| EC50 | 33.2 ± 0.42 | > 128 ± 0.98 | 30.3 ± 3.26 |
Cytotoxic activities of the ethanol extract from leaves of A. megaphylla Hu against cancer cell lines : According to Gao et al. (2010), camellianin A, a flavonoid from leaves of Adinandra nitida, was determined to inhibit proliferation and apoptosis of liver cancer cells (Hep G2) and breast cancer (MCF-7) (Gao et al., 2010). Some heterocyclic compounds containing coumarin-related properties such as anti-inflammatory (El-Haggar and Al-Wabli, 2015), antibacterial (Shi and Zhou, 2011), antiviral (Tsay et al., 2014) and anti-cancer (Jacquot et al., 2007). Moreover, coumarin inhibited Hep2 cell growth and showed typical characteristics of apoptosis including the morphological changes and DNA fragmentation (Mirunalini et al., 2014).
In our study, the A. megaphylla extracts was found to contain flavonoids and coumarin compound, however it is necessary to identify whether or not they are resistant to cancer cells. Therefore, we preliminarily determined the anti-cancer activities of ethanol extract from leaves of A. megaphylla as a basis for efficient purification of flavonoid and coumarin compounds. The cytotoxic activity of ethanol extract against three cancer cell lines, including MDA-MB-231 (breast cancer cell line), AGS (stomach cancer cell line) and A549 (lung cancer cell line) was investigates in this study. The results showed that the extract of A. megaphylla has strong cytotoxic activity against MDA-MB-231, AGS and A549 with IC50 values are 84.46, 67.76 and 77.02 µg mL-1, respectively (Table 3).
Table 3. Cytotoxicity of extract from leaves of A. megaphylla against human cancer cell lines in vitro (IC50, µmol L-1)
| Concentrations (µg mL-1) |
Inhibition of human cancer cell line growth (%) | ||
| A549 | AGS | MDA-MB-231 | |
| 100 | 73.07 ± 0.66 | 84.61 ± 3.20 | 65.06 ± 0.41 |
| 20 | 5.96 ± 3.06 | 8.20 ± 2.62 | 4.64 ± 0.66 |
| 4 | 1.25 ± 1.59 | 3.44 ± 2.13 | 0.90 ± 0.08 |
| 0.8 | -2.63 ± 0.80 | -3.97 ± 0.29 | -1.66 ± 0.74 |
| IC50 | 77.02 ± 5.27 | 67.76 ± 3.31 | 84.46 ± 9.09 |
| Note: IC50 values are means from three independent experiments (average ± SD) in which each compound concentration was tested in three replicate wells. Ellipticine (as a reference compound) was the positive control and assayed at concentrations of 10, 2, 0.4 and 0.08 µg mL-1. | |||
In particular, the inhibitory effect of the extract against stomach cancer cell line is highest, followed by lung cancer cell line and the lowest is the breast cancer cell line. Thus, the extract from A. megaphylla has activity against cancer cell lines, including the breast cancer cell line, the stomach cancer cell line and the lung cancer cell line. This result is the basis for the isolation of pure compounds with anti-cancer properties.
CONCLUSION
Results of thin layer chromatographic analysis using n-hexane/acetone at a 1:1 ratio and dichloromethane/n-hexane solvent system at a 3:1 ratio had been identified to contain polyphenols, coumarin in the extracts from leaves of A. megaphylla Hu. The ethanol and ethyl acetate extracts were able to inhibit B. subtilis, L. plantarum, E. coli, but not inhibit S. lutea, S. macescens. The dichloromethane extract had inhibited B. subtilis, S. marcessens, S. lutea, L. plantarum, E. coli. DPPH free radical activity of the dichloromethane extract is strongest in comparison to the ethanol and ethyl acetate extracts, the EC50 value of dichloromethane extract is 30.3 µg mL-1. The ethanol extract from leaves of A. megaphylla Hu has activity against breast, stomach and lung cancer, the IC50 values reached to 84.46, 67.76 and 77.02 µg mL-1, respectively. Thus, the dichloromethane extract showed stronger biological activities comparing to the ethanol and ethyl acetate extracts. These research results have demonstrated that A. megaphylla contains bioactive and pharmacological compounds, which is a new potential source for isolating these compounds to produce medicine.
ACKNOWLEDGEMENTS
This research was funded by the Ministry of Education and Training under grant number B2019-TNA-08.
REFERENCES
Chen, Y., X. Ma, X. Fub, and R. Yan. (2017) Phytochemical content, cellular antioxidant activity and antiproliferative activity of Adinandra nitida tea (Shiyacha) infusion subjected to in vitro gastrointestinal digestion. RSC Advances 7:50430–50440. doi: 10.1039/c7ra07429h.
El-Haggar, R. and R.I. Al-Wabli. (2015) Anti-Inflammatory screening and molecular modeling of some novel coumarin derivatives. Molecules 20:5374-5391. doi: https://doi.org/10.3390/molecules20045374.
Gao, H., B. Liu, F. Liu, and Y. Chen. (2010) Anti-Proliferative Effect of Camellianin A in Adinandra nitida Leaves and Its Apoptotic Induction in Human Hep G2 and MCF-7 Cells. Molecules 15:3878-3886. doi: 10.3390/molecules15063878.
Hughes, J.P., S. Rees, S.B. Kalindjian, and K.L. Philpott. (2011) Principles of early drug discovery. British journal of pharmacology 162:1239-1249.
Hung, H.D., D.D. Tien, N.T. Ngoan, B.T. Duong, D.Q. Viet, P.G. Dien, and B.K. Anh. (2019) Study on chemical constituents and bioactivities of the fruits of dipterocarpus retusus of Viet Nam. Vietnam Journal of Science and Technology 57:294-299. doi: doi:10.15625/2525-2518/57/3/13249.
Jacquot, Y., I. Laïos, A. Cleeren, D. Nonclercq, L. Bermont, B. Refouvelet, K. Boubekeur, A. Xicluna, G. Leclercq, and G. Laurent. (2007) Synthesis, structure, and estrogenic activity of 4-amino-3-(2-methylbenzyl)coumarins on human breast carcinoma cells. Bioorg. Med. Chem. 15:2269-2282.
Liu, B., Y. Ma, Y. Liu, Z. Yang, and L. Zhang. (2013) Ultrasonic-Assisted Extraction and Antioxidant Activity of Flavonoids from Adinandra nitida leaves. Tropical Journal of Pharmaceutical Research 12:1045-1051.
Liu, B., Y. Zhan, Z.X. Ning, J.H. Gao, and K.Y. Xu. (2008) Characterization and Antioxidant Activity of Flavonoid Extract from Leaves of Adinandra nitida Merr. ex Li. Chemistry and Chemical Engineering 28:6-10.
Min, T.L. and B.B. Bruce. (2007) Theaceae. In: Wu Z. Y., Raven P. H. and Hong D. Y. (eds.), Flora of China. Science Press, Beijing and Missouri Botanical Garden Press, St. Louis 12:435-443.
Minh, T.T., N.T.H. Anh, V.D. Thang, and T.V. Sung. (2010) Study on Chemical Constituents and Cytotoxic Activities of Salacia chinensis Growing in Vietnam. Z. Naturforsch. 65b:1284-1288.
Mirunalini, S., K. Deepalakshmi, and J. Manimozhi. (2014) Antiproliferative effect of coumarin by modulating oxidant/antioxidant status and inducing apoptosis in Hep2 cells. Biomed. Aging Patho. 4:131-135.
Monks, A., D. Scudiero, P. Skehan, R. Shoemaker, K. Paull, D. Vistica, C. Hose, J. Langley, P. Cronise, A. Vaigro-Wolff, and M. Gray-Goodrich. (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Journal of the National Cancer Institute 83:757-766.
Nguyen, H.Q. and T.T.G. Kieu. (2019) Use of matK DNA barcode to identify Adinandra samples collected at Lao Cai, Vietnam. TNU Journal of Science and Technology 197: 205-210.
Nguyen Thai An and Bui The Hung. (2008) Study on Flavonoid and Coumarin components of drugs in dispersion method. Journal of Pharmacy 368:37-40.
Pham Hoang Ho (1999) An Illustrate Flora of Vietnam. vol. 11. Young Publishing.
Shi, Y. and C. Zhou. (2011) Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 21:956-960. doi: https://doi.org/10.1016/j.bmcl.2010.12.059.
Tabart, J., C. Kevers, Pincemail J, J.O. Defraigne, and J. Dommes. (2009) Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem 113:1226-1233.
Tsay, S.C., J.R. Hwu, R. Singha, W.C. Huang, Y. Hsiung, M.H. Chang Hsu, F.K. Shieh, C.C. Lin, K.C. Hwang, J. Horng, E. De Clercq, I. Vliegen, and J.C. Neyts. (2014) Coumarins hinged directly on benzimidazoles and their ribofuranosides to inhibit hepatitis C virus. European Journal of Medicinal Chemistry 63:290-298.
Vu, T.T.T., T.K.L. Vu, H.Q. Nguyen, V.K. Pham, T.D. Nguyen, N.T.N. Nguyen, and H.M. Chu. (2019) Cytotoxic effects of steroidal glycosides isolated from the Paris vietnamensis plant on cancer cell lines and against bacterial strains. Biotechnology & Biotechnological Equipment 33:1516–1524. doi: https://doi.org/10.1080/13102818.2019.1676168.
Wang, Y., W.C. Ye, Z.Q. Yin, and S.X. Zhao. (2008) Triterpene saponins from Adinandra nitida. Yao. Xue. Xue. Bao. 43:504-508.