
Pathological
Communication
Biosci. Biotech. Res. Comm. 11(3): 393-401 (2018)
Isolation, phenotypic and genotypic characterization
of indigenous
Beauveria bassiana
isolates from date
palm infested with
Rhynchophorus ferrugineus
in Hail
region, Saudi Arabia
Khalid A. Asiry
1
*, Abdel Moneim E. Sulieman
2
, Naimah A. Al-Anazi
2
, Vajid N. Veettil
2
,
Mohanad Abdelgadir
2
and Ibrahim Alkhregi
2
1
Department of Arid Land Agriculture, King Abdulaziz University, Jeddah, Saudi Arabia
2
Department of Biology, Faculty of Science, University of Hail, Saudi Arabia
ABSTRACT
The red palm weevil (RPW) Rhynchophorus ferrugineus overrun date palm ranches in many parts of Saudi Arabia,
henceforth causes massive economic losses. Integrated pest Management (IPM) of the RPW by utilizing Entomopath-
ogenic fungi (Beauveria bassiana), which has antagonistic activity against many insect pests, was the main objective
of the current study. In the present study, soil samples, samples of dead red palm weevils (RPW) and palm fronds were
collected according to RPW incidence map of Hail region, Saudi Arabia. Isolates of entomopathogenic fungi were
isolated from the dead RPW adults and larvae. The fungal culture (BSA1, BH-2 and BH-3) was preserved and main-
tained for further analysis. Morphological and biochemical characterization of the antagonist fungi were employed
and con rmed that the fungus belonged to Beauveria spp. Further, this fungal isolates were propagated and prepared
for genetic characterization. Sequencing of internal transcribed spacers ( ITS1 and ITS2) region was shown that three
polymorphic ITS regions. The molecular identi cation of the fungus strain was employed at King Faisal University-
the College of Agriculture and Food Science and con rmed that the fungus identi ed as Beauveria bassiana as the
rst record of this bene cial species in Hail region.
KEY WORDS: DATE PALM, RED PALM WEEVIL, FUNGUS, MORPHOLOGICAL, BIOCHEMICAL, MOLECULAR IDENTIFICATION
393
ARTICLE INFORMATION:
*Corresponding Author: Prof_1974@hotmail.com
Received 17
th
July, 2018
Accepted after revision 24
th
Sep, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.3/7
394 ISOLATION, PHENOTYPIC AND GENOTYPIC CHARACTERIZATION OF INDIGENOUS
BEAUVERIA BASSIANA
ISOLATES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Khalid A. Asiry et al.
INTRODUCTION
The red palm weevil (RPW) Rhynchophorus ferrugineus
Olivier (Coleoptera: Curculionidae), is an overwhelming
palm pest that can cause tremendous nancial losses
worldwide throughout the previous 30 years, as they are
notable to assault several species (more than 200) of palm
including the date palm (Phoenix dactylifera L.) (Mur-
phy and Briscoe, 1999; Barranco et al., 2000; Faleiro,
2006). These large economic losses in date palms could
ascribed to the way that, to-date, there are no powerful
control measures. Rhynchophorus ferrugineus is broadly
geographically distributed in Africa, Asia, Europe, Oce-
ania, and North America (EPPO, 2006; 2007a; 2007b;
2009; Azmi et al., 2017).
The entomopathogenic fungus Beauveria bassiana
(Ascomycota, Hypocreales) has demonstrated use within
insect biocontrol administrations for concealment of
numerous crop. It infects a an extensive variety of insect
pests of socioeconomic importance pests (Bing & Lewis
1991, 1992, Krueger & Roberts 1997; Mulock & Chan-
dler 2000). Recently, B. bassiana, was additionally found
to occur naturally as an endophyte in plant tissues, for
example, as leaves, twigs, wood and bark in speci c
plants like maize, cotton, wild cacao, white pine, coffee
and furthermore been set up as an endophyte arti cially
in speci c crops like, maize, cotton, tomato, opium
poppy, cacao, coffee, date palm, banana, sorghum and
jute. The entomopathogenic fungus B. bassiana isolated
from dead R. ferrugineus cadavers gave more mortality
compared to the other isolates. In the virulence bioassay
two isolates of B. bassiana shown the highest percentage
of larval and adult mortality at all exposure which rec-
ommend that they may be the most effective isolates for
sustainable insect control programs, (Yasin et al.,2017).
Endophytes have several advantages; rstly, they are
inside the plant tissues and constantly shielded from
abiotic push factors. Also, the application cost is less
because of limited application through seed treatment
or seedling dip or foliar spray. Moreover, once settled
as an endophyte, they may offer season-long protection
against the pests that have secretive life cycle by causing
encouraging discouragement or antibiosis (Vega et al.,
2008) or prompting their mortality or less pervasion. All
these properties of fungal endophytes make them rea-
sonable to be utilized as a bio-control agent to protect
crops from the pests. Insect pests such as the European
corn borer (Ostrinia nubilalis) in U.S.A., and the banana
weevil (Cosmopolites sordidus) in Uganda respectively
were effectively controlled by endophytic establishment
of B. bassiana.
Date palms are considered as the image of life in the
leave, since it endures high temperatures, and saltiness
when contrasted with numerous other fruit crop species.
One of the most established relationships that man has
had with a tree has been with date palms, which have
been developed since ancient times (Zohary and Hopf,
2012). One of the character of date palm is that it can
adapt to extreme drought, to heat, and to relatively high
levels of soil salinity. Nevertheless, extreme quantities
of salinity due to irrigation with saline water result to
a signi cant decrease in the productivity of the fruits.
It is important to study the mechanism of tolerance to
these abiotic stress in order to develop future date palm
varieties that can tolerate excessive soil salinity (Yaish
& Kumar 2015 ).
Several insects invade date palm trees, of which the
red palm weevil (RPW), R. ferrugineus (Olivier) (Coleop-
tera: Curculionidae), is a standout amongst the most
essential and harming pests, being a noteworthy risk to
date palm trees everywhere throughout the world. The
red palm weevil is ordinarily well hidden, and numerous
local pervasions have just recently been perceive, mak-
ing the red palm weevil a pest of major economic sig-
ni cance in all Persian Gulf Countries. This study aimed
to isolation and screening of antagonistic B. bassiana
from diverse soils and date palm and plantation crop
ecosystem in Hail region, Saudi Arabia.
MATERIAL AND METHODS
Soil samples, samples of infected and dead red palm
weevil (RPW) insects and date plant materials collected
according to RPW incidence map of Hail, Saudi Arabia
during the period 2016-2017. The B. bassiana (BH-2)
strain was isolated from soil sample collected from Al
Koutha village in Hail region. About 500 grams of soil
sample were placed in a plastic bag. Five larvae (L3-L4)
of the red palm weevil R. ferrugineus were added to the
soil substrate to be infected by the entomopathogenic
fungi. The plastic bag was kept for 2-3 weeks at room
temperature (20-25°C) and a ca. 80% humidity level was
ensured inside the bag by periodic water sprays. Dead
larvae were collected, placed on wet lter paper in Petri
dishes and put in an incubator at 25°C. The mycelium
and spores that emerged on larvae were transferred
directly onto growth medium (PDA – PotatoDextrose
Agar) in Petri dishes and incubated at 25°C for 10 days;
the isolates were then puri ed by repeated transplanting
(Goettel and Inglis, 1997).
The B. bassiana (BH-1, BH-2 and VBM) isolates were
obtained from naturally infected RPW adults collected
in from Jubbah village in Hail region (Fig 1). To promote
conidial growth, mycosed RPW adult cadavers were
placed separately on lter paper soaked daily with water
to achieve ca.100% RH inside Petri dishes. The petri
dishes were incubated at room temperature (20-25C°).

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ISOLATION, PHENOTYPIC AND GENOTYPIC CHARACTERIZATION OF INDIGENOUS
BEAUVERIA BASSIANA
ISOLATES 395
Khalid A. Asiry et al.
Parts of fungal propagules grown on the cadavers were
then transferred, with sterile needles, into Petri dishes
with SDAY1/4 (Sabouraud Dextrose Agar Fluka supple-
mented with yeast extract ¼of concentration) and kept
in an incubator at 25°C. Pure fungal colonies were then
stored on PDA (Potato Dextrose Agar) and MEA (Malt
Extract Agar) slants in bacteriological glass tubes at 4°C.
The B. bassiana isolates were con rmed by sequencing
analyses of the 18SrRNA gene and the internal tran-
scribed spacer (ITS1).
For microscopic identi cation of the fungus, pure fun-
gal culture was used after successive puri cation. With
the help of inoculating needle some portion of growth
of the fungus was teased and place it on the slide then,
70% ethanol was used for washing, then ethanol was
removed by blotting paper. Then, a drop of Lacto-phenol
cotton blue was kept, and the mycelium was spread with
needles, and a cover slip placed and examined under
microscope with high power objective. The morphology
and spore structures were noted.
API 20 C AUX kit was used to identify the strains
of yeast isolated from RPW samples according to the
manufacturer’s instructions. Yeast strains were puri-
ed by culturing them on PDA (Potato Dextrose Agar)
medium and incubated for 72 hours at 25°C. The strains
were puri ed twice before identi cation. After 48 and
72 hours the growth was compared in each cupule to
the 0 cupule, which is used as a negative control. A
cupule more turbid than the control indicates a posi-
tive reaction to be recorded in the result sheet. Each
strain was identi ed with the identi cation software by
manually entering the 7-digit numerical pro le via the
keyboard.
The molecular analyses for the fungal isolates were
performed at King Faisal University- the College of Agri-
culture and Food Science – Pest & Plant Disease Unit.
All isolates were re-grown on other PDA petri dishes
and inoculated on Potato dextrose broth medium for
microscopic examination (Fig 2), also for extraction of
DNA and molecular identi cation.
DNA wa s extracted using modi ed method of Del-
laporta and Hicks, 1983 as the following protocol:
Twenty mgs of frozen- dried mycelium or fresh har-
vested mycelium were ground with Kontes pestles in a
1.5 ml tube with 500 l of Dellaporta buffer (100 mMTris
pH 8. 50 mMethylenediamine-tetraacetate EDTA, 500
mMNaCl, 10 mM beta mercaptoethanol (BME). Thirty
three l of 20% sodium dodecyl sulfate (SDS, w/v) were
added, and incubate the mixture was vortexed and incu-
bated for 10 min at 65°C.160l of 5 M potassium acetate
KoAc (Sigma chemicals) were added and vortexed. The
mixture was spun for 10 min at 10,000 rpm in a micro-
centrifuge tube. 450 l of supernatant were transferred
to a new tube. 450 l phenol, chloroform and isoamyl-
alcohol (PCI) 25:24:1 were added and vortexed for 5min
and then spun for 5 min at 10,000 rpm. 400 l of the
upper phase were removed to a clean micro-centrifuge
tube and 0.5 volumes of isopropanol were added, vor-
texed and spinet for 10 min at 14,000 rpm. The superna-
tant was removed, the total nucleic acid was precipitated
in the bottom of the tube. The pellet was washed with
70% ethanol and spun 5 min at 10,000 rpm. The pellet
was resuspended in 100 l of ddH2O.
Two primer pairs, the forward IT5 primer 5’-GGAA-
GTAAAAGTCGTAACAAGG-3’) and the reverse ITS4
primer (5’-TCCTCCGCTTATTGATATGC-3’) were used to
FIGURE 1. Fungal growth of red palm weevil in Hail region.

Khalid A. Asiry et al.
396 ISOLATION, PHENOTYPIC AND GENOTYPIC CHARACTERIZATION OF INDIGENOUS
BEAUVERIA BASSIANA
ISOLATES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
amplify the entire ITS region (White et al., 1990). PCR
was done in a 25 µl reaction containing 1 µl of the fun-
gal DNA extract (40 ng of total DNA), 2 mM MgCl2, 2.5
of 10x PCR buffer,1.5 µL of 10 µM of each primer, 2.5 µl
of 10 mMdNTPs, 0.3 µl of 5U Taq DNA Polymerase and
the reaction was completed to 25L with Nuclease-free
water. PCR was conducted in the ESCO Swift Maxi Ther-
mal Cycler with initial denaturation at 95°C for 2 min,
followed by 35 cycles of 95°C for 30 sec, 52°C for 30 sec,
and 72°C for 30 sec, and the nal cycle is a polymer iza-
tion cycle performed at 72°C for 10 min. PCR Products
were puri ed using QIAquick® PCR Puri cation Kit (Cat.
No. 28106) according to manufacturing procedures.
The puri ed PCR products were sequenced by Mac-
rogen Inc., (Korea), and sequencing of the puri ed iso-
lates was performed in both directions using ITS5 and
ITS4 primer pairs. Sequence alignments were edited by
MEGA6 (Tamura et al., 2013).
RESULTS AND DISCUSSION
Based on the API 20 AUX test performed on selected iso-
lates of Beauveria spp. (Table 1), Beauveria basiana was
the predominant yeast strain in all samples, also there
was small percentage of Aspergillus spp. On the basis
of the API 20 AUX test, the isolated yeast was identi ed
as Beauveria basiana. Its pro le makes up to 90% of
the strains. As shown in the table, various assimilation
pro les were obtained for Beauveria basiana. Studies on
assimilation pro les were based on the acidi cation of
FIGURE 2. Plates shown Beauveria bassianaafter re-grown in PDA in
PPDUlab (A,B). C and D B. bassianaas received form University of Hail.
Table 1. The biochemical pro le of Beauveria spp.
Sugar Reaction Sugar Reaction Sugar Reaction
esculin - Adonitol + salicin +
D-arabinose - rhamnose + glycogen -
dulcitol - Inositol + dextrose +/-
D-xylose - Lactose + trehalose +
raf nose - sorbitol + maltose +
galactose + dextrine +/- sucrose +
D-fructose + mannitol - dolicitol -
Note: + = Positive reaction
+/- = Weak reaction
- = Negative reaction
Khalid A. Asiry et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ISOLATION, PHENOTYPIC AND GENOTYPIC CHARACTERIZATION OF INDIGENOUS
BEAUVERIA BASSIANA
ISOLATES 397
twenty sugars. The results indicate that galactose, fruc-
tose, rhamnose, maltose, sucrose and trehalose were
assimilated at high degree contrasted with esculin, ara-
binose, xylose, raf nose, mannitol, glycogen and dulci-
tol. Biochemical properties and speci cally their use of
sugars can be utilized to help the morphology and are
valuable for recognizing species of Beauveria. Mugnai
et. al. (1989) contemplated the intra- and interspeci c
variety of 32 isolates appointed to the genus Beauveria.
They presumed that cultural characters were profoundly
factor also, couldn’t be utilized dependably to separate
species.
Depending on cultural and microscopic examination,
fungal isolates belonged to two genera Beauveria and
Aspergillus as shown on Table (2).
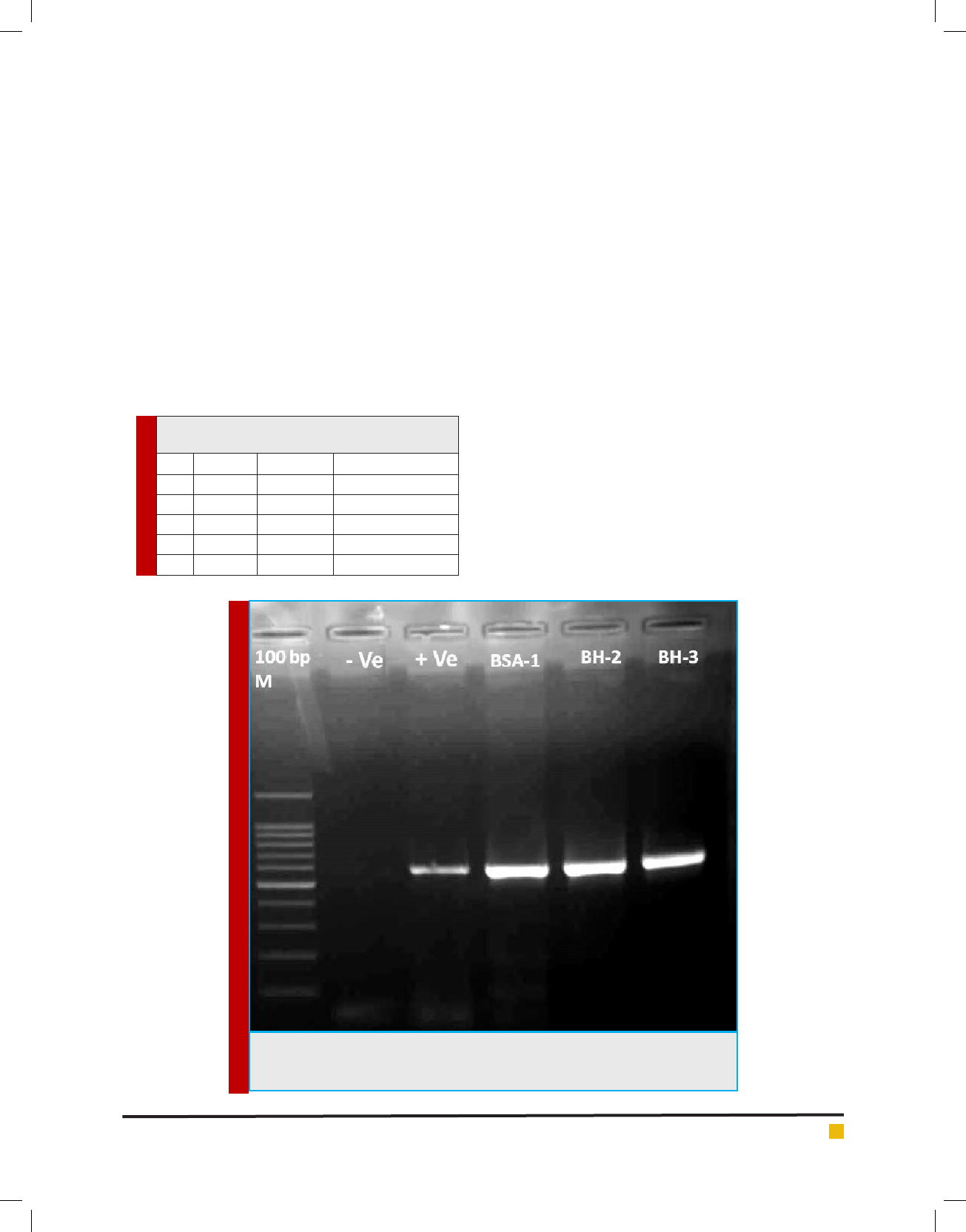
All extracted DNAs from the three fungal isolates
gave a clear bands on the expected size ≈ 600 bp
using primer pairs ITS5&ITS4 (Fig 3). Blast analysis
revealed that the fungal isolates are Beauveria bassiana.
Sequences alignment (Fig 3) showed that all isolates
had same sequence with 100% similarity with chinses
isolates (Accession No. JQ320361). All sequences of the
three isolates were deposited in the Genbank. 16 S rDNA
nucleotide sequence has been sent to the Genbank for
sequence publication.
The red palm weevil R. ferrugineus is a standout
amongst the most serious pests of various date palm
species, including date palms (Giblin-Davis, 2001). The
weevils create inside the tree trunk, wrecking its vascu-
lar system and in the long run causing the crumple and
death of the tree. The pest is generally circulated in Oce-
ania, Asia, Africa and Europe. The RPW makes extreme
harm to coconuts in Southeast Asia (Giblin-Davis, 2001).
It showed up in the Middle East in the 1980s and has
vigorously harmed date production by pulverizing huge
number of date palms (Murphy and Briscoe 1999). Inva-
sion was rst reported in Israel and Jordan in 1999
(Khan and Gangapersad 2001).
The enormous economic losses created by RPW for
date palm trees in Hail region encouraged the authors
Table 2. Fungal speices isolated from R. ferrugineus
in Hail region.
No Label Colour Fungus name
1 BSA-1 white Beauveria bassiana
2 BH-1 brown Aspergillus ssp.
3 BH-2 white Beauveria bassiana
4 BH-3 white Beauveria bassiana
5 VBM green Aspergillus ssp.
FIGURE 3. PCR products of ITS region using ITS5 and ITS4 primer pairs. M: 100 bp
DNA Ladder; -Ve is the non-template control while +Ve is the positive control, BSA-
1, BH-2 and BH-3 are Beauveriabassiana isolates from Hail.

Khalid A. Asiry et al.
398 ISOLATION, PHENOTYPIC AND GENOTYPIC CHARACTERIZATION OF INDIGENOUS
BEAUVERIA BASSIANA
ISOLATES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
to seek for an economically feasible technique to be
employed for eradicating this harmful insect. During
surveying the various infected date palm farms, many
dead RPW were found under the infected trees covered
with whitish material. In addition, farmers informed the
researchers that they do not use any chemical treatment
for pest control in their farms. This information moti-
vated the researchers to try to recognize the causes of
RPW death. Hence, the research has been resumed by
collecting the dead insects are identifying the whitish
material covering their bodies. The morphological tests
indicated that the whitish materials were fungus growth.
Subsequently, the biochemical identi cation tests indi-
cated that the fungus was Beauveria bassiana. Then, the
identi cation was con rmed using molecular biology
tests.
Fungi are the commonest reason of insect disease
in nature. Certain species of entomopathogenic fungi
shown speci city to certain host (Wang, Chengshu &
Wang, Sibao. 2017). Fungi may infect insects by direct
in ltration of the cuticle thus works as contact insecti-
cides. Beauveria is standout amongst other known gen-
era of entomopathogenic fungi and worldwide various
enrolled mycoinsecticide formulations in view Beauveria
bassiana is utilized for control of insect pests (Thomas
and Read, 2007). This fungus has an especially wide host
range (over 700 species) enabling it to be utilized against
vectors of human disease and an extensive variety of
insect pests (de Faria, and Wraight, 2007). For instance
in China, around one million hectares a year are treated
with Beauveria bassiana to control forest insects such
as the pine caterpillar Dendrolimus punctatus (Wang
et al., 2004)
Beauveria is outstanding for creating huge cluster of
biological active secondary metabolites including non-
peptide pigments and polyketides (e.g., oosporein, bassia-
nin and tenellin), nonribosomally synthesized peptides
(e.g., beauvericin, bassianolides and beauveriolides), and
discharged metabolites associated with pathogenesis and
destructiveness (e.g., oxalic acid) that have potential or
acknowledged modern, pharmaceutical and agricultural
uses (Xu et al., 2009). The mechanism of action and bio-
logical function of Oosporein have persisted unclear.B.
bassianahas developed the ability to parasitize insects.
A unique zinc nger transcription factor, BbSmr1 (B.
bassianasecondary metabolite regulator 1), was identi-
ed in a screen for oosporein overproduction. Deletion
of Bbsmr1 resulted in up-regulation of the oosporein
biosynthetic gene cluster (OpS genes) and constitutive
oosporein production, (Fan et al., 2017).
The dominant part of endophyte explore has concen-
trated to date on the vertically-transmitted endophytes
inside the genus Neotyphodium (Clavicipitaceae) that
systemically colonize the over the ground parts of a few
grasses. These clavicipita-ceous endophytes are gener-
ally known to present a variety of potential advantages
to their grass host plants (Kuldau and Bacon, 2008). Less
consideration has been given to the evenly transmitted
non-clavicipitaceous endophytes, which are in nature
and commanded by the Ascomycetes (Arnold and Lut-
zoni, 2007); of which a few genera are fungal ento-
mopathogens (Ascomycota: Hypocreales).
Rising as an energizing
new area of research,
‘fungal ento-mopathogens as endophytes’ has
just
rather recently been incorporated into
a more than
100
old endophyte research base after the
recupera-
tion
of
different
genera of fungal ento-mopathogens
as endophytes from
various
plant species (Vega
et al., 2008). Some of these fungi have been
accounted
for
as
normally happening endophytes
, while others
have been
brought into the plant utilizing distinctive
different inoculation techniques. Spearheading
take a shot at entomopathogenic endophytes was
directed with B. bassiana (Balsamo), a universal
soil-borne fungus that infects an extensive variety
of various insects (>700 insect species; Inglis et al.,
2001) and is a standout amongst the most marketed
fungal biopesticides (de Faria and Wraight, 2007).
Lewis and Cossentine (1986) credited the season-
long concealment of the European corn borer Ostrinia
nubilalis (Hübner) (Lepidoptera: Pyralidae) in maize Zea
mays L. (Poaceae), estimated as reduced tunneling by
the insect, to the establishment of B. bassiana as an
endophyte following utilization of a watery suspen-
sion of the fungus to the plants. Ensuing work by Lewis
and colleagues utilizing a similar model system dem-
onstrated fruitful re-isolation of B. bassiana from inte-
rior plant tissues after utilization of the fungus utilizing
different inoculation methods. These antagonist fun
can have bene cial effects on host plants, e.g., plant
growth promotion, reducing disease severity, inducing
plant defense mechanisms, and producing anti-herbi-
vore products (Arnold and Lewis, 2005, Amatuzzi, et al.,
2017).
In addition to maize, a wide assortment of host plants
(counting both agronomic and weed species) have addi-
tionally been appeared to harbor B. bassiana as an endo-
phyte. As opposed to B. bassiana, the host plant scope of
other fungal entomopathogens is as yet developing. For
example, Verticillium (=Lecanicillium) lecanii (Zimm.)
Viegas has been accounted for as a natural endophyte
in bear-berry Arctostaphylos uva-ursi L. (Ericaceae)
(Widler and Muller, 1984) and ironwood (Bills and Pol-
ishook, 1991). A relatively recent development, the use
of some fungal entomopathogens could function as
biofertilizers. Various inoculation techniques (e.g., foliar
sprays, soil drenching, seed soaking, injections, etc.)
are effective in introducing fungal entomopathogens as

Khalid A. Asiry et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ISOLATION, PHENOTYPIC AND GENOTYPIC CHARACTERIZATION OF INDIGENOUS
BEAUVERIA BASSIANA
ISOLATES 399
endophytes, but colonization appears to be localized and
ephemeral, (Vega 2018).
There is presently considerable con rmation that some
endophytic fungal entomopathogens, especially B. bassi-
ana and Lecanicillium spp. (some time ago Verticillium
lecanii), may likewise exhibit hostile action against plant
pathogens , in addition to their outstanding biocontrol
activity against insect pests. This proposes that these
entomopathogens have a promising potential to be devel-
oped as biopesticides for numerous reasons in IPM meth-
odologies (Goettel et al., 2008; Vega et al., 2009; Ownley
et al., 2010). Beauveria bassiana strain 11–98, applied as
a seed treatment, has been accounted to suppress damp-
ing-off caused by the soil-borne pathogens, Rhizoc-tonia
solani Kuhn (Basidiomycota: Cantharellales) and Pythium
myriotylum Drechsler (Oomycota: Pythiales), in tomato
(Ownley et al., 2004; Clark et al., 2006) and cotton seed-
lings (Grif n, 2007; Ownley et al., 2008). Pre-treatment of
cotton seedlings with the same B. bassiana strain likewise
resulted in reduced seriousness of bacterial blight caused
by Xanthomonas axonopodis pv. malvacearum (Xam)
(Grif n et al., 2006; Ownley et al., 2008).
More recently, several strains of B. bassiana were
found to fundamentally diminish the rate and serious-
ness of the Zucchini yellow mosaic virus (ZYMV; genus
Potyvirus, family Potyviridae) in squash (Jaber and
Salem, 2014) and downy mildew caused by Plasmopara
viticola (Berk. and Curt.) Berl. and de Toni. (Oomycota:
Peronosporaceae) in grapevines (Jaber, 2015) following
foliar inoculation of plants with conidial suspensions of
the tested strains.
CONCLUSION
Strains of Beauveria bassiana (Bals.-Criv.) Vuill. and
Metarhizium anisopliae (Metchn.) Sorokin (Hypocreales:
Clavicipitaceae) have been isolated from wild R. ferrug-
ineus populations (Lo Verde et al., 2015). Beaveria bassi-
ana fungus could be used as one of the biological strate-
gies in controlling red palm weevil (Hajjar et. al., 2014).
Based on the morphological, biochemical and molecu-
lar biological techniques, B. bassiana was isolated and
identi ed as the fungal strain for the rst time in Hail
region, Saudi Arabia in which this fungal strain can be
utilized in the biological control of the red palm weevil
R. ferrugineus which is considered as a destructive pest
of date palms in this region.
ACKNOWLEDGEMENTS
The authors would like to express sincere thanks and
gratitude to the Al-Jomaiah’s Chair of Sustainable
Development in Agricultural Communities at the Uni-
versity of Hail for providing nancial support.
REFER ENCES
Arnold, A.E., Lewis, L.C., (2005). Ecology and evolution of fun-
gal endophytes and their roles against insects. In: Vega, F.E.,
Blackwell, M. (Eds.), Insect-Fungal Associations: Ecology and
Evolution. Oxford University Press, New York, pp. 74–96.
Arnold, A.E., Lutzoni, F., (2007). Diversity and host range of
foliar fungal endophytes: are tropical leaves biodiversity hot-
spots. Ecology 88, 541–549.
Amatuzzi, R.F.; Cardoso, N.; Poltronieri, A.S.; Poitevin, C.G.;
Dalzoto, P.; Zawadeneak, M.A.; Pimentel, I.C. (2017). Poten-
tial of endophytic fungi as biocontrol agents of Duponchelia
fovealis (Zeller) (Lepidoptera: Crambidae). Braz. J. Biol. 78(3)
pp 429-435.
Azmi Wahizatul, Chong, Ju lian Ahamad Zakeri, Hazlina
Yusuf, Norhayati, Wan Omar, Wan Bayani, Kah Wai, Yong,
Nadrah Zulke i, Ainatun and Hussain Mohamad. (2017). The
Red Palm Weevil, Rhynchophorus ferrugineus: Current Issues
and Challenges in Malaysia. Oil Palm Bulletin. 74. 17-24.
Barranco, D., A. Cimato, P. Fiorino, L. Rallo, A. Touzani, C.
Castañeda, F. Serafín, and I. Trujillo. (2000). Catálogo Mundial
de Variedades de Olivo. Consejo Oleícola Intl., adrid, Spain.
Bills, G.F., Polishook, J.D., (1991). Microfungi from Carpinus
caroliniana. Can. J. Bot. 69, 1477–1482.
Bing, L. A. & Lewis, L. C. (1991). Suppression of Ostinia nubila-
lis (Hu$ bner) (Lepidoptera: Pyralidae) by endophytic Beauve-
ria bassiana (Balsamo) Vuillemin. Environmental Entomology
20: 1207-1211.
Bing, L. A. & Lewis, L. C. (1992). Endophytic Beauveria bassi-
ana (Balsamo) Vuillemin in corn: the in uence of the plant
growth stage and Ostrinia nubilalis (Hu$ bner). Biocontrol Sci-
ence and Technology, 2:1, pp. 39-47
Clark, M.M., Gwinn, K.D., Ownley, B.H., (2006). Biological con-
trol of Pythium myriotylum. Phytopathology 96, S25.
de Faria, M. R. & Wraight, S. P. (2007). Mycoinsecticides and
Mycoacaricides: A comprehensive list with worldwide cover-
age and international classi cation of formulation types. Biol.
Control 43, 237–256.
Dellaporta SL, Wood J, Hicks JB. A plant DNA mini-prepa-
ration: version 2. Plant Mol Biol Rep. 1983;1(4):19–21. doi:
10.1007/BF02712670.
EPPO. (2006b). First record of Rhynchophorus ferrugineus in
France and Greece. EPPO Reporting Service 11: 4-5. EPPO.
2007a. First report of Rhynchophorus ferrugineus in Cyprus.
EPPO Reporting Service 2: 23
EPPO. (2007b). First report of Rhynchophorus ferrugineus in
Turkey. EPPO Reporting Service 1: 2.
EPPO. (2009a). First record of Rhynchophorus ferrugineus in
Curaçao, Netherlands Antilles. EPPO Reporting Service 1: 2.
EPPO. (2010). First record of Rhynchophorus ferrugineus in
the USA. EPPO Reporting Service10:3.Frost Tolerance of Eight
Olive Cultivars (PDF Download Available). Available from:
https://www.researchgate.net/publication/279662719_Frost_
Tolerance_of_Eight_Olive_Cultivars [accessed Mar 11 2018].

Khalid A. Asiry et al.
400 ISOLATION, PHENOTYPIC AND GENOTYPIC CHARACTERIZATION OF INDIGENOUS
BEAUVERIA BASSIANA
ISOLATES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Faleiro JR. Fundación Agroalimed. Jornada Internacional
sobre el Picudo Rojo de las Palmeras.Valencia, Spain: (2006a).
Insight into the management of red palm weevil Rhyncho-
phorus ferrugineus Olivier: Based on experiences on coconut
in India and date palm in Saudi Arabia. pp. 35–57. 27–29
November, 2005. ISBN: 84-690-1742-X.
Fan Y., Liu X.,Keyhani N.O., Tang G., Pei Y., Zhang W and
Tong S. (2017). Regulatory cascade and biological activity
of Beauveria bassiana oosporein that limits bacterial growth
after host death. Proceedings of the National Academy of Sci-
ences,114(9)E1578-E1586.
Fernando E. Vega (2018) The use of fungal entomopathogens
as endophytes in biological control: a review, Mycologia,
110:1, 4-30
Giblin-Davis, R.M. (2001). Borers of palms. in: Howard, F.W.,
Moore, D., Giblin-Davis, R.M. and Abad, R.G.[Eds.]Insects on
Palms.CABI Publishing, Wallingford, UK.pp.267-305.
Goettel, M.S., Inglis, D.G., (1997). Fungi: Hyphomycetes. In:
Lacey, L.A. (Ed.), Manual of techniques in insect pathology.
Academic Press, London, UK, pp. 213–249.
Goettel, M.S., Koike, M., Kim, J.J., Aiuchi, D., Shinya, R., Bro-
deur, J., (2008). Potential of Lecanicillium spp. for manage-
ment of insects, nematodes and plant diseases. J. Invertebr.
Pathol. 98, 256–261.
Grif n, M.R., (2007). Beauveria Bassiana, A Cotton Endophyte
With Biocontrol Activity Against Seedling Disease (Ph.D. Dis-
sertation). The University of Tennessee, Knoxville, TN, USA.
Hajjar, M. J. ,Ajlan A. M and Al-Ahmad M. H., (2014) New
Approach of Beauveria bassiana to Control the Red Palm Wee-
vil (Coleoptera: Curculionidae) by Trapping Technique. Journal
of Economic Entomology.108 (2) 425-432.
Inglis, G.D., Goettel, M.S., Butt, T.M., Strasser, H., (2001). Use of
hyphomycetous fungi for managing insect pests. In: Butt, T.M.,
Jackson, C., Magan, N. (Eds.), Fungi as Biocontrol Agents. Pro-
gress, Problems and Potential. CABI Publishing, Oxfordshire,
pp. 23–69.
Jaber, L.R., (2015). Grapevine leaf tissue colonization by the
fungal entomopathogen Beauveria bassiana s.l. and its effect
against downy mildew. Biocontrol 60, 103–112.
Jaber, L.R., Salem, N.M., (2014). Endophytic colonisation of
squash by the fungal entomopathogen Beauveria bassiana
(Ascomycota: Hypocreales) for managing Zucchini yellow
mosaic virus in cucurbits. Biocontrol Sci. Technol. 24, 1096–
1109.
Khan, A. and Gangapersad, G. (2001) Comparison of the effec-
tiveness of three entomopathogenic fungi in the management
of the banana borer weevil, Cosmopolites sordidus (Germar)
(Coleoptera: Curculionidae). Int. Pest Control 43:208-213.
Krueger, S. R. & Roberts, D. W. (1997). Soil treatment with
entomopathogenic fungi for corn rootworm (Diabrotica spp.)
larval control. Biological Control 9: 67-74.
Kuldau, G., Bacon, C., (2008). Clavicipitaceous endophytes:
their ability to enhance resistance of grasses to multiple
stresses. Biol. Control 46, 57–71.
Lewis, L.C., Cossentine, J.E., (1986). Season long intraplant epi-
zootics of entomopathogens, Beauveria bassiana and Nosema
pyrausta, in a corn agroecosystem. Entomophaga 31, 36–69.
Lo Verde G, Torta L, Mondello V, Caldarella CG, Burruano S and
Caleca V., (2015). Pathogenicity bioassays of isolates of Beau-
veria bassiana on Rhynchophorus ferrugineus. Pest Manag Sci
71:323–328.
Mugnai L., Bridge P.D., Evans H.C. (1989). A chemotaxonomic
evaluation of the genus Beauveria. Mycol. Res. 92 (2): 199–
209. Doi: 10.1016/S0953-7562(89)80012-7.
Muhammad Y asin Waqas Wakil Muhammad Usman Ghazanfar
Mirza Abdul Qayyum Muhammad Tahir and Geoffrey O. Bed-
ford. (2017). Virulence of entomopathogenic fungi Beauveria
bassiana and Metarhizium anisopliae against red palm weevil,
Rhynchophorus ferrugineus (Olivier). Entomological research.
doi: 10.1111/ 1748-5967.12260.
Mulock, B. & Chandler, L. (2000) Field-cage studies of Beauve-
ria bassiana (Hyphomycetes: Moniliaceae) for the suppression
of adult western corn rootworm, Diabrotica virgifera virgifera
(Coleoptera: Chrysomelidae). Biocontrol Science and Technol-
ogy 10: 51- 60.
Murphy, S. T., and B. R. Briscoe. (1999). The red palm weevil
as an alien invasive: biology and the prospects for biological
control as a component of IPM. Biocontrol News and Informa-
tion, 20(1):34n-46n.
Ownley, B.H., Grif n, M.R., Klingeman, W.E., Gwinn, K.D.,
Moulton, J.K., Pereira, R.M., (2008). Beauveria bassiana: endo-
phytic colonization and plant disease control. J. Invertebr.
Pathol. 3, 267–270.
Ownley, B.H., Pereira, R.M., Klingeman, W.E., Quigley, N.B.,
Leckie, B.M., (2004). Beauveria bassiana, a dual purpose bio-
control organism, with activity against insect pests and plant
pathogens. In: Lartey, R.T., Caesar, A. (Eds.), Emerging Con-
cepts in Plant Health Management. Research Signpost, Kerala,
pp. 256–269.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S.
(2013). MEGA6: molecular evolutionary genetics analysis ver-
sion 6.0. Molecular biology and evolution, 30(12), 2725-2729
2: 39-47.
Thomas, M. B. & Read, A. F. (2007). Can fungal biopesticides
control malaria Nat Rev Microbiol 5, 377–383.
Vega, F.E., Goettel, M.S., Blackwell, M., Chandler, D., Jackson,
M.A., Keller, S., Koike, M., Maniania, N.K., Monzo’n, A., Own-
ley, B.H., Pell, J.K., Rangel, D.E.N., Roy, H.E. (2009). Fungal
entomopathogens: new insights on their ecology. Fungal Ecol.
2, 149–159.
Vega, F.E., Posada, F., Catherine Aime, M., Pava-Ripoll, M.,
Infante, F., Rehner, S.A., (2008). Entomopathogenic fungal
endophytes. Biol. Control 46, 72–82.
Wang, Chengshu & Wang, Sibao. (2017). Insect Patho-
genic Fungi: Genomics, Molecular Interactions, and Genetic
Improvements. Annual Review of Entomology. 62. 73-90. 10.
Wang, Chengshu & Fan, M & Li, Zengzhi & Butt, Tariq. (2004).
Molecular monitoring and evaluation of the application of

Khalid A. Asiry et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ISOLATION, PHENOTYPIC AND GENOTYPIC CHARACTERIZATION OF INDIGENOUS
BEAUVERIA BASSIANA
ISOLATES 401
the insect-pathogenic fungus Beauveria bassiana in Southeast
China. Journal of applied microbiology. 96. 861-70.
White, T. J., Bruns, T., Lee, S. J. W. T., & Taylor, J. W. (1990).
Ampli cation and direct sequencing of fungal ribosomal RNA
genes for phylogenetics. PCR protocols: a guide to methods
and applications, 18(1), 315-322.
Widler, B., Muller, E. (1984). Untursuchungen uber endo-
phytische Pilze von Arctostaphylos uva-ursi (L.) Sprenger (Eri-
caceae). Bot. Helv. 94, 307–337.
Xu Y,Orozco R,Kithsiri Wijeratne EM,Espinosa-Artiles P,Les-
lie Gunatilaka AA,Patricia Stock S,Molnár I (2009) Biosyn-
thesis of the cyclooligomer depsipeptide bassianolide, an insec-
ticidal virulence factor of Beauveria bassiana. Fungal Genet
Biol. 46,353–364.
Yaish, Mahmoud W., and Prakash P. Kumar. “Salt Tolerance
Research in Date Palm Tree (Phoenix Dactylifera L.), Past, Pre-
sent, and Future Perspectives.” Frontiers in Plant Science 6
(2015): 348. PMC. Web. 21 Sept. 2018.
Zohary, D., Hopf, M., & Weiss, E. (2012). Domestication of
Plants in the Old World: The Origin and Spread of Domesti-
cated Plants in Southwest Asia, Europe, and the Mediterranean
Basin (4th ed.). Oxford: Oxford University Press.