
Medical
Communication
Biosci. Biotech. Res. Comm. 11(3): 347-355 (2018)
The central roles of exosomesin hematological
malignancies: A new frontier review
Bing Xia
1
*, Mengzhen Li
1
,
Ruifang Yang
2
, Xi Wang
1
, Chengtao Shun
1
and Yizhuo Zhang*
1,3
1
Department of Hematology, Tianjin Medical University Cancer Institute and Hospital, National Clinical
Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin; Tianjin’s Clinical
Research Center for Cancer; 300060, China
2
Department of Clinical Laboratory, Tianjin Medical University Cancer Institute and Hospital,National
Clinical Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin; Tianjin’s
Clinical Research Center for Cancer; 300060,China
3
Department of Pediatric Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology
in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong 510060, China
ABSTRACT
Exosomes, which are 30- to 120-nm vesicles, are released by most types of cells, including tumor cells. In hemato-
logical malignancies, exosome-mediated expulsion of a number of key proteins and micro RNAs, resulting in in u-
ence of major tumor-related pathways. Emerging evidence suggests that the component secreted by exosomescan
promote tumor survival,angiogenesis and metastasis, and also mediate tumor microenvironment induced drug resist-
ance and immune escape. Furthermore, exosomes contain a great variety of bioactive molecules andare emerging
as rich reservoirs of hematological tumor-speci c biomarkers for the detection and therapeutics.This comprehensive
review highlights the advancements in understanding of the pathogenesis of exosomes secretion and the consequence
onhematological malignancies development. Full knowledge of the contribution of exosomes to the potential medi-
cal application ofdiagnosis and treatment will depend on the ingenuity of future investigators and their insight into
biological processes.
KEY WORDS: HEMATOLOGICAL MALIGNANCIES; EXOSOMES; DISEASE DEVELOPMENT;DIAGNOSIS; TREATMENT
347
ARTICLE INFORMATION:
*Corresponding Authors: forxiabing@163.com, Bing Xia and
Yizhuo Zhang
Received 12
th
July, 2018
Accepted after revision 19
th
Sep, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.3/1
348 THE CENTRAL ROLES OF EXOSOMESIN HEMATOLOGICAL MALIGNANCIES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Bing Xia etal.
INTRODUCTION
Hematological malignancies, which include lymphoid,
myeloid, histiocytic, and mast cell neoplasms, are a hetero-
geneous group of diseases of diverse incidence, pathogen-
esis and prognosis (Kornblau etal., 1998). The prognosis
of hematological malignancies patients diverges greatly,
largely depending on thepathological types of the patient
(Li et al., 2016). Nonetheless, enhancing understanding
of tumorigenesis mechanism is critical for development
of novel diagnosis and therapeutic strategy. Exosomes
are a kind of extracellular vesicles (EVs), which was rst
reported by Pan and Johnstone in 1983 as unwanted cel-
lular components extruding from reticulocytes. EVs are
medium-sized vesicles, ranging from 30 to 120 nm and
are secreted by different cell types under both physi-
ological and pathological conditions. Exosomes are rich
in cholesterol, sphingomyelin, ceramide lipids, protein,
mRNA, miRNA, and various other signaling molecules
from donor cell. In addition, CD9, CD63, and CD81 are
most frequently detected and are considered asthe classic
markers of exosomes, (
Malla etal., 2018).
Then, the MVB’s can either fuse with lysosomes result-
ing in degradation of intra-luminal contents or they can
secrete their content as exosomes outside the donor cells.
Furthermore, there are several ways by which exosomes
are taken up by recipient cells: receptor- or lipiddraft-
mediated endocytosis, phagocytosis, macropinocytosis,
or fusion with the plasma membrane of a target cell, For
transportation, exosomes mainly originate from multi-
vesicular bodies (MVB’s) in the cells which are produced
by the invagination of endosomal limiting membrane
(Pfrieger etal., 2018).
Recently, it has been reported by many workers that
the potential functions of exosomes contributed to vari-
ous aspects of hematological tumorigenesis, particularly
with a focus on the exosome-mediated tumor progression,
metastasis, drug resistance and immune escape by alter-
ing the function of receiver cells via diverse exosomal car-
goes including proteins, DNA, messenger RNAs (mRNAs),
and microRNAs ) (Whiteside and Boyiadzis, (2017). Fur-
thermore, exosomes are found in most biological fluids
including urine, blood, ascites, saliva and cerebrospinal
fluid, which can be made for very attractive targets for
diagnostic application.Although the known information is
limited, exosomes have been reported to play an emerging
role in various aspects of the tumor survival, metastasis,
drug resistance, and immune escape of hematologic tumor.
In this review, we will focus speci cally on the effects of
exosomes on tumorigenesis, diagnosis and treatment of
hematological malignanciesand the relevantnew prospects.
THE ROLE OF EXOSOMES IN DEVELOPMENT
OF HEMATOLOGICAL MALIGNANCIES
Recently, considerable amount of studies have revealed
that exosomes were secreted by tumor cells or tumor micro-
environment cells and cross-talk in uence tumor prolifera-
tion, angiogenesis, drug resistance and immune escape of
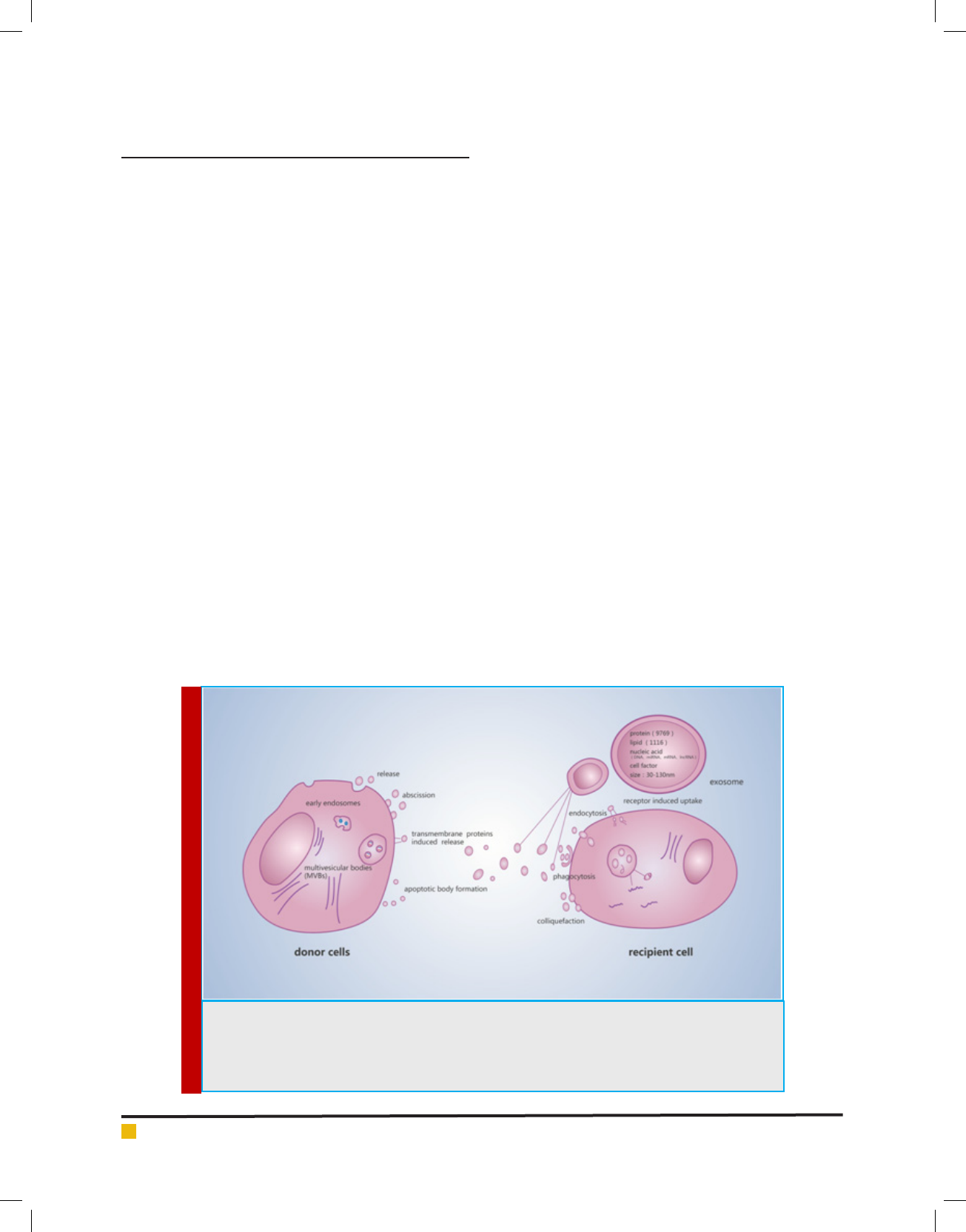
several hematological cell types ( gure1) (Sun, etal., 2018).
FIGURE 1. Biogenesis, transportation and composition of exosome, and the possible communication
mechanism between donor cells and targetcells. Diagram depicting the well-accepted model for exo-
some biogenesis. Exosomes secreted by donor cells included release, shedding, transporter-mediated
release and apoptotic body formation. Receptor cells receive exosomes including endocytosis, phago-
cytosis, fusion and receptor-mediated uptake.

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE CENTRAL ROLES OF EXOSOMESIN HEMATOLOGICAL MALIGNANCIES 349
Bing Xia etal.
Survival and proliferation
One study demonstrated that the level of sera-derived
exosomes in acute myeloid leukemia (AML) patients
are higher than sera-derived exosomes from healthy
controls
[Hong etal., 2014] and the exosomes secreted
by AML cells express higher level of mRNA, such as
GATA1, FOX3, SHIP1, ID1, E2F1, CEBP-, CEBP-, Myc
and MEF2C, which are the group of transcripts of genes
to the development of AML
[Huan J. etal., 2013]. Fur-
thermore, another study showed that levels of active
TGF-1 carried by exosomes obtained at AML diag-
nosis were high and then decreased following induc-
tion chemotherapy. In addition, in long-term remis-
sion of AML patients, TGF-1 levels in plasma-derived
exosomes approach the same levels seen in exosomes
of normal controls. The results indicate that exosomal
protein and TGF-1 levels in AML plasma could serve
as biomarkers of response to chemotherapy.These data
showed that both AML cell lines and primary AML blasts
released exosomes relevant to AML pathogenesis (Huan
etal., 2013 and Long etal., 2017).
For chronic myelocytic leukemia ( CML), CML cell
line LAMA84-derived exosomes increase levels of IL-8
mRNA and protein in HS-5 (bone marrow stromal
cell line), and further promote HS-5 induced CML cell
proliferation , (Zhou et al., 2012) . In another way, the
exosomes also reduce expression of the proapoptotic
genes BAD, BAX and PUMA and increase expression of
anti-apoptotic genes BCL-xL, BCL-w, and BIRC5.Moreo-
ver, exosomes also activate the PI3/AKT and MAPK/ERK
signaling pathways. Collectively, the exosomes secreted
by CML cells both educate the tumor microenvironment
cells and directly affected the proliferation and apopto-
sis of LAMA84 cells and nally promote the CML cells
survival. Human T-lymphotropic virus type 1 ( HTLV1)
infected T cells release exosomes that contain viral Tax
protein and Tax, HBV, and EBV mRNA, which increase
levels of phosphorylated AKT and active NF-kB path-
way, and further facilitate T-cell tumor cells survival.
These results suggest that the exosomes released from
HTLV-1-infected cells play key role in the pathogene-
sis of T-cell leukemia (Jaworski etal., 2014, Raimondo
etal., 2015).
The study by Raphael Koch shows that diffuse large
B-cell lymphoma (DLBCL) possess a self-organized infra-
structure comprising side population (SP) and non-SP
cells, where transitions between clonogenic states are-
modulated by exosomes mediated Wnt signaling. Lym-
phoma SP cells exhibit autonomous clonogenicity and
export Wnt3a via exosomes to neighboring cells, thus
modulating population equilibrium in the tumor (Koch
etal., 2014). The study about the role in MCL observes
that MCL exosomes are taken up rapidly and preferen-
tially by MCL cells. Only a minor fraction of exosomes
was internalized into T-cell leukemia and bone mar-
row stroma cell lines, when these cells werecocultured
with MCL cells. Moreover, MCL patients’ exosomes were
taken up by both healthy and patients’ B-lymphocytes
with no apparent internalization to T lymphocytes and
NK cells. Exosome internalization was not inhibited by
speci c siRNA against caveolin1 and clathrin but was
found to be mediated by a cholesterol-dependent path-
way. These ndings demonstrate natural speci city of
exosomes to B-lymphocytes and ultimately might be
used for therapeutic intervention in B cells malignan-
cies, (Hazan etal., 2015).
EBV infection of B cells in vitro induces the release
of exosomes that harbor the viral latent membrane pro-
tein 1 (LMP1). LMP1 via exosomes actives CD40 sign-
aling and induces proliferation of B lymphocytes and
T cell independent class-switch recombination.Finally,
LMP1 drove B cell differentiation toward a plasmab-
last-like phenotype. In conclusion, the results suggest
that exosomes released by EBV-infected lymphoma
cells include the production of the activation-induced
cytidine deaminase (AID), and further promote tumor
cells aggressive and progression, ( Nanbo etal., 2013).
In addition, Chugh etal. (2013) reported that exosomes
derived from patients with KSHV-associated malignan-
cies and KSHV mouse modelscontained KSHV encoded
microRNAs such as miR-17-92 cluster, which are affect
the targets of KSHV signaling pathways that may there-
fore be part of the paracrine signaling mechanism that
mediates KSHV pathogenesis.
A research about relationship between MSC and
plasma cells demonstrated that the tumor-supportiverole
of BMSC-derived exosomes: Compared with normal mes-
enchymal stem cells, BMSC-derived exosomes in MMex-
presslower level of microRNA15a and microRNA15a
isa tumor-suppress factor thatcontributeto MM disease
progression. In addition, BMSC-derived exosomes in
MM expresshigher content levels bronectin, indicat-
ing that BM-MSC-derived exosomes may differentially
impact MM cell adhesion , (Roccaro etal., 2013). Kim De
Veirman etal. (2016) showed that miRNA-146a in MM-
derived exosomes can be transmitted into mesenchymal
stem cells, resulting in induction of expression for some
cytokines and chemokines including CXCL1, IL6, IL-8,
IP10, MCP-1, and CCL-5, thus lead to increasing vitality
and progression of MM cells.
Angiogenesis and metastasis:Exosomes have been
increasingly recognized as a new mediator for angio-
genesis and metastasis of hematological malignancies.
For example, Umezu etal.(2013) showed that leukemia
cells K562 released the exosomal miRNAs, such as miR-
17-92 cluster, especially miR-92a, into human umbili-
cal vein endothelial cells (HUVECs) ,and target reduced
the expression of integrin 5 in HUVECs by exosomal

Bing Xia etal.
350 THE CENTRAL ROLES OF EXOSOMESIN HEMATOLOGICAL MALIGNANCIES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
miR-92a, indicating that exogenous miRNA via exo-
somal transport can function like endogenous miRNA
in HUVECs, which enhance endothelial cell migration
and tube formation. In the study, which investigated
the angiogenic role of exosomes produced by acute
promyelocytic leukemia cells NB4.PML-R ARA tran-
script has been detected in NB4 exosomes and taken
up by endothelial cells, resulting in decreasing the lev-
els of VEGF and tissue factor (TF) through increasing
IL-8 mRNA and protein content in their EVs, renders
the HUVECs more TF-positive and procoagulant, (Fang
etal., 2016).
A recent study by Hiroko Tadokoro etal. has clearly
demonstrated that hypoxia promotes the release of
exosomes in K562 cells,the amount of exosomal miR-
210, which down-regulated EFNA3, an inhibitor of
angiogenesis , (Tadokoro etal., 2013). The results suggest
that exosomal miRNA derived from cancer cells under
hypoxic conditions may partly affect angiogenic activ-
ity in endothelial cells. Paggetti etal. found that CLL-
derived exosomes are actively incorporated by endothe-
lial and mesenchymal stem cells ex vivo and in vivo
and that the transfer of exosomal protein and microRNA
induces an in ammatory phenotype in the target cells,
which resembles the phenotype of cancer-associated
broblasts (CAFs), (Paggetti etal., 2015).
Exosomeswere uptakenby endothelial cells increased
angiogenesis ex vivo and in vivo, and coinjection of
CLL-derived exosomes and CLL cells promoted tumor
growth in immunode cient mice. These ndings dem-
onstrate that CLL-derived exosomes actively promote
disease progression by modulating several functions
of surrounding stromal cells that acquire features of
cancer-associated broblasts, (Paggetti et al., 2015).
Another similar study found that EBV-positive Burkitt’s
lymphoma cells Raji released exosomes with miR-155
inducing angiogenesis in remote recipient cells, whereas
no major difference was found in co-culture with EBV-
negative Burkitt’s lymphoma cells, (Yoon etal., 2016).
Thus, it would be reasonable to believe that speci c
viral exosomal microRNAs contribute to angiogenesis
of vascular endothelial cells, subsequently leading to
pathophysiologic angiogenesis. In accord with a study
by Umezu etal. (2014), miRNA-135b from MM-derived
exosomes accelerated HIF-1 transcriptional activity via
inhibition of FIH-1, which is called the HIF-FIH signal-
ing pathway, exertinganangiogenesis in uence . Collec-
tively, these results suggest that hematological malig-
nancy cells release exosomes that could promote tumor
metastasis and the formation of pre-metastatic niches
to create a microenvironment favorable to survival and
proliferation of tumor cells themselves.
Drug resistance: Like many other tumors, increasing
evidences also revealed that the tumor microenvironment
( TME) has crucial impact on hematological malignancies
initiation and progression (Rizzo etal., 2011). Given the
central role of exosomes in cellular communication, it
is undoubtable that exosomes also contribute to micro-
environment-induced drug resistance. One intuitive
mechanism involving exosomes would be the sequestra-
tion of cytotoxic drugs in the intracellular vesicles and
subsequent expulsion, to negate drug effect within the
cells. Notably, emerging evidence suggests that both the
exosomes released from TME cells and the tumor cells
themselves help the hematological malignancy cells to
resist chemotherapy, (Isidori etal., 2014). One prominent
example, exosomes from both AML-BMSC and healthy
controls protect MOLM-14 FLT3 internal tandem dupli-
cation (FLT3-ITD+) AML cells from cytarabine and stro-
mal exosomes alter chemo-resistance in AML cells. Fur-
ther, only AML-BMSC exosomes signi cantly protected
AML cells from the FLT3 inhibitor AC220 after exposure.
The protection might be associated with elevated level
of miRNA-155, miRNA-375, cytokine epidermal growth
factor (EGF) and TGF-1 (Viola etal., 2016).
Finally, these data imply a few novel approaches to
overcome drug resistance on AML blasts by either block-
ing exosome-leukemia cell communication, or inhibiting
tumor microenvironment exosome production. Moreo-
ver, several studies unveiled that galectin-3 was upregu-
lated and stabilized anti-apoptotic Bcl-2 family proteins
in survival leukemia cells, which facilitates escape from
apoptotic stimuli through activation of Wnt/-Catenin
signaling pathway and the PI3K/Akt pathway, (Cheng
etal., 2011, Hu K. etal., 2015).
In another study about lymphoma, the results revealed
that high levels of adenosine triphosphate (ATP)-binding
cassette (ABC) transporter A3 (ABCA3) were related to
drug resistance, especially by drug expulsion which
might be modulated by microparticles (Chapuy et al.,
2008, Steinbach, et al., 2006). Oksvold et al. (2014)
showed a similar observation that exosomes secreted
by B-cell lymphoma reduced rituximab-induced cyto-
toxicity. These studies indicated a novel mechanism
of drug resistance in lymphoma, which is linked to an
ABCA3-dependent pathway of exosome secretion, (Oks-
vold et al., 2014). In particular, it was suggested that
increased expression of cellular galectin-3 and elevated
concentration of galectin-3 in circulating system may
contribute to tumor progression and drug resistance.
Regarding ALL, the levels of exosomal Gal-3 mRNA and
protein, which originated from stromal cells in B-ALL
were in relation with the appearance of drug-resistance
through activated MEK/ERK pathway, (Fortuna et al.,
2014). Galectin-3 is enriched in OP9 exosomes, and exo-
somal galectin-3 can be internalized by ALL cells, and
activates NFB signaling pathway, whichisoften linked
to anti-apoptosis and drug resistance (Hu etal., 2015).

Bing Xia etal.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE CENTRAL ROLES OF EXOSOMESIN HEMATOLOGICAL MALIGNANCIES 351
Another study showed that exosomal evasion of
humoral immunotherapy in aggressive B-cell lymphoma
wasmodulated by ATP-binding cassette transporter
A3. B-cell lymphoma cells released exosomes that car-
ried CD20, bound therapeutic anti-CD20 antibodies,
consumed complement, thereby impairing antibody-
dependent cell mediated cytotoxicity (ADCC) and pro-
tected target cells from antibody attack through linked
in an ABCA3-dependent pathway of exosome secretion.
Additionally, removing exosomes from plasma samples
of B-NHL resulted in considerable improvements in
the effect of rituximab against lymphoma cell in vitro,
(Aung etal., 2011). In multiple myeloma, the integrin-
mediated interaction of cancer cells with MSC, upregu-
lates the secretion of soluble factors such as IGF-1, FGF,
IL-6 and others that provide growth advantage and drug
resistance to multiple myeloma cells (Isidori etal., 2014,
Greco etal., 2009).
Another study by Wang etal. (2014) indicated that
bone marrow stromal cell–derived exosomes in MM can
promote proliferation, migration, and survival of MM
cells, andcan also induce chemotherapy drug resistance
to bortezomib, in uencing several pathways including
c-Jun N-terminal kinase, p38, p53, and Akt which are
relevant to survival of MM cells.All these studies suggest
that exosome mediate drug resistance through both the
direct shuttling of drugs out of the cells and the horizon-
tal transfer of molecules/molecular signals that bestow
drug resistance to the otherwise sensitive cells.
Immune escape: Hematological malignancy cells can
evade host immune surveillance, a well-known phenom-
enon called as immune evasion or immune escape, which
is also a hallmark of tumor. Many research groups have
demonstrated abundant quantity of exosomes released
by tumor cells exerting an immunosuppressive effect
that helps them evade immune response. Dysfunctions
of natural killer (NK) cells, which are a major component
of the anti-tumor immune response, have been reported
in various hematologic malignancies, including chronic
lymphocytic leukemia (CLL). BAG6, which issecreted by
tumor cell, activate receptor NKp30 on the surface of NK
cells, further increase NK cell cytotoxicity and promote
NK cells to kill cancer cells , and is suggested that down-
regulated or absent of exosomal BAG6 in CLL patients
evasion of CLL cells from NK cell anti-tumor activity,
(Reiners etal., 2013).
TGF-1 is a potent immunosuppressive molecule that
inhibits NK cell cytotoxicity. The serum concentration
of exsomal TGF-1 in the newly diagnosed AMLwas-
signi cantly higher than that of the normal control.
Furthermore, NK cell differentiation is IL-15 dependent,
and IL-15 plays a major role in NK-cell expansion and
promotes NK-cell survival, IL-15 is also able to counter-
act immunosuppressive effects mediated by TGF- car-
ried on exosomes from AML patients. Taken together,
these evidence suggest IL-15 can enhance anti-tumor
effects of NK cells in AML patient s, (Greco etal., 2009,
Hong et al., 2014). In virus-related lymphomas, EBV+
lymphoma cells are embedded in non-neoplastic by
standers: B and T cells, macrophages. There is increasing
evidence that the indirect actions (i.e. immunosuppres-
sion and TME components) of different viruses also play
signi cant roles in lymphomagenesis, (Esau, 2017).
For example, EBV and HIV-1 are proved capable
of inducing the overexpression of PD-L1 on antigen-
presenting cells, thus resulting in immunosuppression
by the increased apoptosis of T cells . Furthermore, the
EBV-miRNAs in the exosome secreted from EBV posi-
tive lymphoma cells were transferred to macrophage and
promote the development of lymphoproliferative disease
(LPD) in vivo mouse model, (Lichterfeld et al., 2008).
Another study showed that the exosomes present in the
serum of CHB patients contain both HBV nucleic acids
and HBV proteins, HBV might in uence NK-cell func-
tion via exosomes through upregulating immunosup-
pressive factors, such as TGF-, in HBV-infected CHB
patients. These observations suggest that exosomes may
serve as important regulators of HBV transmission and
may be involved in escaping innate immunity.
THE ROLE OF EXOSOMES IN DIAGNOSIS
AND PROGNOSIS OF HEMATOLOGICAL
MALIGNANCIES
Early cancer detection and disease strati cation or clas-
si cation are critical to successful treatment. Accessible,
reliable, and informative hematological malignancies
biomarkers can be medically valuable and can provide
some relevant insights into cancer biology [Elsherbini
and Bieberich., 2018]. Early detection of cancer could
be easily performed using exosomes isolated from body
uids such as blood plasma, serum, and urine, which
is allowing for a non-invasive method of detection of
hematological malignancies, (Kang etal., 2018).
Microvesicles are found in higher concentrations in
the sera of different types of hematological malignan-
cies, such as AML, AL L, CML, CLL, diffuse large B‐cell
lymphoma ( DLBCL), Hodgkin’s lymphoma (HL) and MM
patients and abundantly express surface proteins unique
to their cell of origin, which is rarely observed from serum
microvesicles of normal controls [Kang etal., 2018]. AML
biomarkers NPM1, FLT3, CXCR4, MMP9 and IGF-IR are
also present in AML cell derived exosomes, along with
mRNAs involved in leukemia development(Boyiadzis,
2016). Hong. etal (2014). indicated that exosomal TGF-
1 levels and relative levels of the three TGF-1 forms
(TGF-1 pro-peptide, latency-associated peptide (LAP),
and mature TGF-1 were distinct in AML patients in

Bing Xia etal.
352 THE CENTRAL ROLES OF EXOSOMESIN HEMATOLOGICAL MALIGNANCIES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Table 1. The role of exosome in the development of hematologic tumor.
Targets Type Disease Function Reference
TGF-1 MICA,
MICB,ULBP1,ULBP2,
BAG6
protein AML Reduce the ability of
natural killer (NK)
cells to kill leukemic
cells.
Cheng etal., 2011
Jaworski etal., 2014
GATA1, FOX3, SHIP1,
ID1, E2F1, CEBP-,
CEBP-, Myc, MEF2C
protein AML Induce development
of leukemia.
Malla etal., 2018.
PI3/AKT,MAPK/ERK,
NF-kB,TGF-1
protein CML Enhance cell survival
of LAMA84 cell
Sun etal., 2017
Tax, AKT, Rb, cFLIP,
NF-kB
ATL Enhance cell survival
in murine and human
T-cell cell lines.
Hong etal., 2014
Galectin-3, NF-kB protein ALL Promote ALL drug
resistance.
Hong etal., 2014
SRC, TSP-1, IL-8 protein CML Promotes endothelial
cell angiogenesis.
Nanbo etal., 2013
MiR-210 microRNA CML Promote the vascular
activity of CML.
Nanbo etal., 2013
Wnt3a protein DLBCL Promote the growth of
DLBCL cells.
Huan etal., 2014
IL-6, CCL2,
bronectin
protein MM Promote the growth of
MM cells.
Raimondo etal., 2015
miR-135b microRNA MM Promoteendothelial
vessel formation.
De etal., 2016
miR-34a, miR-125b- 5p,
miR-146a, miR-15a, miR
-137/197, miR- 21
microRNA MM Facilitate multiple
myeloma growth and
progression
Nanbo etal., 2013
Jaworski etal., 2014
AML: acute myeloid leukemia; CML: chronic myelocytic leukemia; ATL: T-cell acute lymphoblastic leukemia; ALL:
acute lymphoblastic leukemia; DLBCL: diffuse large B-cell lymphoma; MM: multiple myeloma
Table 2. Circulating vesicles as a general hematological malignancies biomarker.
Biomarker Type Source
Hematological
Malignancies
Reference
NPM1, FLT3, CXCR4, MMP9
and IGF-IR
protein serum
AML
Wang etal., 2014
MiR-92a MicroRNA serum ALL Hong etal., 2014
TGF-1 protein serum AML Reiners etal., 2014
miR155 MicroRNA serum
AML, CLL, WM,
MDS, MM
Elsherbini &
Bieberich 2018
Src protein serum CML Hong etal., 2014
MiR-155, MiR-210, MiR-21 MicroRNA serum DLBCL Rekker etal., 2014
MiR-22 MicroRNA plasma HL Caviano etal., 2014
MiR-15a, let-7b miR-18a,
MicroRNA plasma MM Caviano etal., 2014
AML: acute myeloid leukemia; ALL: acute lymphoblastic leukemia; CLL: chronic lymphocytic leukemia; WM:
macroglobulinemia of Waldenstrom; MDS: myelodysplastic syndrome; MM: multiple myeloma; CML: chronic
myelocytic leukemia; DLBCL: diffuse large B-cell lymphoma; HL: Hodgkin’s lymphoma.
different stages of chemotherapy[Hong C.S. etal., 2014].
This stability makes exosomes as suitable mines for
hunting reproducible and consistent biomarkers. ALL-
associated expression of miRNA92a can be detected in
the circulating vesicles of a majority of ALL patients
(Rekker K. etal., 2014)
In CML, Mineo etal. (2012), showed that a co-evolu-
tion between endothelial cells and CML cells are essential

Bing Xia etal.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE CENTRAL ROLES OF EXOSOMESIN HEMATOLOGICAL MALIGNANCIES 353
for leukemia progression and resistance to therapy. This
is possible because of the fact that K562 malignant cells
secret growth factors and various miRNAs and transport
these ‘endothelial inducing factors’ via exosomes. As a
result, tube formation is stimulated, even when treating
with imatinib, a tyrosine kinase inhibitor that targets the
Philadelphia chromosome-positive (Ph+) myeloid leuke-
mia cells. In this case, the development of angiogenesis
was reported to regulate the progression and dissemina-
tion of this hematological malignancy, (Wojtuszkiewicz
etal., 2016 Caivano etal., 2017).
Parikh etal. (2016) found the EV miR155 level may
serve as a promising prognostic/predictive biomarker in
CLL, independent of clinical stage . In conjunction with
the previously reported data, Caivano etal. (2017) found
that the EV miR155 levels were signi cantly higher in
CLL, AML and Waldenström’smacroglobulinemia (WM)
cases compared to controls (Parikh et al., 2016, Zhu
et al., 2018). Conversely, they also found that the EV
miR155 levels were signi cantly lower in myelodys-
plastic syndrome (MDS) and MM cases, (Caivano etal.,
2017). In addition, they found that high EV miR155 lev-
els correlated with high white blood cell counts in AML
patients. In conclusion, this study indicated that EV
miR155 may serve as an attractive new, non-invasive
diagnostic biomarker in human hematologic malignan-
cies. Levels of exosomal miR-155, miR-210 and miR-21
in serum from DLBCL patients (n=60) were higher than
control sera (n=43)(P=0.009), (Mineo etal. 2012).
Monique et al. found that EV-associated miR21-5p,
miR127-3p, let7a-5p, miR24-3p, and miR155-5p signals
were higher in primary and relapsed classic Hodgkin’s
lymphoma (cHL) patients compared with healthy indi-
viduals [van Eijndhoven M.A. etal., 2016]. However, they
also detected that miR21-5p and miR155-5p is small,
but signifcant, decreases (2-fold, P = 0.016) in plasma
after therapy. Nevertheless, the decrease in miR155-5p
was more pronounced in the EV fraction, (4-fold, P =
0.016). They detected high levels of miR127-3p in EVs
produced by HRS cells in plasma EVs of cHL patients but
less abundant levels in healthy control EVs, which sug-
gests that the pool of miR127-3p detected in the protein
fraction is unrelated to cHL tumor tissue and is derived
from other sources [van Eijndhoven M.A., etal., 2016].
miR-15a is lower in mesenchymal stromal cells derived
vesicles of MM patients compared with healthy subject,
and miRNA-rich exosomes secreted from MM-mesen-
chymal stromal cells facilitate MM progression , (Manier
etal., 2017).
Lower expression of let-7b or miR-18a was signi -
cantly associated with a high ISS stage. However, both
let-7b and miR-18a were independent predictors after
adjusting for the ISS and speci c cytogenetic abnormal-
ities. The effect of the two miRNAs on PFS and OS was
illustrated by Kaplan-Meier curves with dichotomized
miRNAs at the median [Manier S. etal., 2017]. These data
indicate that speci c miRNAs can be critical in de n-
ing worse prognosis in patients with newly diagnosed
MM. Nevertheless, to establish circulating exosomes as
biomarkers, well-designed clinical trials are required. So
far, there is no trial registered that is relevant to hema-
tology and investigates circulating exosomes as a pre-
dictive marker in hematological malignancies. Exosomes
are currently viewed as tumor cell surrogates or ‘liquid
biopsies’ and as a promising non-invasive metrics of
cancer. Exosomes might emerge as the most informative
non-invasive predictors of cancer outcome orresponse
to therapy.
EXOSOME-BASED HEMATOLOGIC
MALIGNANCIESTHERAPEUTICS
Exosome-based therapies serve as attractive strategy
against hematologic malignancies and solid tumors.
Exosome-based delivery methods have been tested in
the clinic successfully and were found to be well toler-
ated in patients. Being autologously generated within
the host, they can be engineered to carry drugs or tar-
get proteins without invoking immunogenic response.
A number of different strategies have been applied to
harness the potential of these exosomes. Nano injections
of RNAi in dendritic-derived exosomes allowed delivery
to the brain without invoking immune response. These
ndings were con rmed when Gurwitz et al. showed
that siRNAs can be delivered across the blood brain
barrier in a mouse model using systemic injections of
exosomes , (Gurwitz, 2016).
In another study, tumor-derived exosome-pulsed DCs,
tumor-derived exosomes, and exosomes isolated from
malignant ascites all have been investigated for their
ability to elicit antitumor immune response in patients,
(Liu et al., 2018). Likewise, their ef cacy on exosome
release or against exosomes has also been tested in dif-
ferent laboratories independently. These are some of the
studies highlighting the bene t of applying nanobased
assays in the design of exosomes drug therapies for
cancer. However, its clinical utility needs to be tested
in future studies. At present, there are several clini-
cal studies that utilize an exosome-based regimen for
solid tumors, (Que etal., 2016), but there is no clinical
trial about exosome-based agents against hematologic
malignancies ( ClinicalTrials.gov Website keyword search
exosome). These studies clearly point to an unexplored
area of research where researchers can nd answers to
some of the unexplained mechanisms attributed to the
multifaceted natural agents against hematologic malig-
nancies. Altogether, exosomes have multiple potential
clinical uses including the development of vaccines for

Bing Xia etal.
354 THE CENTRAL ROLES OF EXOSOMESIN HEMATOLOGICAL MALIGNANCIES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
targeting tumors; also, tumor-derived exosomes may be
useful as surrogate endpoints in evaluating therapeutic
and preventive approaches to hematological tumors.
CONCLUSIONS
Success in diagnosis and treatment of complex hema-
tological malignancies is dependent on our full under-
standing of the intricacies of interactions between dif-
ferent components within tumors. On the one hand, a
number of studies showed exosomes are emerging as
major players in inter- and intracellular communica-
tions. Exosomes have been shown to secrete diverse
biological molecules, which are in the context of tumor
cells survival, metastasis, drug resistance and immune
evasion. On the other hand, tumor relevant exosomes
play the important role in the areas of diagnostics and
drug therapy, regenerative medicine, and vaccines.
Taken together, technology and biology will inevitably
pave the way for the future use of exosomes analysis in
many preclinical research and clinical applications.
FUNDING
This work was supported by grants 81600163 and
81570201 from the National Natural Science Foundation
of China (NSFC) and Shandong Provincial Natural Sci-
ence Foundation (ZR2017PH057).
REFERENCES
Kornblau SM, etal. (1998). The role of apoptosis in the patho-
genesis, prognosis, and therapy of hematologic malignancies.
Leukemia, 12(1), 41-6.
Li, X, H. Zhong, etal. (2016). The diagnosis, prognosis, and
therapeutic application of MicroRNAs in haematological
malignancies. Hematology, 21(5): p. 263-71.
Rama Rao Malla, Santhi Pandrangi, Seema Kumari, et al.
(2018), Exosomal tetraspanins as regulators of cancer progres-
sion and metastasis and novel diagnostic markers. Asia Pac J
Clin Oncol, DOI: 10.1111/ajco.12869.
Pfrieger, F.W. and N. Vitale. (2018), Cholesterol and the journey
of extracellular vesicles. Journal of lipid research, 59(9).
Whiteside, T.L., M. Boyiadzis, (2017), Response commentary:
exosomes vs microvesicles in hematological malignancies.
Leukemia, 31(10): p. 2277.
Sun Z, S Yang, Q Zhou, etal. (2018), Emerging role of exo-
some-derived long non-coding RNAs in tumor microenviron-
ment. Mol Cancer, 17(1): p. 82.
Hong, C.S., etal. (2014), Plasma exosomes as markers of thera-
peutic response in patients with acute myeloid leukemia. Front
Immunol, 5: 160.
Huan, J., etal. (2013), RNA traf cking by acute myelogenous
leukemia exosomes. Cancer Res, 73(2):. 918-29.
Huan, J.,etal. (2013), RNA traf cking by acute myelogenous
leukemia exosomes. Cancer Res, 73(2): 918-29.
Long, Q., etal. (2017), Intranasal MSC-derived A1-exosomes
ease in ammation, and prevent abnormal neurogenesis and
memory dysfunction after status epilepticus. Proc Natl Acad
Sci U S A,114(17): E3536-E3545.
Zhou, J., etal. (2012), The pro-metastasis tyrosine phosphatase,
PRL-3 (PTP4A3), is a novel mediator of oncogenic function of
BCR-ABL in human chronic myeloid leukemia. Mol Cancer,
11: 72.
Raimondo, S., etal. (2015), Chronic myeloid leukemia-derived
exosomes promote tumor growth through an autocrine mecha-
nism. Cell Commun Signal, 13:8.
Jaworski, E., etal. (2014), Human T-lymphotropic virus type
1-infected cells secrete exosomes that contain Tax protein. J
Biol Chem, 289(32): 22284-305.
Koch, R., etal. (2014), Populational equilibrium through exo-
some-mediated Wnt signaling in tumor progression of diffuse
large B-cell lymphoma. Blood, 123(14): 2189-98.
Hazan-Halevy, I., et al. (2015), Cell-speci c uptake of man-
tle cell lymphoma-derived exosomes by malignant and non-
malignant B-lymphocytes. Cancer Lett, 364(1): 59-69.
Nanbo, A., etal. (2013), Exosomes derived from Epstein-Barr
virus-infected cells are internalized via caveola-dependent
endocytosis and promote phenotypic modulation in target
cells. J Virol, 87(18): 10334-47.
Chugh, P.E., et al. (2013), Systemically circulating viral and
tumor-derived microRNAs in KSHV-associated malignancies.
PLoS Pathog, 9(7): e1003484.
Roccaro, A.M., et al. (2013), BM mesenchymal stromal cell-
derived exosomes facilitate multiple myeloma progression. J
Clin Invest, 123(4): 1542-55.
De Veirman, K., etal. (2016), Induction of miR-146a by mul-
tiple myeloma cells in mesenchymal stromal cells stimulates
their pro-tumoral activity. Cancer Lett, 377(1): 17-24.
Umezu, T., etal. (2013), Leukemia cell to endothelial cell com-
munication via exosomal miRNAs. Oncogene, 32(22): 2747-
55.
Fang, Y., etal. (2016), PML-RARa modulates the vascular sig-
nature of extracellular vesicles released by acute promyelo-
cytic leukemia cells. Angiogenesis, 19(1): 25-38.
Tadokoro, H., et al. (2013), Exosomes derived from hypoxic
leukemia cells enhance tube formation in endothelial cells. J
Biol Chem, 288(48): 34343-51.
Paggetti, J., etal. (2015), Exosomes released by chronic lym-
phocytic leukemia cells induce the transition of stromal cells
into cancer-associated broblasts. Blood, 126(9): 1106-17.
Yoon, C., etal. (2016), Delivery of miR-155 to retinal pigment
epithelial cells mediated by Burkitt’s lymphoma exosomes.
Tumour Biol, 37(1): 313-21.
Umezu, T., etal. (2014), Exosomal miR-135b shed from hypoxic
multiple myeloma cells enhances angiogenesis by targeting
factor-inhibiting HIF-1. Blood, 124(25): 3748-57.

Bing Xia etal.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE CENTRAL ROLES OF EXOSOMESIN HEMATOLOGICAL MALIGNANCIES 355
Rizzo, M.T., et al. (2011), Cyclooxygenase-2 in oncogenesis.
Clin Chim Acta, 412(9-10): 671-87.
Isidori, A., et al. (2014), The role of the immunosuppressive
microenvironment in acute myeloid leukemia development
and treatment. Expert Rev Hematol, 7(6): 807-18.
Viola, S., etal. (2016), Alterations in acute myeloid leukaemia
bone marrow stromal cell exosome content coincide with gains
in tyrosine kinase inhibitor resistance. Br J Haematol, 172(6):
983-6.
Cheng, Y.L., etal. (2011), Increased galectin-3 facilitates leuke-
mia cell survival from apoptotic stimuli. Biochem Biophys Res
Commun, 412(2): 334-40.
Hu, K., etal. (2015), Galectin-3 mediates bone marrow micro-
environment-induced drug resistance in acute leukemia cells
via Wnt/beta-catenin signaling pathway. J Hematol Oncol,
8:1.
Fortuna-Costa, A., et al. (2014), Extracellular galectin-3 in
tumor progression and metastasis. Front Oncol, 4: 138.
Chapuy, B., etal. (2008), Intracellular ABC transporter A3 con-
fers multidrug resistance in leukemia cells by lysosomal drug
sequestration. Leukemia, 22(8): 1576-86.
Steinbach, D., etal. (2006), ABCA3 as a possible cause of drug
resistance in childhood acute myeloid leukemia. Clin Cancer
Res, 2006. 12(14): 4357-63.
Oksvold, M.P., etal. (2014), Expression of B-cell surface anti-
gens in subpopulations of exosomes released from B-cell lym-
phoma cells. Clin Ther, 36(6): 847-862.
Aung, T., et al. (2011), Exosomal evasion of humoral immu-
notherapy in aggressive B-cell lymphoma modulated by ATP-
binding cassette transporter A3. Proc Natl Acad Sci U S A,
108(37): 15336-41.
Greco, C., et al. (2009), Reduction of serum IGF-I levels in
patients affected with Monoclonal Gammopathies of unde-
termined signi cance or Multiple Myeloma. Comparison with
bFGF, VEGF and K-ras gene mutation. J Exp Clin Cancer Res,
28: 3
Wang, J., et al. (2014), Bone marrow stromal cell-derived
exosomes as communicators in drug resistance in multiple
myeloma cells. Blood, 124(4): 555-66.
Reiners, K.S., etal. (2013), Soluble ligands for NK cell receptors
promote evasion of chronic lymphocytic leukemia cells from
NK cell anti-tumor activity. Blood, 121(18): 3658-65.
Hong, C.S., etal. (2014), Plasma exosomes as markers of thera-
peutic response in patients with acute myeloid leukemia. Front
Immunol, 5: 160.
Esau, D. (2017), Viral Causes of Lymphoma: The History of
Epstein-Barr Virus and Human T-Lymphotropic Virus 1. Virol-
ogy (Auckl), 8: 1178122X17731772.
Lichterfeld, M., etal. (2008), Telomerase activity of HIV-1-spe-
ci c CD8+ T cells: constitutive up-regulation in controllers
and selective increase by blockade of PD ligand 1 in progres-
sors. Blood, 112(9): 3679-87.
Elsherbini, A., E. Bieberich. (2018), Ceramide and Exosomes: A
Novel Target in Cancer Biology and Therapy. Adv Cancer Res,
140: 121-154.
Kang, K.W., etal. (2018), The Potential of Exosomes Derived
from Chronic Myelogenous Leukaemia Cells as a Biomarker.
Anticancer Res, 38(7): 3935-3942.
Boyiadzis, M.,T.L. (2016), Whiteside, Plasma-derived exosomes
in acute myeloid leukemia for detection of minimal residual
disease: are we ready? Expert Rev Mol Diagn, 16(6): 623-9.
Hong, C.S., etal. (2014), Plasma exosomes as markers of thera-
peutic response in patients with acute myeloid leukemia. Front
Immunol, 5: 160.
Rekker, K., etal. (2014), Comparison of serum exosome isola-
tion methods for microRNA pro ling. Clin Biochem, 47(1-2):
135-8.
Mineo, M., SH Gar eld, S Taverna, et al. (2012), Exosomes
released by K562 chronic myeloid leukemia cells promote angi-
ogenesis in a Src-dependent fashion. Angiogenesis,15(1), 33–45.
Caivano, A.,
et al. (2017), MicroRNA-155 in serum-derived
extracellular vesicles as a potential biomarker for hematologic
malignancies - a short report. Cell Oncol (Dordr), 40(1): 97-103.
Wojtuszkiewicz, A., etal. (2016), Exosomes Secreted by Apop-
tosis-Resistant Acute Myeloid Leukemia (AML) Blasts Harbor
Regulatory Network Proteins Potentially Involved in Antago-
nism of Apoptosis. Mol Cell Proteomics, 15(4): 1281-98.
Parikh, S.A., T.D. (2016), Shanafelt, Prognostic factors and risk
strati cation in chronic lymphocytic leukemia. Semin Oncol,
43(2): 233-40.
Zhu, H.Y., etal. (2018), [Prognostic signi cance of CLL-IPI for
Chinese patients with chronic lymphocytic leukemia]. Zhong-
hua Xue Ye Xue Za Zhi, 39(5): 392-397.
Caivano, A., et al. (2017), MicroRNA-155 in serum-derived
extracellular vesicles as a potential biomarker for hematologic
malignancies - a short report. Cell Oncol (Dordr), 40(1): 97-103.
Mineo, M., etal. (2012), Exosomes released by K562 chronic
myeloid leukemia cells promote angiogenesis in a Src-depend-
ent fashion. Angiogenesis, 15(1): 33-45.
van Eijndhoven, M.A., etal. (2016), Plasma vesicle miRNAs for
therapy response monitoring in Hodgkin lymphoma patients.
JCI Insight, 1(19): e89631.
Manier, S., etal. (2017), Prognostic role of circulating exoso-
mal miRNAs in multiple myeloma. Blood, 129(17): 2429-2436.
Gurwitz, D. (2016), MicroRNAs as CNS Drug Targets. Drug Dev
Res, 77(7): 331-335.
Liu, H., etal. (2018), Dendritic cells loaded with tumor derived
exosomes for cancer immunotherapy. Oncotarget, 9(2): 2887-
2894.
Que, R.S., et al. (2016), Increasing the immune activity of
exosomes: the effect of miRNA-depleted exosome proteins on
activating dendritic cell/cytokine-induced killer cells against
pancreatic cancer. J Zhejiang Univ Sci B, 17(5): 352-60.