Environmental
Communication
Biosci. Biotech. Res. Comm. 9(4): 865-871 (2016)
Removal of cadmium from industrial waste water
by adsorption zeolite clinoptilolite
Leila Dstan
1
and Mohsen Dehghani
2,
*
1
Water and Wastewater Master’s Graduates, Islamic Azad University Bandar Abbas
2
Department of Environmental Engineering, Islamic Azad University Bandar Abbas Branch Hormozgan, Iran
ABSTRACT
Today, with the expansion of industry and industrial development, the risk of heavy metals pollution is more than
before. With due attention to the increasing of environmental pollution by heavy metals, using appropriate methods
is essential for removal of these elements from environment. In this study, the absorption of the Zeolite clinoptilolite
was used to remove Cd
+2
from industrial waste. For this purpose, the effect of pH parameters, contact time, adsorbent
dosageand initialconcentrationof cadmium was studied on the removal of cadmium ef ciency. The results showed
that, the optimal pH, optimal contact time and optimal adsorbent dosage is equal to 4 and 15 minutes and 1 g/L
respectively. The results of Langmuir, Freundlich, Temkin and Dubinin–Radushkevich adsorption isotherms showed
that Freundlich model is the best adapted model to the equilibriumexperimentdata for absorbent. According to the
ef ciency of natural zeolite clinoptilolite for removal of cadmium from industrial waste with an ef ciency of 98%
and also due to low cost, available and less environment effects of this absorbent than many of kinetics absorbent is
proposed that industrial units use this kind of absorbent in waste water treatment.
KEY WORDS: REMOVAL, CADMIUM, CLINOPTILOLITE, INDUSTRIAL WASTEWATER
865
ARTICLE INFORMATION:
*Corresponding Author: Dehghani933@gmail.com
Received 2
nd
Aug, 2016
Accepted after revision 20
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
The risk of environmental pollution, resulting from human
activities has become a global concern (Alvarez-Ayuso,
2003).Heavy metals are also an important group of pol-
lutants in the environment. Today, with the development
of industry and technology the risk of heavy metals pol-
lution has been created in the environment more than
before (Inglezakis et al., 2004). Most of the heavy metals
are very soluble at low concentration and they have thea-
bility to access to vital components of living things. In the
nature, heavy metals are in the group of rate elements and
generally have been formedless than one percent of the
Earth’s crust (Erdem et al., 2004). Removal of heavy metals
from wastewater has become a major concern nowadays
because of its ability to contaminate water bodies.
866 REMOVAL OF CADMIUM FROM INDUSTRIAL WASTE WATER BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Leila Dstan and Mohsen Dehghani
Many processes have been proposed for removal of
heavy metals, including chemical sedimentation, mem-
brane ltration, ion exchange, reverse osmosis and
adsorption by the activated carbon which is the most
common of these methods (Buerge-Weirich et al., 2002).
Researchers for the aim of environment protection are
looking for appropriate solutions, scienti c and eco-
nomical to minimize the harmful effects of ef uent.
In this regard, the using of methods and materials that
have minimal side effects are in top priority. Zeolite with
extraordinary structure, high cation exchange capacity,
keeping structure in high temperature, low cost and abun-
dant distribution of it in the world has been caused to
consider as a remover (Cecille and Toussaint, 1989, Bable
et al., 2003, Rosales et al., 2012 and Kalantry et al 2014).
These minerals are used primarily in the high range
in the industry as an adsorbent for removal of oil and
metals (Inglezakis et al., 2004, Papaioannou et al., 2005).
clinoptilolite is an inorganic and natural compound and
safe, non toxic and harmless that using of that does not
have sideeffects on the environment (Wu, 2008). In this
study, with due regard to the previous studies for remov-
ing heavy metals from waste water and industrial water,
it is tried to present best conditions to remove cadmium
ion from simulated aqueous solution in the waste water
by using of zeolite.
MATERIAL AND METHODS
In this study, zeolite was studied as adsorbent for the
removal of cadmium ions from industries waste water
to evaluate the effect of studied process of pH, con-
tact time, concentration of cadmium, adsorbent mass
and interferences effect. Utilizing Zeolitein this study
was obtained from mines of Semnan in the central of
Iran. pH meter system multi 340 I and shaker system
FG model with regulating capability of round 50 to 600
was used for determining pH and for samples disorder
respectively. The solution of centrifuge system centrion
model was used to separate absorbent. This study was
conducted non continuous in laboratory scale at tem-
perature of 25ºC and.
1) The Preparation of absorbent
Zeolite clinoptilolite samples were obtained from Sem-
nan mines and they were used without any chemical
modi cations. At rst, by using a sieve 1 & 3 mm, Par-
ticles absorbent have been grouped, and in different
experimentation processes were used from these Parti-
cles in the size of 1-3 mm of absorbent.
2) Preparing an aqueous solution ofcadmium ion
At rst, stock standard production was provided
1000 m/g per liter cadmium from sulfate cadmium
(CdSO
4.
8H
2
O) and then standard solutions was made
from it with concentrations1, 2.5, 5, 10.
3) The adsorption experiments stages
Experiments for determining the effects of pH on the
adsorption process:
At this stages, cadmium solution was prepared with a
concentration of 15mg per liter and its pH was regulate
by 1 and 0.1 HCl and NaOH on the values of 4,5,6,7,8,
and 9. Then Zeolite adsorbent was added with mass of
1g. The samples were analyzed for 120minutes on a
shaker with speed of 180rpm and were centrifuged for
20minutes and nally the remaining cadmium concen-
tration was measured by atomic absorption spectrom-
etry. At rst, the concentrations of 1, 2.5, 5, and 10 mg/l
of cadmium was prepared from stock solution and after
regulating pH, about 1 g of adsorbent was added to
the samples. All of the laboratorial pipes settled on the
shaker to shake and then after separating the solution
from adsorbent with centrifuge, the remaining value of
Cadmium was measured in the solution. After determin-
ing the optimal pH and concentration of cadmium, the
effect of adsorption value was carried out for Zeolite
clinopetilolite adsorbent in the masses of 0.1, 0.25, 0.5
and 1g in the solution and then the remaining concen-
tration Cadmium was measured.
For determining the optimal time, 20 ml of Cadmium
solution was prepared separately with concentration of
2.5 ppm at the optimal pH. The value of 1 g of adsor-
bent (optimal adsorbent) was added to the sample. Solu-
ble were separately on the shaker in contact times of
5,10,15,20,30,60 minutes with absorbent with around
180rpm, and after separation of solution, the remaining
concentration of cadmium ion was measured by using
atomic absorption system.In order to evaluate the effect
of other ions on cadmium on the zeolite, according to
the analysis of industrial units `ef uent, an simulation
ef uent was prepared and ions Cu
+2
, Mg
+2
, Zn, Ca
+2
, Pb
+2
,
Cl
-
, NH
4+
, and sulfate was selected and studied as the
experimental matrix.
In this study, Langmuir, Freundlich, Temkin and
Dubinin–Radushkevich isotherms models were used
for modeling process of cadmium adsorption. Lang-
muir isotherm based on a uniform (homogenous) and
monolayer of adsorb material with the same energy is
on all the levels of the adsorbent. Freundlich isotherm
unlike Langmuir is on the adsorbent, based on multi-
layer adsorption and heterogeneous adsorbent material.
Temkin isothermal include one factor that shows clearly
interactions between adsorbent and adsorb particles
and Dubinin–Radushkevich model is more public than
Langmuir, because in this model the monotonousness
of adsorption sites is not needed (Crittenden et al., 2012,
Naghizadeh, et al, 2011).
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS REMOVAL OF CADMIUM FROM INDUSTRIAL WASTE WATER 867
Leila Dstan and Mohsen Dehghani
Freundlich isotherm model:
Equation 1 represents the mathematical model of Frend-
lich isotherm.
(1)
Mass ratio of the solid phase which is the mass of
adsorbent material, ratio to the mass of absorbent mate-
rial.
C
e
=Concentration in equilibrium
Kempirical constant (Freundlich equation coef cients)
Langmuir isotherm model:
The mathematical model of this isotherm, have been
shown in Equation 2:
(2)
B and qm are empirical constants
Ce and q parameters are resemblance to Frendlich iso-
therm.
Temkin Isotherm:
The general from of Temkin isotherm is as follows:
By considering B=RT/B a linear form of Temkin isother-
mal will be as follow:
In this equation A in terms of mg/l is equivalent to
bond constant associated with maximum energy, and
b in terms of mol/J is Temkin isothermal constant,
and b (no unit) constant is proportional to the heat of
adsorption.
Dubinin Rudoshkevich isotherm model
In this equation:
qe is the amount of dissolved material that is on the
adsorbent mass unit and qm is the capacity of absorp-
tion of adsorbent in per unit mass.
K is Dubinin–Radushkevich constant adsorption and
is potential.
RESULTS
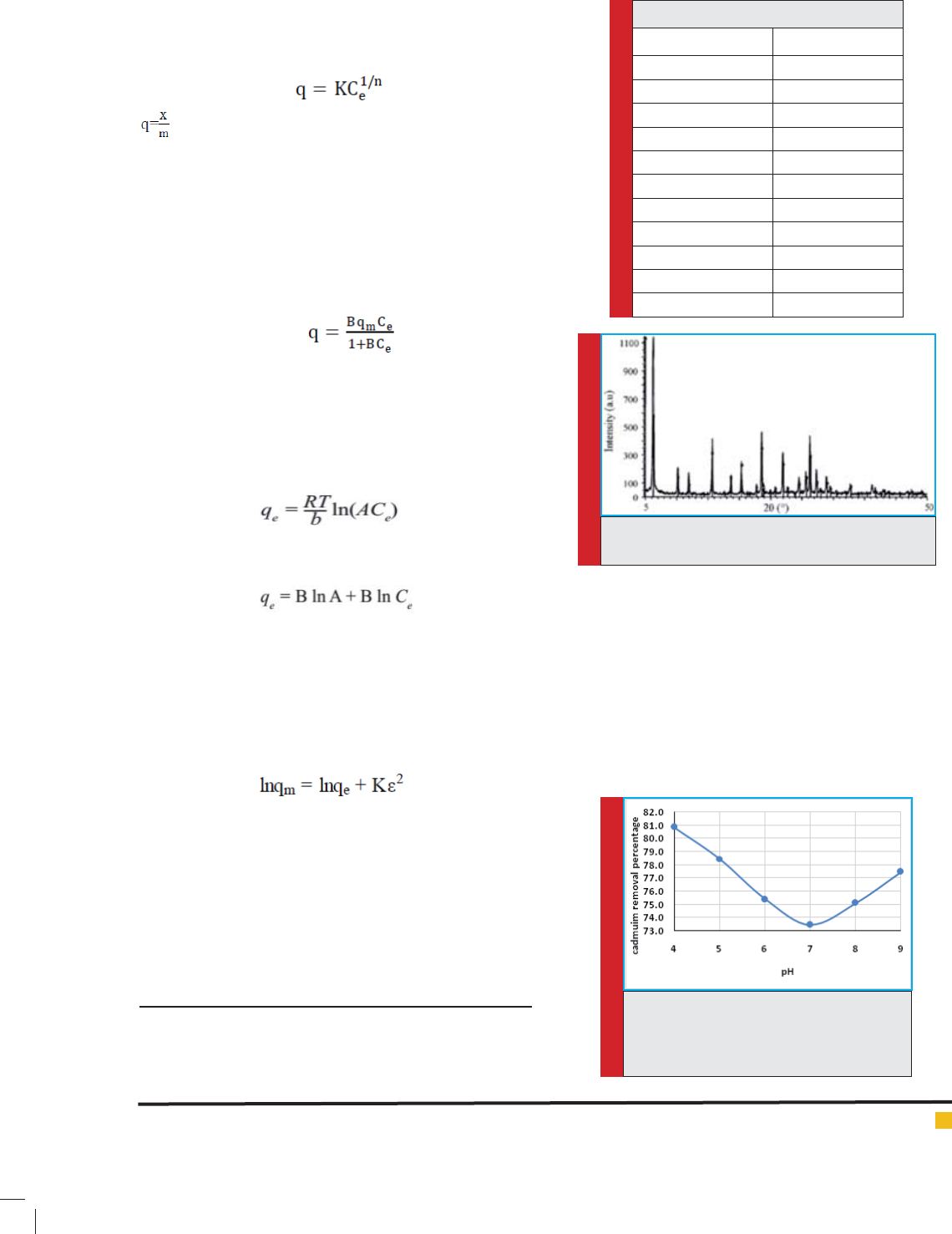
Clinoptilolite mineral analysis and mapping of x-ray
diffraction of this mineral are presented in table1 and
gure 1.
The effect of pH on cadmium adsorption:
The effect of pH solution on the adsorption of cadmium
ions showed that the adsorptions process of the Zeo-
lite have better conditions in acidic pHs and with the
increasing of pH from 4 to 9 the value of removal is
decreased and then by increasing of pH from 7 to 9
adsorption will increased. The presented results in gure
2 shows that the highest adsorption ef ciency of cad-
mium was observed at pH=4.
FIGURE 1. The radiation of X-ray related to the
zeolite powder samples.
FIGURE 2. The effect of pH parameter on
cadmium adsorption ef ciency at tempera-
ture of 25ºC and 15 minutes, the concen-
tration of 1gr/L of adsorbent.
Table 1: Analysis of zeolite clinoptilolite
PercentageComposition
61.91SiO
2
11.02Al
2
O
3
0.99Fe
2
O
3
0.17TiO
2
0.32CaO
0.79MgO
6.75Na
2
O
2.47K
2
O
0.01P
2
O
5
14.34LOIa
98.78Total
868 REMOVAL OF CADMIUM FROM INDUSTRIAL WASTE WATER BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Leila Dstan and Mohsen Dehghani
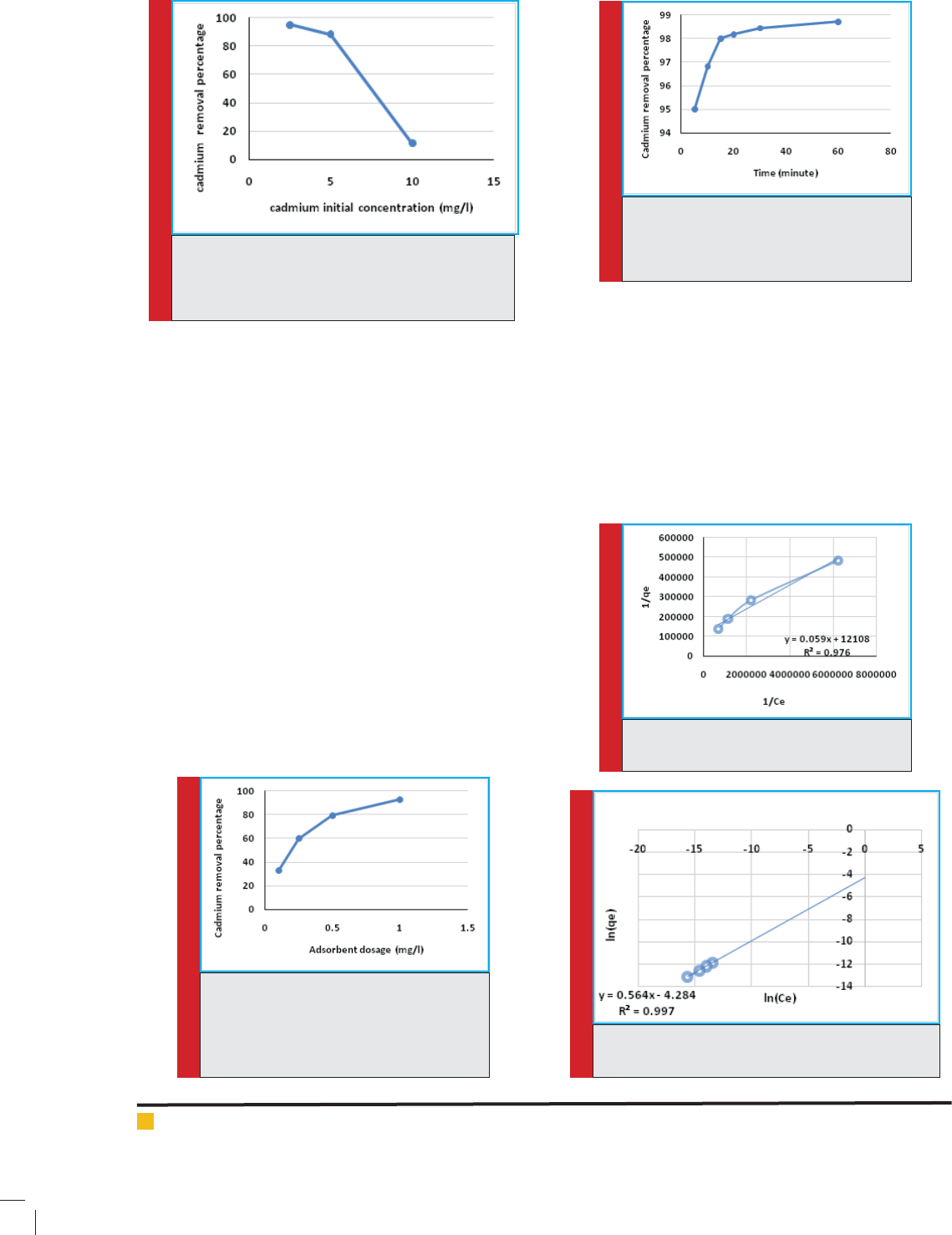
FIGURE 4. The effect of adsorbent value on
the cadmium removal ef ciency in optimal
pH4, at temperature 25ºC,cadmium optimal
concentration of 2.5 ppm in 15 minutes
and 1 gr/L concentration of adsorbent.
Initial concentration effect on cadmium adsorbent
In gure 3, adsorption data have been shown in differ-
ent initial concentrations of cadmium ion. As you can
see the value of adsorption is at the highest by zeolite in
concentration of 2.5 mg/l.
The effect of zeolite clinoptilolite adsorbent for the
removal of cadmium
The results showed that, by increasing of 0.1 to 1g of
adsorbent, the removal rate has increased from 32.70
to 92.77, which according to obtained result with the
weight of 1g per liter is selected as optimal weight of
adsorbent to absorb cadmium. (Figure 4).
The effect of time on the adsorption process.
Figure 5 shows the effect of time on the absorption pro-
cess. At the initial times the rate of absorption is very
high and by passing time, the removal rate has increased
and after 15 minutes of contact time, the removal rate-
will change with low slope.
Interferences effect on the process of cadmium adsorption
The comparison of removal rate of cadmium in the terms
of without interference ions and at the presence of inter-
fering ions shows that at the presence of interference
ions did not have signi cant impact on the process of
removing cadmium by using zeolite clinoptilolit. The
obtained results in this study correspond with the results
of Motsi et al(2009). So that the removal of cadmium
was 97% at the presence of while this amount for solu-
tions without interfering ions was 98.1%.
FIGURE 3. SThe effect of different concentration on
removal ef ciency inoptimal pH 4 at the tempera-
ture of 25ºC, in 15 minutes and concentration of 1
gr/L adsorbent.
FIGURE 6. Langmuir isotherm curve for
cadmium absorption in optimal condition.
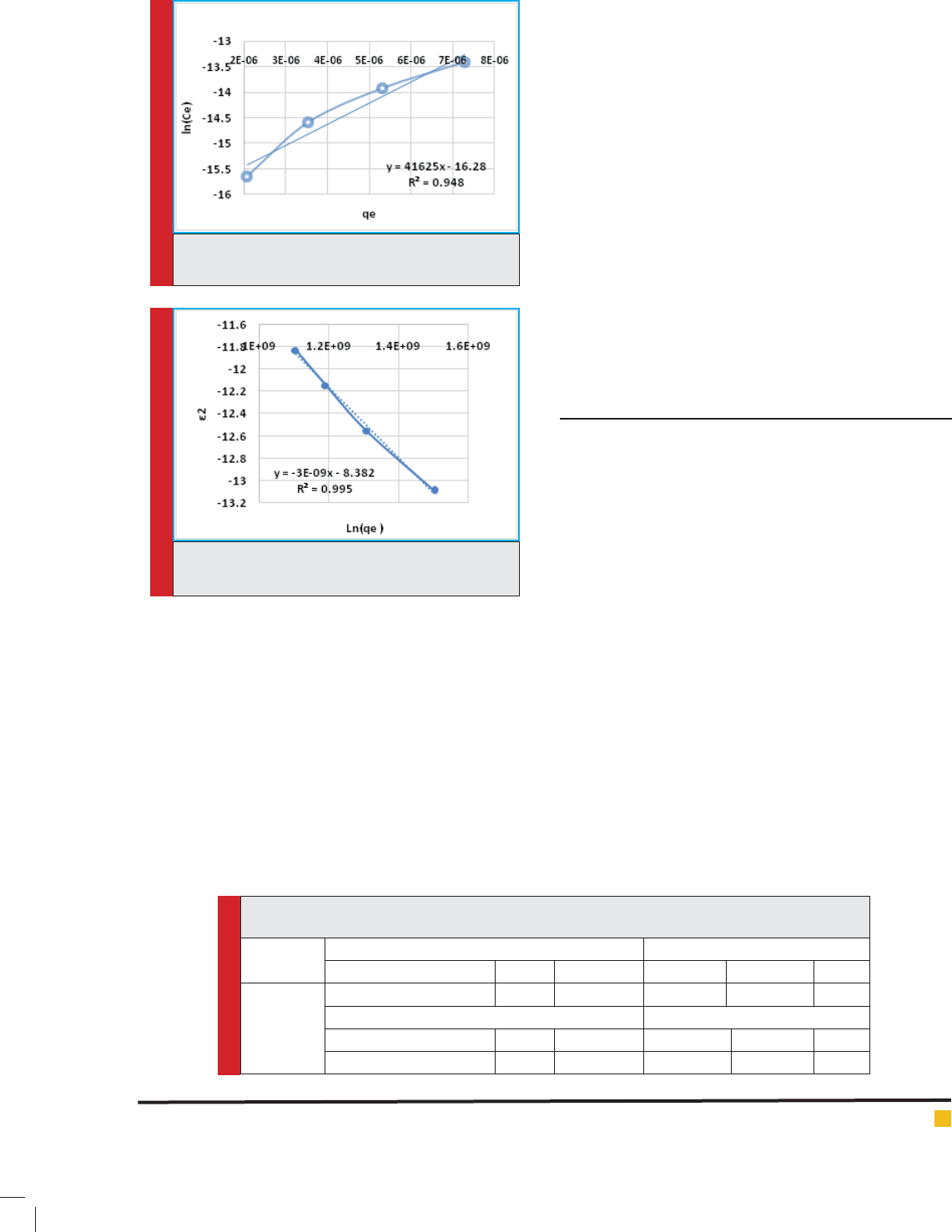
FIGURE 7. Freundlich isotherm curve for adsorption
of cadmium in optimal condition.
FIGURE 5. The effect of contact time
changes on cadmium removal ef ciency
inoptimal pH (4), the concentration of 2.5
ppm cadmium at a temperature of 25ºC.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS REMOVAL OF CADMIUM FROM INDUSTRIAL WASTE WATER 869
Leila Dstan and Mohsen Dehghani
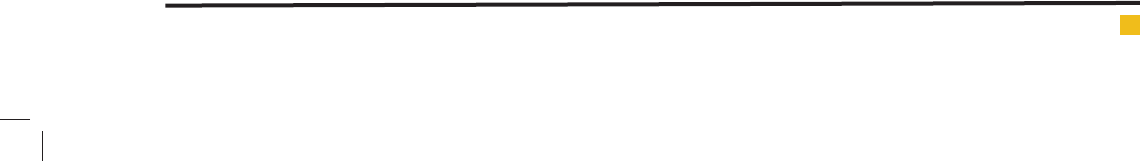
Table 2: The results of adsorption isotherm coef cients for the removal of cadmium from
aqueous by zeolite clinoptiolite.
LangmuirFreundlich
Adsorbent
R
2
KL (L/mg)q
max
(mg/g)R
2
NK(mg/g)(mg/l)
n
0.9764.9316.730.9981.770.013
Zeolite
TemkinDubinin–Radushkevich
R
2
KBR
2
KE(kJ/mol)
0.9495.142.270.995109×312.91
The results of adsorption isotherms for zeolite
clinoptilolite adsorbent.
The gures of 6, 7, 8, and 9 show the Langmuir, Fre-
undlich, Temkin and Dubinin–Radushkevich isotherms
for studied adsorbent respectively. According to the
obtained results, it is observed that cadmium adsorp-
tion by zeolite clinptilolite from Freundlich model with
a correlation coef cient R
2
=0.998 have better ef ciency
than other isotherm models.
In Langmuir isotherm model, the coef cients of q
max
and K
L
will be calculated respectively through the slope
and intercept of linear graph versus Ce.
In Freundlich isotherm model, we have constants of
(n) and (K
f
) that n is desirability of index in the adsorp-
tion process (densities) and K
f
is the absorption capacity
in terms of mg/g(1/mg)
1/n
. In this model, the values of n
less than 1, indicates poor absorption and the values of
1-2 and 2-10 represent optimal and average adsorption
respectively.
In Temkin isotherm model, the amount of coef cients
of B and K
t
will be determined through slope and inter-
cept linear graph q
e
versus LnC
e
respectively.
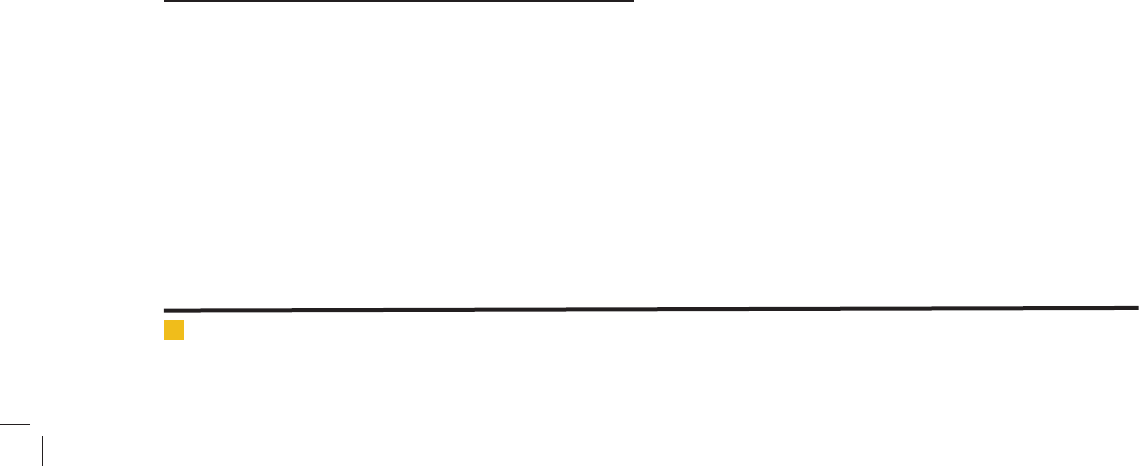
E Index is used for Radushkevich isotherm to evalu-
ate the capability of using equation. This parameter pro-
vides information related to adsorption process mecha-
nism (physical, chemical), in such a way that the values
of E between 8 and 16 kJ/mol indicate that adsorption
process has been follow a chemical mechanism and for
values less than 8 kJ/mol, the mechanism had physical
natural adsorption process (RezaeiKalantary et al., 2014).
DISCUSSION
The obtained results of initial pH effect of solution
showed that the removal ef ciency of cadmium by zeo-
lite clinoptilolite, will be affected by the solution pH. In
adsorption process, oH
-
and H
+
are two determiners ions
for surface charge. (Lu et al, 2009). Natural zeolite pref-
erably tends to absorb H
+
ions from solution in compari-
son with heavy metals ions (Inglezakis et al., 2004). So
by increasing of acidic conditions the adsorption of H
+
ions will increase from solution that includes the most
ef ciency for removing of cadmium, which its results
correspond with the results of Moreno et al. (2001). In
Low concentration of cadmium, speci c surface and the
sites ofadsorbent adsorption were more and cadmium
ions are able to react to each other with available absorp-
tion opportunities on adsorbent surface and therefore
the removal rate will increase. The removal ef ciency
of cadmium ions from solution
has reverse relation by
using of zeolite clinoptilolite with concentration more
than 2.5 mg per liter and the results correspond with the
results of Motsi et al (2009).
The study of balance time effect, showed that the
removal of cadmium in contact time of 15 minutes have
FIGURE 8. Temkin isotherm curve for cadmium
adsorptionin optimal conditions.
FIGURE 9. Dubinin–Radushkevich isotherm curve
for cadmium adsorption in optimal condition.
Leila Dstan and Mohsen Dehghani
870 REMOVAL OF CADMIUM FROM INDUSTRIAL WASTE WATER BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
maximum ef ciency by zeolite clinoptilolite. The cause
of high absorption rate in the initial moments of reac-
tion is a large number of absorption active sites (Gho-
rai 2005). These results correspond with the results of
Shawabkeh (2004).The results of adsorbent dosage effect
on the adsorption process showed that, by increas-
ing of adsorbent value, the value of cadmium absorp-
tion will be increased, that is because of increasing the
number of levels at the available of adsorbent because
of its increasing, which leads to an increasing in the
level of contact and increasing in the free bonds on
the level of adsorbent (Wajima et al., 2009; Ramdani
et al., 2010).These obtained results correspond with the
results of Shawabkeh, 2004 and Shamohammadi et al.,
2008).
Rate and value of adsorption is subject to charge
and mass of adsorbent. At all multipartite soluble con-
centrations, the amount of total absorb heavy metals
ions increased in per unit mass of natural zeolite in
comparison with the amount of absorbed article from
partial solutions. This shows that the difference in the
mechanism of surface absorption may include surface
absorption per action. The obtained results correspond
with Motsi et al (2009).In Freundlich model has been
supposed that the adsorbed exit on heterogeneous sur-
faces will be occurred by adsorption on multi layers.
By performed calculation it showsthat the value of R
2
is equal to 0.998 and n is equal to 1.77 and it indicates
that this adsorption is optimal and the reaction follows
from Freundlich equation. Also in this study, based on
Radushkevich model E is equal to 12 kJ/mol (between
8 and 16 kJ/mol). So adsorption process is a chemical
reaction. In a study that carried out by Motsi et al on
the removal of Mn
2
by natural zeolite, also the obtained
results by Wanga and Peng (2010) as well as the results
of Mishra and Patel (2009) on the modi ed zeolite in
aqueous environment, this process follows Freundlich
model that corresponds with the results of the removal
of cadmium zeolite clinoptilolite.
CONCLUSION
The results of this study shows that natural zeolite for
removal of cadmium from industrial waste water under
optimal conditions (times of 15 minutes, pH=4, adsor-
bent dosage of 1 g per liter and concentration of 2.5
ppm cadmium) has very good ef ciency. The bene ts of
this natural, low cost, mineral article, is easy access to
it. Moreover this absorbent is more advantageous than
common absorbents such as activated carbon or opera-
tional procedures such as electro dialysis and exists in
the numerous mines in Iran, including Semnan, Kerman
and Azarbaijan.
REFERENCES
Cecille, L., Toussaint, J. (1989) Future Industrial Prospects of
Membrane Processes. Springer Netherlands.
Alvarez-Ayuso, E., Garcia-Sanchez, A., Querol, X., (2003) Puri-
cation of metal electroplating waste waters using zeolites.
Water Res. 37, 4855–4862.
Babel. S, Kurniawan. T.A, (2003), Low-cost adsorbent for heavy
metals uptakefrom contaminated water: a review, J. Hazard.
Mater. B97. 219–243.
Crittenden, J. Trussell, R. Hand, D. Howe, K, Tchobanoglous,
G. (2012) Water Treatment: Principles and Design. New York:
John Wiley and Sons.
Erdem, E., Karapinar, N., Donat, R. (2004). The removal of
heavy metal cations by natural zeolite. J. Colloid Interface Sci.
280, 309–314.
Ghorai S, Pant KK (2005) Equilibrium, kinetics and break-
through studies for adsorption of uoride on activated alu-
mina. Separation and puri cation technology 42 (3), 265-271
Inglezakis V.J. and Grigoropoulou H.P. (2004) Effects of oper-
ating conditions on the removal of heavy metals by zeolite in
xed bed reactors, J. Hazard. Mater., B112 37–43.
Lu J, Li Y, Yan X, Shi B, Wang D, Tang H (2009) Sorption of
atrazine onto humic acids (HAs) coated nanoparticles. Colloids
Surf A Physicochem Eng Asp 347:90–96.
Moreno N, Querol X., Ayora C., Pereira C.F.(2001) Utiliza-
tion of zeolites synthesized from coal y ash for the puri -
cation of acid mine waters. Environ Sci Technol. 2001 Sep
1;35(17):3526-34.
Motsi, T., Rowson, N.A. and Simmons, M.J.H. (2009) Adsorp-
tion of Heavy Metals from Acid Mine Drainage by Natural
Zeolite. International Journal of Mineral Processing, 92, 42-48.
Naghizadeh, A. Nasseri, S. Nazmara, Sh.(2011) Removal of
Trichloroethylene from Water by adsorption on to Multiwall
Carbon Nanotubes, Iran. J. Environ. Health. Sci. Eng., vol. 8,
No. 4, pp. 317-324.
Papaioannou D., Katsoulos P.D., Panousis N. and Karatzias H.
(2005). The role of natural and synthetic zeolites as feed addi-
tives on the prevention and / or the treatment of certain farm
animal diseases: a review. Micropor. Mesopor. Mat. 84, 161-
170.
Ramdani A, Taleb S, Benghalem A, Ghaffour N.(2010) Removal
of excess uoride ions from Saharan brackish water by adsorp-
tion on natural materials. Desalination 250 (1), 408-413.
Wajima T, Umeta Y, Narita S, Sugawara K.(2009) Adsorption
behaviour of uoride ions using a titanium hydroxide-derived
adsorbent. Desalination; 249:323–30.
Buerge-Weirich D., Hari R., Xue H., Behra P.(2002) Adsorp-
tion of Cu, Cd, and Ni on goethite in the presence of natural
groundwater ligands Environ Sci Technol.1;36(3):328-36.
Shamohammadi, Z., Moazed H., Jaafarzadeh, N., and Haghighat
Jou, P. (2008). Removal of low concentrations of cadmium
from water using improved rice husk. Journal of Water and
Wastewater, Vol. 19 No.3 (67), 27-33.
Leila Dstan and Mohsen Dehghani
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS REMOVAL OF CADMIUM FROM INDUSTRIAL WASTE WATER 871
Shawabkeh. R.A (2004) Synthesis and characteriza-
tion of activated carbo-aluminosilicate material from oil
shale. Microporous and Mesoporous Materials 75(1):107-
114·
Rezaei Kalantary R, JonidiJafari A., Kakavandi B., Nasseri S.,
Ameri A., Azari A (2014) Adsorption and Magnetic Separation
of Lead from Synthetic Wastewater Using Carbon/Iron Oxide
Nanoparticles Composite. J Mazandaran Univ Med Sci 24(113):
172-183.
Rosales, E., Pazos M., Sanromán, M. A., & Tavares, T. (2012).
Application of zeolite-Arthro bacter viscosus system for the
removal of heavy metal and dye: Chromium and Azure B.
Desalination, 284, 150–156.
Mishra P.C., Patel R.K.(2009) Removal of lead and zinc ions
from water by low cost adsorbents, J. Hazard. Mater. 168
(2009) 319–325.
Wanga S., Peng Y. (2010) Natural zeolites as effective adsor-
bents in water and wastewater treatment, Chemical Engineer-
ing Journal. 156 11–24.
Wu D., Sui Y., He S., Wang, Li C., Kong H.(2008) Removal of
trivalent chromium from aqueous solution by zeolite synthe-
sized from coal y ash, J. X. Hazard. Mater. 155 415–423.