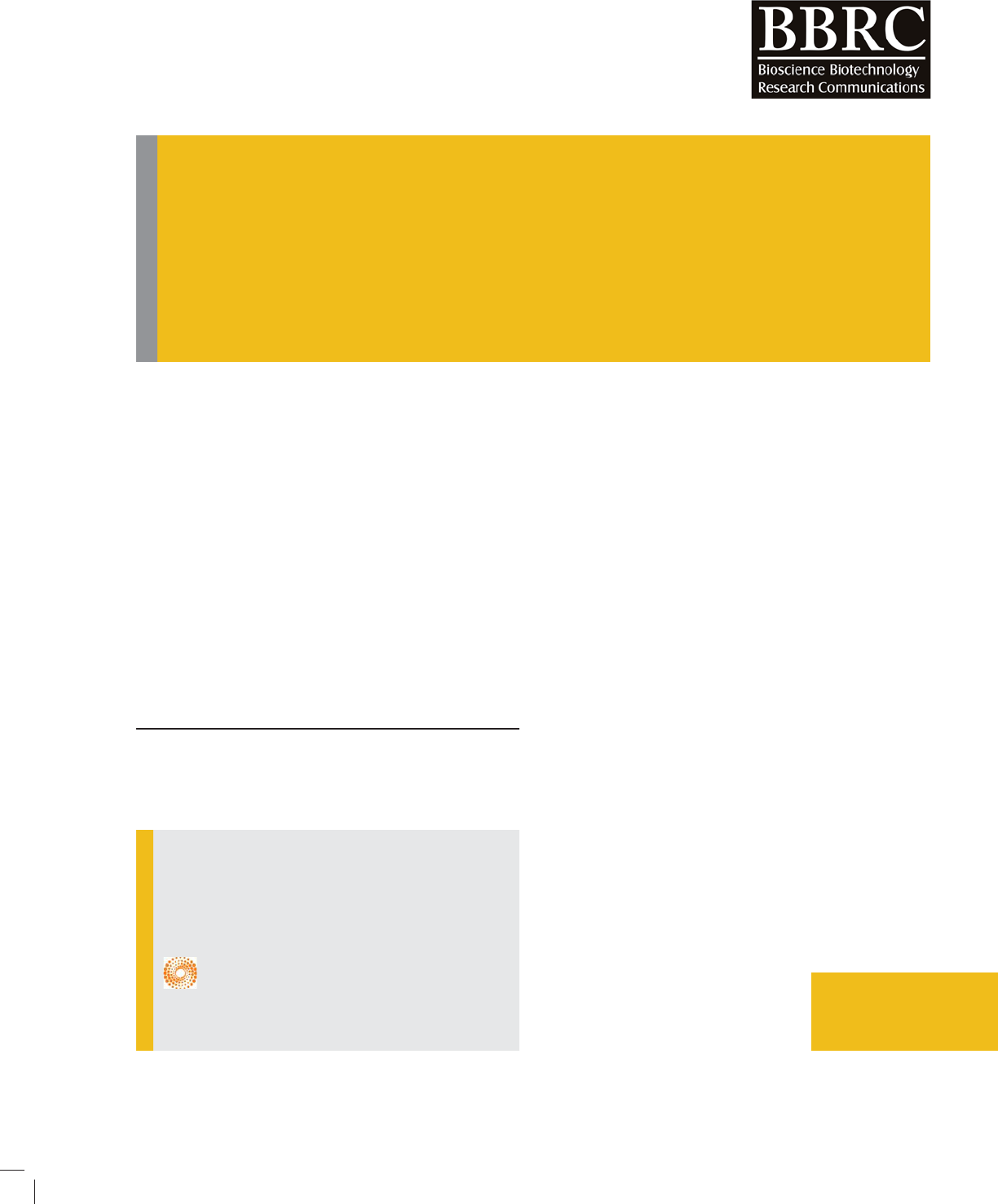
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 9(4): 769-775 (2016)
Synthesis of green ZnO/SiO
2
nanocatalyst and its
application to reduce acenaphthylene from re nery
waste water
Rajani Bharati and S. Suresh*
Green Catalysis and Process Technology Research laboratory, Department of Chemical Engineering, Maulana
Azad National Institute of Technology, Bhopal-462 051
ABSTRACT
Plants extract have a vital role for the synthesis of different nanocatalysts like TiO
2
, ZnO, Fe
2
O
3
etc. due to its non-
toxicity, low cost. Many of these nanocatalysts have been used for the degradation of water pollutants. Some of the
plants extract are used as reducing and stabilizing agent in the synthesis of nanoparticles. No work has been found
on Butea monosperma (Palash) ower extract with ZnO nanoparticles. Phenol is found in many industrial ef uents
and excessive exposure of its may cause coma, cyanosis and even death. This research work focuses on the syn-
thesis of ZnO/SiO
2
with palash ower extract nanocatalyst by using single pot process for degradation of phenol
from aqueous solution. The synthesized nanocatalyst of ZnO/SiO
2
palash ower extract was characterized by using
BET surface area, FEG-SEM, EDAX, XRD, and FTIR. BET surface area of nanocatalyst is 157.23 m2/g and FEG-SEM
micrograph shows that spot of nanorange particles. Optimum percent removal of acenaphthylene was found to be
81% at 0.5g/500ml catalyst loading, 30oC temperature and 4h reaction time. Synthesis of ZnO/SiO
2
nanocatalyst with
palash ower extract by single-pot process is eco-friendly and cost effective method than other chemical methods.
KEY WORDS: SYNTHESIS, ZNO/SIO
2
, NANOCATALYST, PALASH, FLOWER, EXTRACT, ACENAPHTHYLENE, DEGRADATION
769
ARTICLE INFORMATION:
*Corresponding Author: rajniem000@yahoo.co.in
Received 27
th
Nov, 2016
Accepted after revision 27
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Palash tree is also known as Butea monosperma, is
a very use full tree which is easily available in India
(Hegde et al., 2014). From literature it has been found
that palash ower has many biomolecules some are trit-
erpene, avonoids, glycosides carbohydrate and protein
(Jamkhade et al., 2013). It has been found that solubility
of palash ower is more in water than other solvents for
extractive value (Hegde et al., 2014). It has been found

770 SYNTHESIS OF GREEN ZNO/SIO
2
NANOCATALYST AND ITS APPLICATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Rajani Bharati and S. Suresh
that using plants extract as reducing and stabilizing
agents for nanocatalysts synthesis is eco-friendly and
cheap method than other methods which use chemicals
(Dizaji et al., 2015).
Single pot method also known as one step method
is the bioreduction processes in which plant extracts are
used as reducing and stabilizing agent to reduce metal
ions in nano range (Gdikandula et al., 2016). Palash
ower extract contains a broad variability of biomole-
cules so this method is faster than other methods (Kumar
and Yadav, 2012).
No research article has been found in the literature
for the synthesis of ZnO/SiO
2
based nanocatalyst by
using an aqueous palash ower extract as reducing
and stabilizing agent. This work is different from other
works because palash ower extract contains many
metals naturally which increases catalysts photocata-
lytic activity. SiO
2
has been found naturally in palash
ower extract. Incorporation of ZnO with SiO
2
increases
its photocatalytic activity for visible light. So ZnO/SiO
2
nanocatalyst synthesized by this method will be more
effective under sunlight to remove pollutant than ZnO
nanocatalyst synthesized by other methods. We can
use this nanocatalyst to treat ef uents from industries
under sunlight also. ZnO/SiO
2
with palash ower extract
nanocatalyst was used for acenaphthylene removal from
re nery wastewater in an annular reactor. We have
found Hexagonal crystal structure of ZnO nanocatalyst
which is the most stable in the environment. Synthe-
sized nanocatalysts were characterized by Brunauer-
Emett-Teller (BET), energy dispersive atomic X-ray
(EDAX), Field Emission Gun-Scanning electron micro-
scope (FEG-SEM), and X-ray diffraction (XRD) and Fou-
rier transform infrared spectroscopy ( FTIR) to determine
their Surface area, size, shape, particle distribution and
functional group.
MATERIALS AND METHODS
ZnO bulk material (Molychem from Mumbai, 99.5%
purity), acenaphthylene (Ranbaxy ne chemicals from
New Delhi, 99% purity),Sodium hydroxide (A. B. Enter-
prises, Mumba,, 98% purity),HCL (Central drug house
Ltd. From Delhi, 35% purity), Palash owers, (Butea
monosperma) were collected from MANIT Bhopal cam-
pus, Double distilled water. ZnO bulk material was used
as a precursor to make 1mM ZnO aqueous solution; HCL
acid was used to making 1 M solution to dissolve ZnO in
water. NaOH to make the 1N solution to maintained pH.
Phenol was used to make aqueous solution of Phenol
to remove by catalyst and nd Percentage removal of
Phenol by HPLC.
All chemicals used for the experimental work were
analytical grade (AR). Palash owers were collected
from the Campus of MANIT Bhopal, Madhya Pradesh,
India. The collected owers were cleaned by washing
many times with double distilled water and then dried
in sunlight. Dried ower were powdered in a mixer and
stored in a container to prevent from moisture and this
powder was preserved for experimental work. 250 mL
Erlenmeyer ask has been taken for boiling 10 g of
palash ower powder and 200 mL of double distilled
water for 1 h (Yadav and Khurana, 2015). Whatman l-
ter paper (pore size >0.5μm) has been used for ltration
of extract and it has been stored at 4°C in a deep freezer
which was used later for experimental work. We have
chosen water for extracting agent due to an extractive
value of palash ower in water is 17.5 ± 0.5% which is
comparatively maximum than other solvents.
ZnO has been bought from Molychem, Mumbai, and
the aqueous palash ower extract has been used for
the bioreduction process. To make nanoparticles from
palash ower 35 mL of the aqueous ower extract was
carefully added to 90 mL of 1 mM aqueous ZnO solution
in 250 mL Erlenmeyer asks. Colour of the solutions has
changed from brownish yellow to dark brownish, con-
rming the green synthesis of ZnO. We have performed
this experiment at pH 9.30; at this pH we got more pre-
cipitate of ZnO nanocatalyst. After the reaction, nano-
particles in the form of the precipitate had been settled
at the bottom of the conical asks. We separated ZnO
nanoparticles in the form of precipitate by centrifuging
it at 10000 rpm for 15 min (Awwad et al., 2013). Then we
have used these nanoparticles for characterization and
experimental work.
Characterization of ZnO nanocatalyst with palash
ower extract was done by standard methods (Sarma
and Sarma, 2014). Brunauer–Emmett–Teller (BET) sur-
face area of the nanoparticle was found by nitrogen
adsorption at 77.15K using an automatic pulse chem-
isorptions system (Micromeritics Chemisorb 2720) using
the software available with the instrument. To determine
the surface morphology and chemical composition, SEM
and EDAX tests were done in the SAIF, IIT Mumbai. The
phase identi cation and crystal structure have been
analysed by X-ray powder diffraction (XRD) test. Func-
tional groups present in synthesized nanocatalyst were
found by FTIR test. The XRD and FTIR tests have been
done in central laboratory of North Maharashtra Univer-
sity, Jalgaon, India.
Removal of acenaphthylene by synthesized nanocatalyst
Concentric Annulus photocatalytic reactor has been
used for acenaphthylene removal from re nery waste-
water by synthesized nanocatalyst. In this batch reac-
tor, which we have used for experiment; two coaxial
cylinders are there. The inner with reaction zone and
lamp is placed on the symmetry axis. The total volume
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SYNTHESIS OF GREEN ZNO/SIO
2
NANOCATALYST AND ITS APPLICATION 771
Rajani Bharati and S. Suresh
of the reactor is 1 litre and working volume is 0.7 litres.
Practically, all the photons emitted by UV lamp reach
the reaction medium if the outer walls of the reactor
are re ective. This geometry has been selected for the
photoreactor used in this study and offers a number
of advantages viz. ef cient photon captures, practical
to uidize the photocatalyst in a cylindrical geometry
and an outer jacket can be used for meeting the cooling
requirements of UV lamp. At the bottom of the reactor,
the magnetic stirrer is tted for uniform mixing. The
temperature was maintained around 28±2
o
C.
Acenaphthylene is one of the polycyclic aromatic
hydrocarbons that is found as pollutant in re nery
wastewater (Uddeen et al., 2011). In this experimental
work for treatment of re nery waste water, 500 ml re n-
ery wastewater has been treated in concentric annular
reactor with 1000 ml capacity. For this 0.5/500 ml cata-
lyst loading was used for photocatalytic reaction. The
solution of 500 ml was used to treat in a 1000ml of the
total volume of the reactor. The mixture solution was
placed into an annular reactor and started the UV light
(6W) to study the degradation or removal for duration of
6 h. The temperature was maintained at 30
o
C. The sam-
ples were withdrawn from annular reactor periodically
in an interval of 0.5, 1.5, 1, 2, 3, 4, 5, 6 hrs. After appro-
priate degradation, the samples were ltered and then
obtained residual concentrations of acenaphthylene
were analysed with the help of high performance liquid
chromatography (HPLC) (Waters Pvt. Ltd., India) C-18
column.The removal of acenaphthylene (%) was deter-
mined by following relationships (Hassan et al.,2015).
Acenaphthylene degradation (%) = 100 (C
i(0)
-C
f(t)
)/C
i(0)
Where, C
i(o)
is the initial acenaphthylene concentra-
tion (mg/l), C
f(t)
is the nal acenaphthylene concentration
(mg/l) at times t.
RESULTS AND DISCUSSION
The mechanism of ZnO nanocatalyst synthesis with
palash ower extract is that we have taken 1mM ZnO
solution for synthesis ZnO particles in nano range. Ini-
tially, ZnO (Bulk powder) was insoluble in water but
when we added few drops of one molar HCL acid, it
was completely soluble in water. The ZnO was degraded
by acid and formed ZnCl
2
. ZnCl
2
salt worked here as
precursor of Zn
+2
ions.Zn
+2
ions were reduced in nano
range by biomolecules present in palash ower extract.
Than functional groups present in palash ower extract
naturally worked as the capping agent for stabilization
of synthesized nanoparticles (Akhtar et al., 2013). The
mechanism and role of palash ower extract to synthe-
size ZnO/SiO
2
nanocatalyst in nanorange is shown in
Fig. 1. And SiO
2
was obtained from palash ower extract
which is present in palash ower naturally.
Intensity
Range in energy (KeV)
FIGURE 1. EDAX spectra of ZnO/SiO
2
palash ower extract nanoparticles.
ZnO(Powder) + 2HCL ZnCl
2
+ H
2
O
Zn
+2
+ 2OH
-
Zn(HO)
Zn(HO)
2
ZnO(nanocatalyst) + H
2
O
BET surface area of nanocatalyst has been found
157.23 m
2
/g. Reduction of Zn
+2
ions into nanorange
by palash ower extract can be seen by colour change.
When we added palash ower extract and mixed it
with the aqueous solution of the ZnO, the change in
colour of solution from brownish yellow to dark brown-
ish was observed and it has been con rmed that for-
mation of ZnO nanocatalyst has been occurred (Shukla
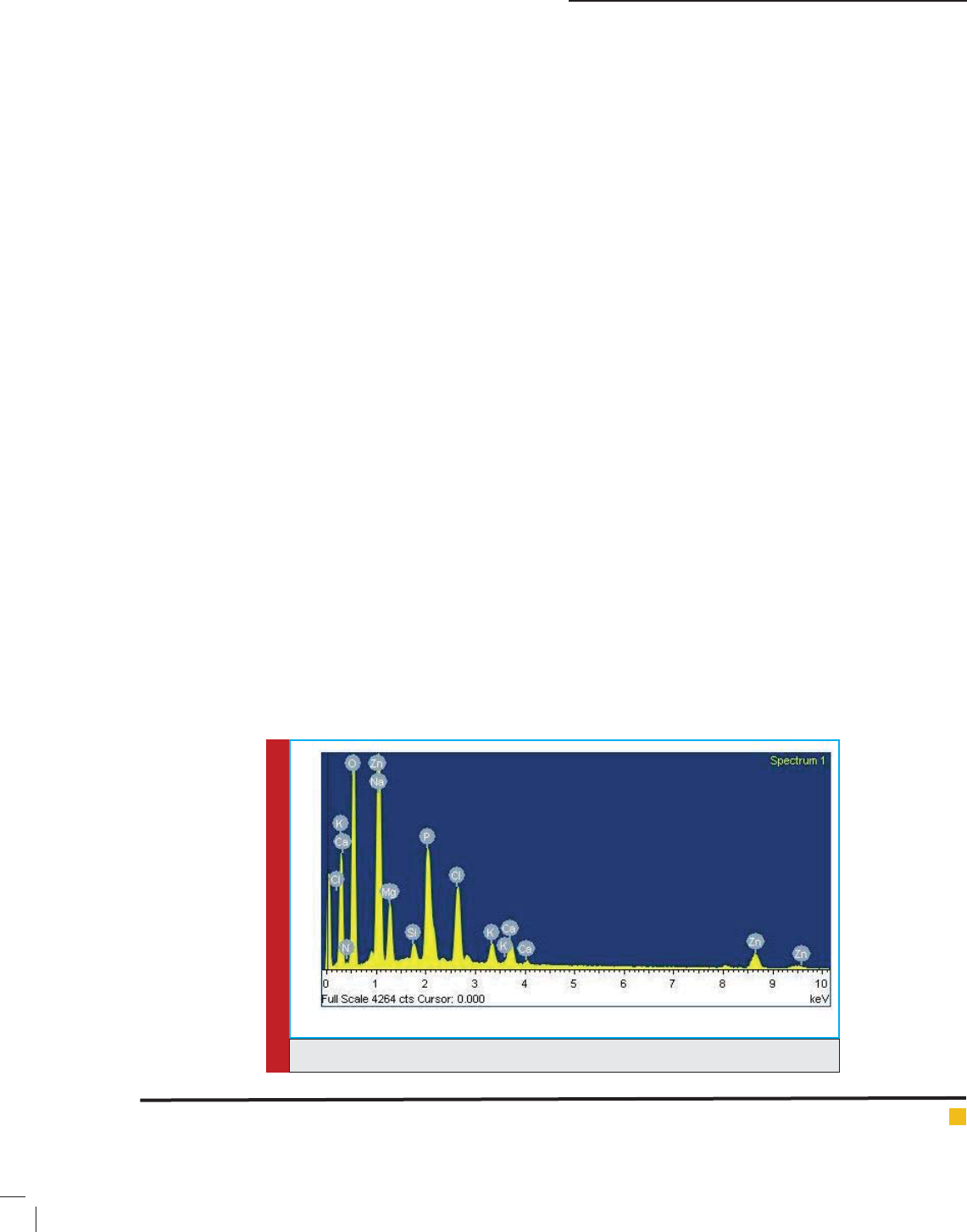
and Vankar, 2012). Figs.1-2 shows the EDAX spectra and
FEG-SEM images of synthesised ZnO/SiO
2
with palash
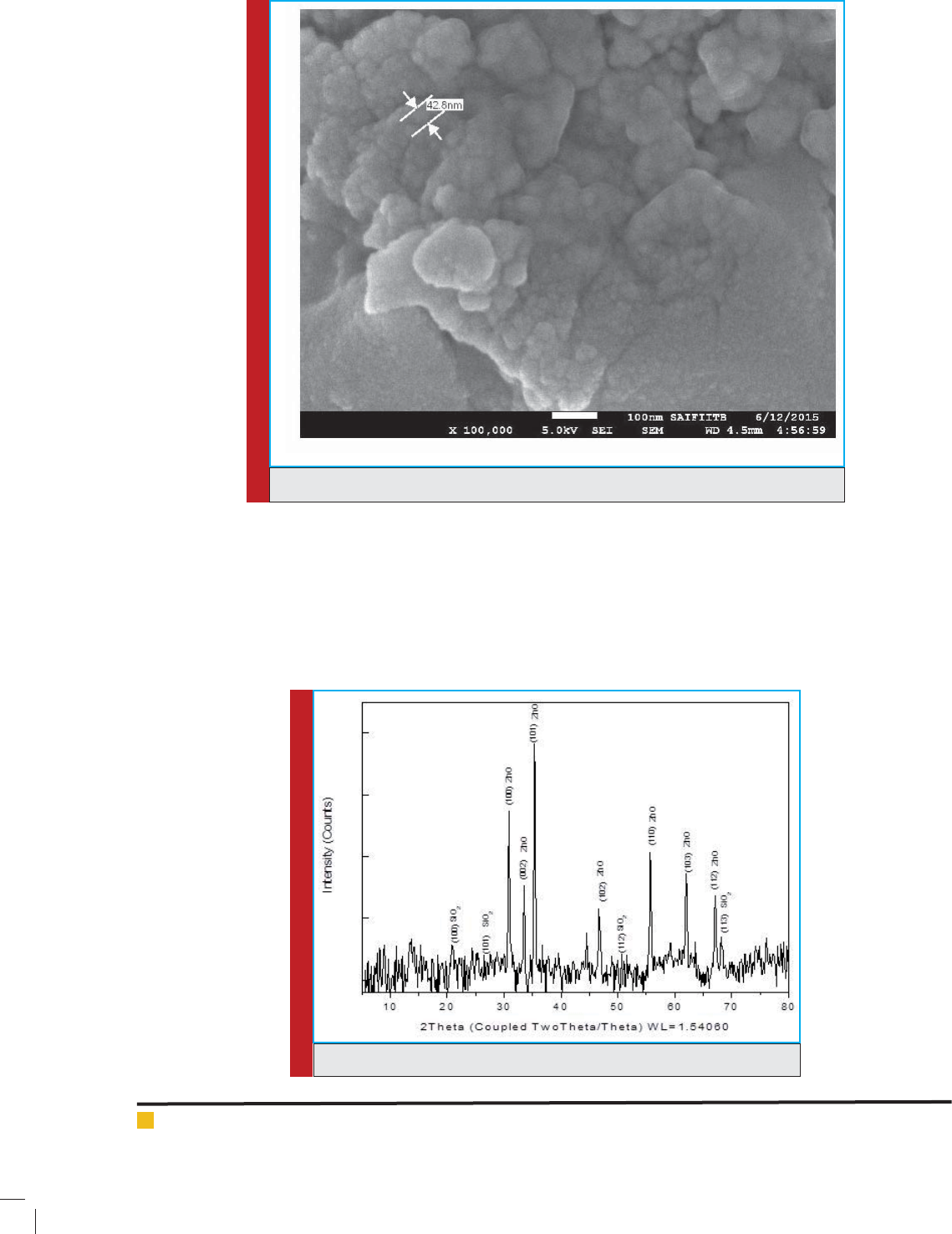
ower extract. The Hexagonal Crystal structure of ZnO
nanoparticles has been found by XRD pattern. The FEG-
SEM image shown in Fig.2 Shows spherical shape of
772 SYNTHESIS OF GREEN ZNO/SIO
2
NANOCATALYST AND ITS APPLICATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Rajani Bharati and S. Suresh
nanoparticles has been formed and Fig2-3 shows that
nanocatalysts have been found in the range of 3 nm to
45 nm with 23 nm mean particle diameter (Khalil et al.,
2014). At some places aggregation of nanoparticles have
been shown in FEG-SEM image Fig.2.
EDAX test has been done to nd chemical compo-
sition of ZnO/palash ower extract nancatalyst .EDAX
spectra found for a sample of ZnO/palash ower extract
nanoparticles and the weight percentage of Zn and O are
found to be 15.79 % and 38.05% respectively (Khalil et
al., 2014). From EDAX it can be seen that Si is also pre-
sent palash ower extract. It con rms that we have got
ZnO/SiO
2
with Palash ower extract nanocatalyst. It has
been shown in EDAX spectra that we have got strong
Diameter
No. of particles
FIGURE 2. FEG-SEM image of ZnO/SiO
2
palash ower extract nanocatalyst.
FIGURE 3. XRD pattern of ZnO/ palash ower extract nanocatalyst.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SYNTHESIS OF GREEN ZNO/SIO
2
NANOCATALYST AND ITS APPLICATION 773
Rajani Bharati and S. Suresh
Percent Transmittance
IR Frequency
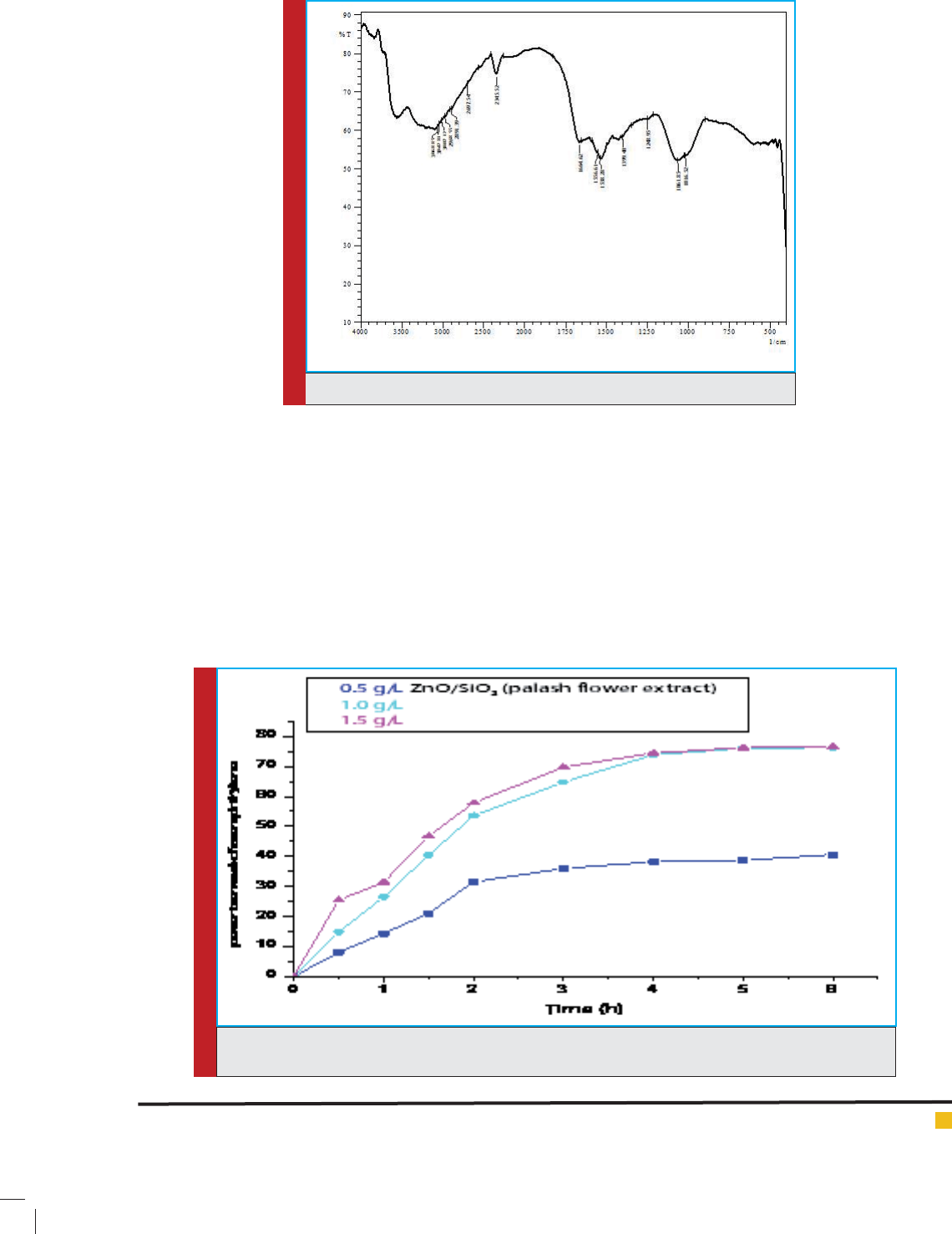
FIGURE 4. FTIR spectra of ZnO/palash ower extract nanocatalyst.
FIGURE 5. Degradation of acenaphthylene from re nery wastewater with time by ZnO/SiO
2
with palash
ower extract nanocatalyst.
signal of energy peaks for Zn and O atoms in the range
0.5–1.5 keV and weaker signals for Zn atoms in the
range 8-10 keV (Khalil et al., 2014; Sarma et al., 2014).
It has been also found that we have got weaker signals
for N, Mg, Si, P, Cl, K, and Ca, which were present in
palash ower extract and due to presence of many met-
als naturally in palash ower extract it increases photo-
catalytic activity of ZnO/SiO
2
with palash ower extract
nanocatalyst.
XRD peaks shown in Fig.3 con rmed the presence
of ZnO corresponding to PDF No. 80-0075. The lattice
parameters a and c were calculated to be about 3.25 A
0
and 5.209 A
0
respectively. The minor amount of Si (SiO
2
)
was also found in the XRD pattern. The crystal structure
of the SiO
2
was found to be matching with low quartz.
The peaks of Si, as well as ZnO, were found to be broad-
ened indicating nanocrystalline or amorphous nature
of the crystal. In our experiment, the X-ray pattern of
synthesized ZnO nanoparticles matches the hexagonal
structure of the ZnO nanoparticles with the broad peaks
at 2 = 36.243, 56.568. These are corresponding to (101),
(110) and (103), respectively. Similar peaks were found in
774 SYNTHESIS OF GREEN ZNO/SIO
2
NANOCATALYST AND ITS APPLICATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
the ZnO based nanoparticles (Rekha et al., 2010, Khalil et
al., 2014 and Sarma and Sarma, 2014).
Fig. 4 Shows FTIR spectra of ZnO/palash ower
extract nanocatalyst , which has been done to nd vari-
ous functional groups present in nanocatalyst .IR fre-
quency from 2697.54 to 3068.85 is due to O-H stretch-
ing , 1061.85 and 1248.95 for Si-O-Si starching ,1538.28
to 1664.62 for C=O stretching ,1016.52 for ZnO stretch-
ing , this stretching is slightly shifted from 1000 which
is for metal oxide due to the presence of other functional
groups(Uddeen et al.,2011).
Hence XRD pattern con rms that ZnO nanoparticles
have been formed by this bioreduction process. Phases of
the sample were matched with standard XRD pattern of
ZnO nanoparticles. From EDAX, XRD and FTIR test it has
been found that SiO
2
has been also found in the sample
which increases photocatalytic activity of this catalyst.
Percentage removal of pollutant
ZnO/SiO
2
nanocatalyst made by biosynthesis or green
single pot method are very good nanocatalyst for degra-
dation of acenaphthylene from re nery wastewater. Fig.
5 shows the degradation of phenol from aqueous solu-
tion by using ZnO/SiO
2
palash ower extract. Acenaph-
thylene degradation from re nery wastewater has been
done in an annular photocatalytic reactor and it has
been found that optimum percent removal of acenaph-
thylene was found to be 81% at 0.5g/500ml, 30
o
C and
4h of catalyst loading, temperature and reaction time
respectively. Fig. 6 shows optimum percent removal of
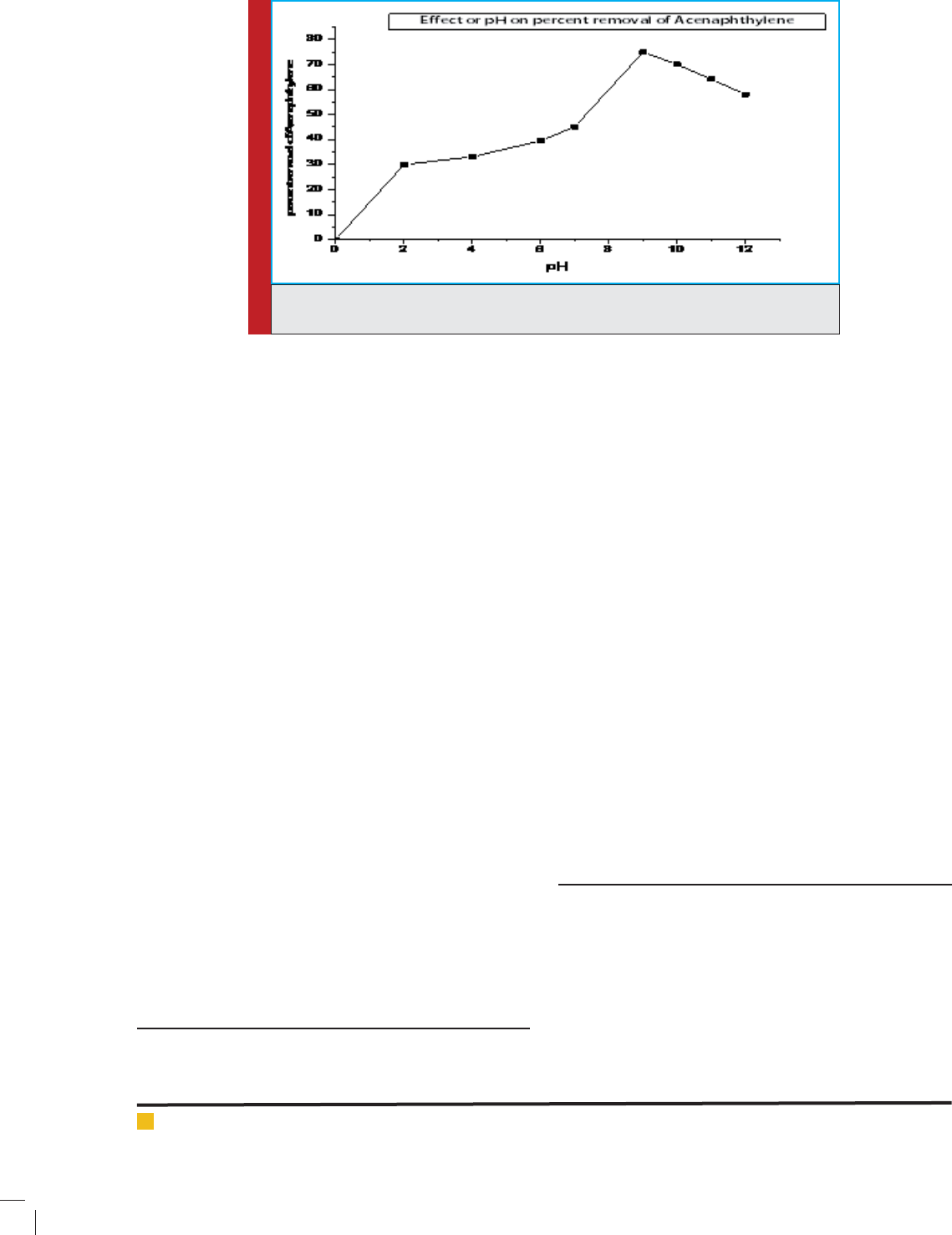
acenaphthylene with pH at 9.5 pH.
CONCLUSION
In present research work the characterization of nano-
catalyst shows that we have synthesized ZnO/SiO
2
nano-
catalyst with palash ower extract in nanorange. We
have used palash ower extract as reducing and sta-
bilizing agent in place of chemical to synthesize ZnO
nanocatalyst so it is eco-friendly method. Palash ower
contains many biomolecules which are responsible for
reduction and stabilization of Zn
+2
ions in nanorange so
it is more fast method than other methods. It contains
SiO
2
naturally catalyst synthesized by palash ower
extract is more ef cient to remove pollutant than other
methods. So Palash ower is easily available in India at
low cost and we need very simple techniques to synthe-
size nanocatalyst by this method so it is cheap process
than other processes. We have found from EDAX and
XRD test that many metals present naturally in palash
ower extract so incorporation of these metals with
ZnO nanocatalyst increases its photocatalytic activity
.Results shows that ZnO/palash ower extract nanocata-
lyst is very good photocatalyst to remove phenol from
an aqueous solution of phenol. The percent removal of
phenol can be optimized by controlling affecting param-
eters such as reaction time, catalyst size, catalyst load-
ing, pH and temperature. The optimum percent removal
of phenol has been found 81% at 0.5/500ml catalyst
loading at 30
o
C temperature and 4h reaction time.
ACKNOWLEDGMENT
The authors wish to thank Ministry of Human Resource
Development, Government of India (MHRD) and Maul-
ana Azad National Institute of Technology (MANIT)
Bhopal, India to provide fund and facilities to carry out
experimental work.
REFERENCES
Ahmed, S.; Ahmad, M.; Swami, B. L.; Ikram, S. (2016): A review
on plants extract mediated synthesis of silver nanoparticles
FIGURE 6. Degradation of acenaphthylene from re nery wastewater at different pHby
ZnO/SiO
2
with palash ower extract nanocatalyst.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SYNTHESIS OF GREEN ZNO/SIO
2
NANOCATALYST AND ITS APPLICATION 775
for antimicrobial applications. A green expertise, Journal of
Advanced Research, 7(1), 17–28.
Akhtar, M. S.; Panwar, J.; Yun, Y.S. (2013): Biogenic Synthesis
of Metallic Nanoparticles by Plant Extracts. American Chemi-
cal Society Sustainable Chemistry & Engineering, 1, 591−602.
Awwad, A. M.; Salem, N. M. and Abdeen, A. O. (2013): Green
synthesis of silver nanoparticles using carbon leaf extract and
its antibacterial activity. International Journal of Industrial
Chemistry, 4(1):29, 1-6.
Balachandran, Y. L.; Peranantham, P.; Selvakumar, R.; Arno,
C.; Girija, G. S. (2012): Size-Controlled Green Synthesis of Sil-
ver Nanoparticles using Dual Functional Plant Leaf Extract at
Room Temperature. International Journal of Green Nanotech-
nology, 4(1), 310–325.
Dizaji, A. N.; Yilmaz, M.; Piskin, E. (2015): Silver or gold depo-
sition onto magnetite nanoparticles by using plant extracts as
reducing and stabilizing agents. Arti cial Cells, Nanomedicine,
and Biotechnology, Early Online, 1–7.
Gudikandula, K. and S. C., Maringanti (2016): Synthesis of
silver nanoparticles by chemical and biological methods and
their antimicrobial properties. Journal of experimental nano-
science, doi: 10.1080/17458080.2016.1139196.
Hassan, S. E. D.; Desouky, S. E.; Fouda, A.; Mamdouh, S. E.
and Ahmed A. (2015): Biodegradation of Phenanthrene by
Klebsiella sp Isolated from Organic Contaminated Sediment.
Journal of Advances in Biology & Biotechnology, 4(4), 1-12.
Hegde, S. V.; Hegde, G. R.; Mannur, S.; Poti, S. S. (2014):
Pharmacognostical studies on Butea monosperma (Lam.) Taub
(Faboideae) ower. International Journal of Pharmaceutical
and Phytopharmacological Research, 4(1), 2250-1029.
Jamkhande, P. G.; Patil, P.H.; Tidke, P. S. (2013): In Vitro Anti-
oxidant activity of Butea monosperma Flowers Fractions. Inter-
national Journal Drug, Delivery& Research, 5 (3), 245-255.
Khalil, M. I.; M. Qunaibit, M. A.; Zahem, A. M. A.; Labis, J. P.
(2014): Synthesis and characterization of ZnO nanoparticles by
thermal decomposition of a curcumin zinc complex. Arabian
Journal of Chemistry, 7(1), 1178–1184.
Kumar, V. and S. K., Yadav (2012): Characterization of gold
nanoparticles synthesized by leaf and seed extract of Syzygium
cumini L. Journal of Experimental Nanoscience , 7(4), 440–451.
Rekha, K.; Nirmala, M.; Nair, M. G.; Anukaliani, A. (2010):
Structural, optical, photocatalytic and antibacterial activity
of zinc oxide and manganese doped zinc oxide nanoparticles,
Physica B, 405 (1), 3180–3185.
Sarma, H.; Chakrabortty, D.; Sarma, K.C. (2014): Structural
and Optical Properties of Zno Nano Particles. IOSR Journal of
Applied Physics, 6 (4), 08-12.
Sarma, H.; Sarma, K.C. (2014): X-ray Peak Broadening Anal-
ysis of ZnO Nanoparticles Derived by Precipitation method.
International Journal of Scienti c and Research Publications,
4(3), 2250-3153.
Shukla, D. and P. S., Vankar (2012): Synthesis of Plant Parts
Mediated Gold Nanoparticles. International Journal of Green
Nanotechnology, 4(1), 277–288.
Uddeen, B.H.D.; Daud, W.M.A.W.; Aziz, A.R.A.(2011): Treat-
ment technologies for petroleum re nery ef uents. A review,
Process safety and environmental protection, 89(1), 95-105.
Wang, P.; Menzies, N. W.; Lombi, E.; Sekine, R.; Blamey, F. P.
C.; Soriano, M. C. H.; Cheng, M.; Kappen, P.; Peijnenburg, W. J.
G. M.; Tang, C.; Kopittke P. M. (2015): Silver sul de nanopar-
ticles (Ag
2
S-NPs) are taken up by plants and are Phytotoxic.
Nanotoxicology, Early Online: 1–9.
Yadav S. and Khurana, J. M. (2015) Cinnamomum tamala leaf
extract-mediated green synthesis of Ag nanoparticles and their
use in pyranopyrazles synthesis. Chinese Journal of Catalysis,
36 (1), 1042-1046.