Department of Zoology, Shivramji Moghe Arts, Commerce and Science College, Kelapur (Pandharkawada),Yavatmal, Maharashtra (India)
Corresponding author email: ramzan_virani@yahoo.co.in
Article Publishing History
Received: 12/05/2020
Accepted After Revision: 23/06/2020
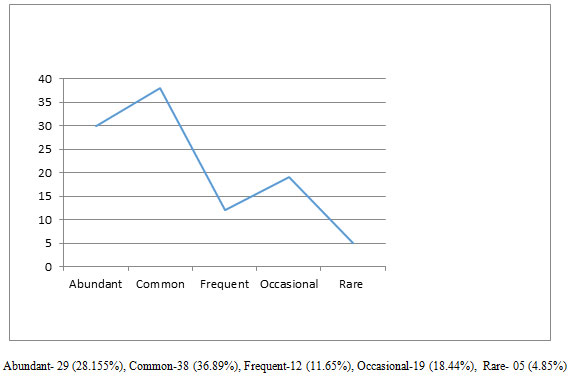
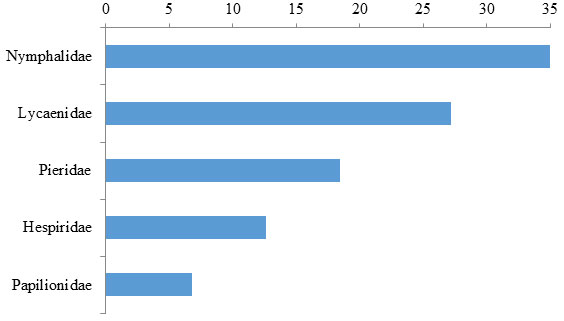
A study was conducted to estimate the butterfly diversity in the Pandharkawada Forest Division of Maharashtra, India. The study revealed presence of 103 species of butterflies belong to 5 family dominated by family Nymphalidae (34.95 %), Lycaenidae (27.18 %) followed by Pieridae (18.45 %), Hesperiidae (12.62 %) and Papilionidae (6.80 %). On the basis of Occurrence of species in study area 28.155 % species was categorized as abundant species whereas 36.89 % species was common, 11.65 % species was frequent, 18.44 % was occasional, and 4.85 % species was rare. On the basis of level of protection provided by Indian Wildlife Protection Act, 1972, 16 species recorded from study area belong to different Schedules of this act of which 5 species are in schedule 1. It appears that the butterfly abundance increased from monsoon to winter while decreased in the summer and pre-monsoon possibly due to the unavailability of the nectar and changes in temperature and humidity of the habitats concerned. The results of the study prove that the Pandharkawada forest division, Maharashtra has a healthy environmental setup that accommodates rich butterfly diversity.
Abundance, Butterfly, Diversity, Occurrence, Pandharkawada
Virani R. S. Assessment of Butterfly Diversity of a Tropical Forest Division of Maharashtra, India. Biosc.Biotech.Res.Comm. 2020;13(2).
Virani R. S. Assessment of Butterfly Diversity of a Tropical Forest Division of Maharashtra, India. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2XJIxkm
Copyright © Virani This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Pandharkawada forest division is situated in Yavatmal District of Indian state of Maharashtra. This area lies at south eastern part of the district, located between the geographical confines of east longitude 78°14′ and 79°13′ East and 19°45′ and 20°20′ North, spread over the area of 655.336 sq. km. The climatic condition of this area is characterized by a hot summer, well-distributed rainfall during the south-west monsoon season and generally dry weather during rest of the year. The cold season is from December to February (Yavatmal Gazetteer 2019). Area constitutes honey comb pattern with compact patches of vegetation, meadows, open-scrubs, waterbodies and seasonal wetlands intersperse with agriculture. These varied ecosystems show great utility for conservation of biological diversity. Varied habitats and seasonal variation in floral composition of this dry deciduous forest attract verities of insect species. The diversity of insects plays an important role in the terrestrial and aquatic ecosystems by providing ecosystem services such as pollination, pest control, nutrient decomposition, and maintenance of ecosystem (Koh and Sodhi 2004; Losey and Vaughan 2006). Among insects, butterflies are the most attractive elements of the universe. They perform prominent roles in pollination (Tiple et al., 2006; Tiple 2018).
Adult butterflies are dependent on nectar and pollen as their food while the caterpillars are dependent on specific host plants for foliage (Nimbalkar et al., 2011), this facilitate pollination. Butterflies are considered as the best indicators of the health of any kind of ecosystem. They bear a history of long-term co-evolution with plants. (Thomas 2005; Bonebrake et al., 2010). Butterflies are therefore treated as an important model group to study ecology of any landscape and its conservation status (Watt and Boggs 2003; Ehrlich and Hanski 2004; Mukherjee et al., 2015). Temperature and relative humidity are the important factors in distribution and assemblage of Butterfly species (Gupta et al., 2019 )
Many butterfly species are vulnerable due to the habitat loss caused by modern agricultural practices and urbanisation, other major negative impacts are due to the widely increasing global environmental change. In the view of this changing scenario to ensure essential ecosystem services rendered by butterflies, it is essential to document these ecologically important vividly hued winged beauties. This study is design to estimation butterfly diversity in the Pandharkawada forest division, Maharashtra, India. This will work as biological instrument in devising sustainable conservation strategies for these beautiful creatures and to understand their role in maintaining ecological dynamics of this landscape.
MATERIALS AND METHODS
Study Area:Study was conducted at Shibla Forest (canopy covered with associated grassy belts), Gopalpur Nursery (Forest Nursery), Shindola Forest (Scrub with Sandy Soil), Nilgiri Ban (Eco-Park), Saykheda (Water Reservoir and Seasonal Wetland). Ecological conditions of every study site are different than other.
Survey method :The butterflies were observed and photographed in the sampling sites for a period of 1 year between January 2019 and December 2019. During the survey, an efficient protocol was adopted. The survey was made using a “Pollard Walk” method (Pollard 1977; Pollard and Yates 1993) with necessary modifications. Study area was visited twice a month/Study site from morning 8 AM to afternoon 11 AM during good weather periods.
Species identification :After detection, a specimen was photographed (Nikon D7100; Nikon Inc., Tokyo, Japan) and identified with the help of visible structural features. For identification and comparative studies of observed specimens, keys and methods suggested by Evans (1932), Wynter-Blyth (1957), Haribal (1992), Kunte (2000) and Kehimkar (2008) were adopted.
Data analysis :Species occurrence analysis was carried out by Microsoft excel program with using the following formulas. Relative Dominance (RD) of species was calculated as [RD=Ni × 100/Nt] where, Ni is number of individuals of species and Nt is total number of individuals all species (Basavarajappa 2006; Joshi 2014). Relative Occurrence (RO) of family was calculated as [RO= Ns × 100/Nt] where, Ns is number of species of each family and Nt is total number of all species (Basavarajappa 2006; Joshi 2014). Mean percent occurrence (M%) for month was calculated as [M% = Nm × 100 /Nt] where, Nm is number of individuals in each month and Nt is total number of individuals during complete study tenure (Basavarajappa 2006; Joshi and Tantarpale 2016). The mean values of the pooled species occurrence data were used to calculate the monthly diversity and to categorize the local status of species.
The diversity assessment enabled highlighting the observed species richness pattern of the butterfly species. The diversity indices were quantified with the help of PAST Version 1.60 software (Palaeontological Asso., Norway; Hammer et al., 2001). The species diversity was calculated using Shannon diversity index that calculated as [ ], where Pi is proportion of the first species which is given by Pi= ni/N (Magurran 1988); species richness was obtained by using Margalef equation [R= (S-1)/ log N], Where, R is Index of species richness, S is Total number of species and N is Total No. of individuals (Magurran, 1988); while Species equitability was determined by equation of Pielou [J= N1/N0] where N1 is Number of abundant species in the sample and N0 is Number of species in the sample (Hammer et al., 2001). The similarity association matrix upon which the cluster based was computed using the nearest neighbour pair linkage algorithm of Euclidean distance index for presence and absence data (Hammer et al., 2001). The differences between the diversity and evenness indices of with species occurrence among different study months were statistically analysed by using Analysis of variance (ANOVA). The statistical analyses were performed following Zar (1999) using the SPSS version 10 (SPSS Inc., Chicago, Il, USA; Kinnear and Gray 2000).
RESULTS AND DISCUSSION
During this study, 103 butterfly species under five families were recorded in study area (Table 1). Based on value of butterfly relative dominance in study area, 28.155 % species was categorized as abundant species whereas 36.89 % species was common, 11.65 % species was frequent, 18.44 % was occasional, and 4.85 % species was rare (Figure 1). The maximum number of butterfly species were recorded under family Nymphalidae (34.95 %), Lycaenidae (27.18 %) followed by Pieridae (18.45 %), Hesperiidae (12.62 %) and Papilionidae (6.80 %) (Figure 2).
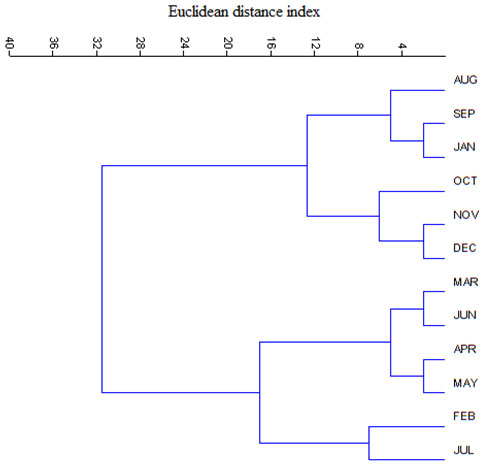
A dendrogram developed by Euclidean distance cluster analysis was observed to be multifaceted and showed variation in the level of similarity in the number of butterfly species in 12 months. The months with the minimum to moderate number of species belong to one cluster, whereas the rest of the months with moderate to maximum number of species formed another cluster (Figure 3). It appears that the butterfly abundance increased from monsoon to winter while decreased in the summer and pre-monsoon possibly due to the unavailability of nectar and the changes in temperature and humidity of the habitats concerned
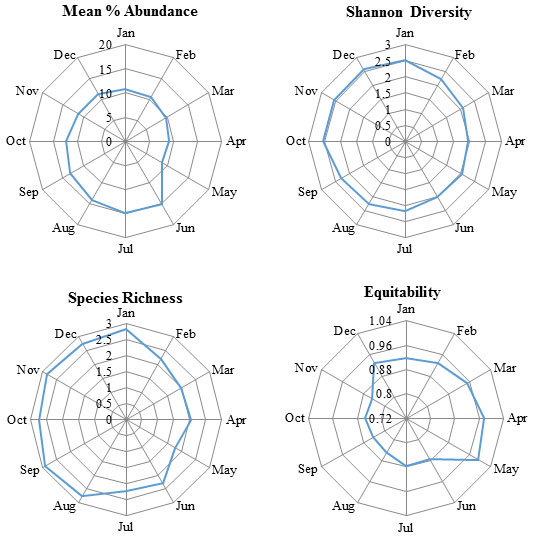
Mean percent abundance of butterflies was significantly different (F = 145.5, df = 11, p < 0.05); Shannon diversity values of butterflies were significantly different (F= 189.2, df = 11, p < 0.05); species evenness among different months was significantly different (F= 196.3, df = 11, p < 0.05) while species richness among the study months was significantly different (F = 188.3, df = 11, p < 0.05). A trend in mean % abundance, Shannon diversity, species richness and species equitability showed the contradictory patterns (Figure 4).
Table 1. Diversity of Butterflies during January 2019 to December 2019 in the Pandharkawada Forest Division, Maharashtra, India
| Common Name | Scientific Name | Relative Dominance |
Local Status |
IUCN Status |
WPA Status |
| Family: Papilionidae | |||||
| Tailed Jay | Graphium agamemnon (Linnaeus, 1758) | 1.163 | Common | NE | |
| Common Jay | Graphium doson (Felder and Felder, 1864) | 1.098 | Common | NE | |
| Common rose | Pachliopta aristolochiae (Fabricius, 1775) | 0.994 | Common | LC | Sch.I |
| Crimson rose | Pachliopta hector (Linnaeus, 1758) | 0.941 | Common | NE | |
| Lime Butterfly | Papilio demoleus (Linnaeus, 1758) | 1.321 | Abundant | NE | |
| Common Mormon | Papilio polytes (Linnaeus, 1758) | 1.237 | Abundant | NE | |
| Spot Swordtail | Graphium nomius (Esper, 1793) | 0.599 | Occasional | NE | |
| Family: Pieridae | |||||
| Common Albatross | Appias albino (Fabricius, 1775) | 1.039 | Common | NE | Sch.II |
| Indian Pioneer | Belenois aurota (Fabricius, 1793) | 1.407 | Abundant | NE | |
| Common Emigrant | Catopsilia pomona (Fabricius, 1775) | 1.220 | Abundant | NE | |
| Mottled Emigrant | Catopsilia pyranthe (Linnaeus, 1758) | 0.920 | Common | NE | |
| Common Gull | Cepora nerissa (Fabricius, 1775) | 1.368 | Abundant | NE | Sch.II |
| Small salmon Arab | Colotis amata (Butler, 1870) | 0.604 | Occasional | NE | |
| Large Salmon Arab | Colotis fausta (Olivier, 1804) | 0.531 | Occasional | NE | |
| Crimson Tip | Colotis danae (Fabricius, 1775) | 0.578 | Occasional | NE | |
| Small Orange Tip | Colotis etrida (Boisduval, 1836) | 1.051 | Common | NE | |
| White Orange Tip | Ixias Marianne (Cramer, 1775) | 1.024 | Common | NE | |
| Yellow Orange Tip | Ixias pyrene (Linnaeus, 1764) | 0.712 | Occasional | NE | |
| Common Jezebel | Delias eucharis (Drury, 1773) | 1.114 | Common | NE | |
| One Spot Grass Yellow | Eurema andersoni (Moore, 1865) | 1.148 | Common | LC | |
| Three Spot Grass Yellow | Eurema blanda (Boisduval, 1836) | 1.003 | Common | NE | |
| Small Grass Yellow | Eurema brigitta (Stoll, 1780) | 1.131 | Common | LC | |
| Common Grass Yellow | Eurema hecabe (Linnaeus, 1758) | 1.294 | Abundant | NE | |
| Spotless Grass Yellow | Eurema laeta (Boisduval, 1836) | 1.359 | Abundant | NE | |
| Psyche | Leptosia nina (Fabricius, 1793) | 0.703 | Occasional | NE | |
| Common Wanderer | Pareronia valeria (Cramer, 1776) | 1.116 | Common | NE | |
| Family: Nymphalidae | |||||
| Tawny Castor | Acraea violae (Fabricius, 1775) | 0.976 | Common | NE | |
| Angled Castor | Ariadne ariadne (Linnaeus, 1763) | 1.157 | Common | NE | |
| Common Castor | Ariadne merione (Cramer, 1779) | 1.077 | Common | NE | |
| Common Sergeant | Athyma perius (Linnaeus, 1763) | 0.502 | Occasional | NE | |
| Plain Tiger | Danaus chrysippus (Linnaeus,1758) | 1.389 | Abundant | NE | |
| Striped Tiger | Danaus genutia (Cramer, 1779) | 1.270 | Abundant | NE | |
| Common Crow | Euploea core (Cramer, 1780) | 1.439 | Abundant | LC | |
| Double Branded crow | Euploea Sylvester (Fabricius, 1793) | 0.490 | Occasional | NE | |
| Baronet | Euthalia nais (Cramer, 1779) | 0.935 | Common | NE | |
| Common Baron | Euthalia aconthea (Cramer, 1777) | 0.304 | Rare | NE | |
| Great Eggfly | Hypolimnas bolina (Linnaeus, 1758) | 1.065 | Common | NE | |
| Danaid Eggfly | Hypolimnas misippus (Linnaeus, 1764) | 0.959 | Common | NE | Sch.II |
| Peacock Pansy | Junonia almana (Linnaeus, 1758) | 1.312 | Abundant | LC | |
| Grey Pansy | Junonia atlites (Linnaeus, 1763) | 1.056 | Common | NE | |
| Yellow Pansy | Junonia hierta (Fabricius, 1775) | 1.110 | Common | LC | |
| Chocolate Pansy | Junonia iphita (Cramer, 1779) | 0.970 | Common | NE | |
| Lemon Pansy | Junonia lemonias (Linnaeus, 1758) | 1.184 | Abundant | NE | |
| Blue Pansy | Junonia orithya (Linnaeus, 1764) | 1.418 | Abundant | NE | |
| Common Evening Brown | Melanitis leda (Linnaeus, 1758) | 1.249 | Abundant | NE | |
| Dark Evening Brown | Melanitis phedima (Cramer, 1780) | 0.724 | Occasional | NE | |
| Common Bush Brown | Mycalesis perseus (Fabricius, 1775) | 0.947 | Common | NE | |
| Dark Brand Bush Brown | Mycalesis mineus (Linnaeus, 1758) | 0.788 | Frequent | NE | |
| Common Sailer | Neptis hylas (Linnaeus, 1764) | 0.929 | Common | NE | |
| Common Leopard | Phalanta phalantha (Drury, 1773) | 1.032 | Common | LC | |
| Blue Tiger | Tirumala limniace (Cramer, 1775) | 1.213 | Abundant | NE | |
| Commander | Moduza procris (Cramer, 1777) | 1.140 | Common | NE | |
| Painted Lady | Synthia cardui (Linnaeus, 1764) | 0.911 | Common | NE | |
| Joker | Byblia ilithyia (Drury, 1773) | 0.902 | Common | NE | |
| Common Three Ring | Ypthima asterope (Klug, 1832) | 1.143 | Common | NE | |
| Large Three Ring | Ypthima nareda (Kirby, 1871) | 0.831 | Frequent | LC | |
| Common Four Ring | Ypthima huebneri (Kirby, 1871 ) | 0.782 | Frequent | LC | |
| Common Five Ring | Ypthima baldus (Fabricius, 1793) | 0.791 | Frequent | NE | |
| Anomalous Nawab | Polyura agrarian (Linnaeus, 1764) | 0.674 | Occasional | NE | |
| Common Nawab | Polyura athamas (Drury, 1773) | 0.481 | Occasional | NE | Sch.II |
| Black Rajah | Charaxes solon (Fabricius, 1793) | 0.546 | Occasional | NE | Sch.II |
| Towny Rajah | Charaxes bernardus (Fabricius, 1793) | 0.680 | Occasional | NE | Sch.II |
| Family: Lycaenidae | |||||
| Pointed Ciliate Blue | Anthene lycaenina (C. Felder, 1868) | 0.758 | Frequent | NE | Sch.II |
| Large Oak Blue | Arphopala amantes (Hewitson, 1862) | 0.368 | Rare | NE | |
| Dull Babool Blue | Azanus uranus (Butler, 1886) | 0.795 | Frequent | NE | |
| Bright Babool Blue | Azanus ubaldus (Stoll, 1782) | 1.023 | Common | NE | |
| Lime Blue | Chilades lajus (Stoll, 1780) | 1.430 | Abundant | NE | |
| Gram Blue | Euchrysops cnejus (Fabricius, 1798) | 1.199 | Abundant | NE | Sch.II |
| Pea Blue | Lampides boeticus (Linnaeus, 1767) | 1.229 | Abundant | NE | Sch.II |
| Zebra Blue | Leptotes plinius (Fabricius, 1793) | 1.377 | Abundant | NE | |
| Dingy Line Blue | Petrelaea dana (de Niceville, 1884) | 1.033 | Common | NE | |
| Tailless Line Blue | Prosotas dubiosa (Semper, 1879) | 1.018 | Common | NE | Sch.II |
| Common Line Blue | Prosotas nora (Felder, 1860) | 1.125 | Common | NE | |
| Guava Blue | Virachola isocrates (fabricius, 1793) | 0.659 | Occasional | NE | Sch.I |
| Dark Grass Blue | Zizeeria karsandra (Moore, 1865) | 1.258 | Abundant | NE | |
| Lesser Grass Blue | Zizina otis (Fabricius, 1787) | 1.181 | Abundant | NE | |
| Tiny Grass Blue | Zizula hylax (Fabricius, 1775) | 1.318 | Abundant | NE | |
| Plum Judy | Abisara echerius (Moore, 1901) | 0.688 | Occasional | NE | |
| Common Pierrot | Castalius rosimon (Fabricius, 1775) | 0.786 | Frequent | NE | Sch.I |
| Forget-Me-Not | Catochrysops strabo (Fabricius, 1793) | 1.282 | Abundant | NE | |
| Plains Cupid | Luthrodes pandava (Horsfield, 1829) | 0.864 | Frequent | NE | |
| Indian cupid | Cupido lacturnus (Godart, 1824) | 0.985 | Common | NE | |
| Grass Jewel | Freyeria trochylus (Freyer, 1845) | 1.344 | Abundant | NE | |
| Common Cerulean | Jamides celeno (Cramer, 1775) | 1.359 | Abundant | NE | |
| Indian Red Flash | Rapala airbus (Fabricius, 1787) | 0.305 | Rare | NE | |
| Slate Flash | Rapala manea (Hewitson, 1863) | 0.229 | Rare | NE | |
| Common Silverline | Spindasis vulcanus (Fabricius, 1775) | 0.755 | Frequent | NE | |
| Common Shot Silverline | Spindasis ictis (Hewitson, 1865) | 0.567 | Occasional | NE | |
| Rounded Pierrot | Tarucus extricates (Kollar, 1848) | 1.175 | Abundant | NE | |
| Peacock Royal | Tajuria cippus (Fabricius, 1775) | 0.163 | Rare | NE | Sch.II |
| Family: Hespiridae | |||||
| Brown awl | Badamia exclamationis (Fabricius, 1775) | 1.338 | Abundant | LC | |
| Plain Banded Awl | Hasora vita (Cramer, 1780) | 0.792 | Frequent | NE | SchIV |
| Bevan’s Swift | Borbo bevani (Moore, 1878) | 0.534 | Occasional | NE | |
| Rice swift | Borbo cinnara (Wallace, 1866) | 1.427 | Abundant | NE | |
| Blank Swift | Caltoris kumara (Moore, 1878) | 0.543 | Occasional | NE | |
| Small branded swift | Pelopidas mathias (Fabricius,1798) | 1.226 | Abundant | NE | |
| Conjoined Swift | Pelopidas conjuncta (Moore, 1878) | 0.878 | Common | NE | |
| Paintbrush Swift | Baoris farri (Moore, 1878) | 0.810 | Frequent | NE | SchIV |
| Common Straight Swift | Parnara guttatus (Bremer and Gray, 1853) | 1.104 | Common | LC | |
| Indian Palm bob | Suastus gremius (Fabricius, 1798) | 0.896 | Common | NE | |
| Dark Palm-Dart | Telicota ancilla (Moore, 1878) | 1.045 | Common | NE | |
| Indian skipper | Spialia galba (Fabricius, 1793) | 0.751 | Frequent | LC | |
| Grass Demon | Udaspes folus (Cramer, 1775) | 0.635 | Occasional | NE |
Figure 1: Relative occurrence of butterfly Species in Pandharkawada forest division
Figure 2: Relative dominance of butterfly families in the Pandharkawada forest division, Maharashtra, India
Figure 3: Dendrogram showing similarity in number of butterfly species composition among the studied month during January 2019 to December 2019
Figure 4: The values of the diversity indices in different months observed through the random sampling of butterflies in the Pandharkawada forest division, Maharashtra, India
The butterflies are the ecologically important creature that serves as indicators of environmental conditions (Stefanescu et al., 2004). Observations on the butterfly diversity provide the information about variations in the species richness and the abundance in relation with the vegetation along the landscape and the species interactions (Öckinger and Smith 2006; Öckinger et al 2006; Mutmainnah and Santosa 2019).
In this context, the diversity of Butterflies in the Pandharkawada forest division, Maharashtra, India was studied during January 2019 to December 2019. The study area was dominated by the dense vegetation with variety of plant species that host the butterfly populations. The earlier studies showed that heterogeneity of the habitats in terms of the available plant species supports the rich butterfly diversity (Kuussaari et al 2007; Mukherjee et al., 2015). Studies on the butterfly diversity in the forest landscape contrast to the urban and suburban regions show that the richness increased with the availability of the green space and the heterogeneity of the habitats in terms of the available plant species (Öckinger et al., 2009; Mukherjee et al., 2015). Consistent with these studies the present observation records a total of 103 species belonging to five families from study area.The maximum number of butterfly species was recorded under family Nymphalidae, Lycaenidae followed by Pieridae, Hesperiidae and Papilionidae. Among these 103 species Based on value of butterfly relative dominance in study area, 28.155 % species was categorized as abundant species whereas 36.89 % species was common, 11.65 % species was frequent, 18.44 % was occasional, and 4.85 % species was rare. The rare species recorded are Rapala airbus Rapala manea Tajuria cippus Euthalia aconthea Arphopala amantes.
Out of these 103 butterfly species, 16 species specified under Indian Wildlife (Protection) Act, 1972 were encountered in good numbers. The butterflies Pachliopta hector Castalius rosimon and Virachola isocrates are placed in Schedule I Part IV, the species Appias albino, Cepora nerissa, Hypolimnas misippus, Polyura athamas, Charaxes bernardus, Anthene lycaenina, Charaxes solon, Euchrysops cnejus, Lampides boeticus. Prosotas dubiosa and Tajuria cippus are protected under Schedule II Part II, while Hasora vita and Baoris farri are categorized as Schedule IV. It is established that the butterfly abundance increased in monsoon as population is at its peak in June and July. It decreased in the summer and pre-monsoon possibly due to the unavailability of nectar and the changes in temperature and humidity of the habitats concerned, as temperature and relative humidity are the important factors in distribution and assemblage of butterfly species (Gupta et al., 2019)
Observations on the monthly variations of butterfly species encounter indicates peak from August to November and December while low from January to May. The present observations remain consistent with the records and views of the butterfly species in different parts of the world (Wilson et al 2004; Tiple et al., 2006; Sodhi et al., 2010; Tiple 2018). The number of species observed in the present study remained similar to the observations on the species in different parts of India bearing similar landscape patterns (Roy et al 2012; Harsh 2014; Saikia 2014; Mukherjee et al., 2015). As revealed through the present study, 103 butterfly species are available in different numbers across the study area. Dominance of the butterflies of the family Nymphalidae is similar to that observed in other parts of the world (Mutmainnah and Santosa 2019).
In parity with the species diversity observed in Pandharkawada Forest Division, Maharashtra, India, it may be assumed that the butterflies play diverse functional roles for the sustenance of the ecosystems. The richness in species composition in study area was also prominent in present investigation. The availability of the vegetation, seasonal wetlands and allied factors render stability to the butterfly population and assemblages in the landscapes, these are possibly important contributors to the observed variations in the butterfly species recorded in the present study. The observations on the diversity of the butterflies in the study area suggested that the intensive conservation management is required to ensure sustenance of ecosystem services derived from the butterflies.
The present diversity study was confined to a limited area and selected habitats. There is, in the future, a chance of more species being reported because of few pockets and habitats in the studied area requiring more extensive exploration.
ACKNOWLEDGMENTS
The author is thankful to Mrs. K.M. Abharna, Deputy Conservator of Forest, Pandharkawada Forest Division for giving permission and for extending support during the conduct of this study.
REFERENCES
Basavarajappa S. (2006) Avifauna of agro-ecosystem of Maidan area of Karnataka. Zoo’s Print Journal 21:2217-2219.
Bonebrake TC, Ponisio C, Boggs CL. et al. (2010) More than just indicators: a review of tropical butterfly ecology and conservation. Biological Conservation 143:1831-1841.
Ehrlich PR, Hanski I. (2004) On the wings of checkerspots: a model system for population biology. Oxford: Oxford University Press. p. 408.
Evans WH. (1932) The identification of Indian butterflies. Bombay: Bombay Natural History Society. p. 464.
Gupta H., Tiwari C. and Diwakar S. (2019) Butterfly diversity and effect of temperature and humidity gradients on butterfly assemblages in a sub-tropical urban landscape. Tropical Ecology 60:150-158
Hammer Ø, Harper DAT, Ryan PD. (2001) Paleontological statistics software package for education and data analysis. Paleontologia Electronica 4:1-9.
Haribal M. (1992) The butterflies of Sikkim Himalayas and their natural history. Gangtok: Sikkim Nature Conservation Foundation (SNCF). p. 217.
Harsh S. (2014) Butterfly diversity of Indian institute of forest management, Bhopal, Madhya Pradesh, India. Journal of Insects 2014:1-4.
Joshi PS, Tantarpale VT. (2016) Diversity of Saurian fauna in the Buldhana district, Maharashtra, India. Journal of Asia-Pacific Biodiversity 9: 306-311
Joshi PS. (2014) Diversity and population dynamics of ophidian fauna from Buldhana District, Maharashtra, India. A thesis submitted to S.G.B. Amravati (India): Amravati University. pp. 1-260.
Kehimkar I. (2008) The book of Indian butterflies. Mumbai: Bombay Natural History Society and Oxford University Press. p. 497.
Kinnear PR, Gray CD. (2000) SPSS for windows made simple. Release 10. Sussex. UK: Psychology Press. p. 432.
Koh LP, Sodhi NS. (2004) Importance of reserves, fragments, and parks for butterfly conservation in a tropical urban landscape. Ecological Applications 14:1695-1708.
Kunte K.( 2000) Butterflies of Peninsular India. Hyderabad: Universities Press (India) Limited. p. 254.
Kuussaari M, Heliölä J, Luoto M, Pöyry J. (2007) Determinants of local species richness of diurnal Lepidoptera in boreal agricultural landscapes. Agriculture, Ecosystems and Environment 122:366-376.
Losey JE, Vaughan M. (2006) The economic value of ecological services provided by insects. BioScience 56:311-323.
Magurran AE. (1988) Ecological diversity and its measurement. London: Chapman and Hall. p. 192.
Mukherjee S, Banerjee S, Saha G, Basu P, Aditya G. (2015) Butterfly diversity in Kolkata, India: An appraisal for conservation management. Journal of Asia-Pacific Biodiversity 8: 210-221.
Mutmainnah AR, Santosa Y. (2019) Impact of oil palm plantation on the butterfly diversity: a Case study in KGP & CNG, Ketapang, West Kalimantan. IOP Conf. Series: Earth and Environmental Science 336: 012032.
Nimbalkar RK, Chandekar SK, Khunte SP. (2011) Butterfly diversity in relation to nectar food plants from Bhor Tahsil, Pune District, Maharashtra, India. Journal of Threatened Taxa 3:1601-1609.
Öckinger E, Dannestam Å, Smith HG. (2009) The importance of fragmentation and habitat quality of urban grasslands for butterfly diversity. Landscape and Urban Planning 93:31-37.
Öckinger E, Eriksson AK, Smith HG. (2006) Effects of grassland management, abandonment and restoration on butterflies and vascular plants. Biological Conservation 133:291-300.
Öckinger E, Smith HG. (2006) Landscape composition and habitat area affect butterfly species richness. Oecologia 149:526-534.
Pollard E, Yates TJ. (1993) Monitoring butterflies for ecology and conservation. London: Chapman and Hall. p. 292.
Pollard E. (1977) A method for assessing changes in the abundance of butterflies. Biological Conservation 12:115-134.
Roy US, Mukherjee M, Mukhopadhyay SK. (2012) Butterfly diversity and abundance with reference to habitat heterogeneity in and around Neora Valley National Park, West Bengal, India. Our Nature 10:53-60.
Saikia MK.( 2014) Diversity of tropical butterflies in urban altered forest at Gauhati University Campus, Jalukbari, Assam. Journal of Global Biosciences 3:452-463.
Sodhi NS, Koh LP, Clements R, et al. (2010) Conserving Southeast Asian forest biodiversity in human-modified landscapes. Biological Conservation 143:2375-2384.
Stefanescu C, Herrando S, Páramo F. (2004) Butterfly species richness in the northwest Mediterranean Basin: the role of natural and human-induced factors. Journal of Biogeography 31:905-915.
Thomas JA. (2005) Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philosophical Transactions of the Royal Society B 360:339-357.
Tiple A. (2018) Butterflies (Lepidoptera Rhopalocera) of the Bor wildlife sanctuary, Wardha, Maharashtra, Central India. Biodiversity Journal 9: 171-180.
Tiple AD, Deshmukh VP, Dennis RLH. (2006) Factors influencing nectar plant resource visits by butterflies on a university campus: implications for conservation. Nota Lepidopteralogica 28:213-224.
Watt WB, Boggs CL. (2003) Synthesis: butterflies as model systems in ecology and evolution present and future. In: Boggs CL, Watt WB, Ehrlich PR, editors. Butterflies: ecology and evolution taking flight. Chicago: The University of Chicago Press. pp. 603e613.
Wilson EO. (1997) Introduction. In: Reaka-Kudla ML, Wilson DE, Wilson EO, editors. Biodiversity II. Washington, D.C: Henry Press. pp. 1-3.
Wilson RJ, Thomas CD, Fox R, et al. (2004) Spatial patterns in species distributions reveal biodiversity change. Nature 432:393-396.
Wynter-Blyth MA. (1957) Butterflies of the Indian region. Mumbai: Bombay Natural History Society. 523 p.
Yavatmal Gazetteer. (2019) Database of Buldhana District redirected from official website of Buldhana District, Maharashtra, India. Available at: www.yavtmal.nic.in [Date accessed: 01 January 2019].
Zar JH. (1999) Biostatistical analysis. 4th ed. New Delhi (Indian Branch): Pearson Education (Singapore) Pte. Ltd.. p. 667.