1Department of Biotechnology, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Guntur, AP, India–522502
Corresponding author Email: kkchandrika@kluniversity.in
Article Publishing History
Received: 02/01/2019
Accepted After Revision: 22/03/2019
Adsorption of C-Phycocyaninalong with its recovery and purity was tested with various adsorbents. Evaluation of experimental results shows that activated charcoal has a higher adsorption when compared to Graphite, Chitosan, and Sawdust and filter aid. Langmuir and Freundlich adsorption isotherms were taken into consideration to screen the best adsorbent. Although, Langmuir and Freundlich isotherms follow a similar sequence of ranking for adsorbents, Langmuir ranking had shown significant variation in ‘qm’ (Adsorption capacity) over the ‘KF’ (Binding constant) of Freundlich model. Nevertheless, both models predicted activated charcoal as the best adsorbent with the maximum specific adsorption. Discrete step optimization for Phycocyanin recovery and purification shows 1% activated charcoal retained 79.3% of CPC while adsorbing 64.8% of contaminant protein with 2.1 purity index. FTIR spectroscopic studies reveal that the C-PC after adsorption with 1% charcoal showed a significant reduction in the concentration of other components characteristic of the crude sample. Thus, this work suggests a quick and reliable way to screen adsorbents based on binding constants and FTIR studies.
Ethanol Fermentation, Molasses, Immobilization, Activators, Reducing Sugar
Chandrika K, Deviprasanth S, Reddy D. P. K, Sultana F, Mandava C. Application of Langmuir and Freundlich Adsorption Isotherms in Screening Suitable Adsorbents and The Role of FTIR in Confirmation of C Phycocyanin Purification. Biosc.Biotech.Res.Comm. 2019;12 (1).
Chandrika K, Deviprasanth S, Reddy D. P. K, Sultana F, Mandava C. Application of Langmuir and Freundlich Adsorption Isotherms in Screening Suitable Adsorbents and The Role of FTIR in Confirmation of C Phycocyanin Purification. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2WvC7Ww
Copyright © Chandrika et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
C-Phycocyanin (C-PC), is a water-soluble protein pigment derived from the alga Arthrospira platensis. The pigment besides being a natural colorant is known as a potential antioxidant, anti-inflammatory agent. Studies also show C-PC is known for its platelet aggregation inhibitor. It has also shown to have hepato-protective and neuroprotective properties (Gupta, 2012). In pharmaceutical applications, the purity of C-PC should be (A620/A280) ≥ 4. Cosmetic and food industry requires a purity ratio of A620/A280 ≥ 2. On the other hand the analytical grade (‘Medical’ or ‘Research’ grade) prefers a purity ratio of 2.5-3.5 (Chaiklahan, 2011).
Phycocyanin is a protein complex which can be purified by a simple adsorption technique. Adsorption is a physical phenomenon in which one can notice an adhesion of atoms, ions and molecules on to a solid surface (Siegbahn, 1990). The adsorbate creates a film on the surface of the adsorbent. The process of adsorption can be explained with classical adsorption models such as the Langmuir and Freundlich isotherms. During a typical adsorption, before equilibrium, the amount of adsorbate on the adsorbent is a function of its pressure (if gas) or concentration (if liquid) at constant temperature. The amount of adsorbed material is the normalization of adsorbent mass, so that a comparison of different adsorbent material can be made during the screening of adsorbents. Freundlich and Langmuir isotherm models are useful tools for comparison of adsorption efficiency of the adsorbents.
A study (Alves, 2010) shows the adsorption of proteins such as bovine serum albumin, human serum albumin (HSA) and human fibronectin (HFN). In their study it was shown that albumin adsorption fits the Freundlich model. Several studies show enzyme (protein) adsorption onto the polymer particles (Tetsuya Yamamoto, 2017).
The recovery and yield of a desired product is important during extraction process. At present, several expensive unit operations are routinely adopted for purification of C-Phycocyanin. About 50-90% of the production costs are included in the purification steps. Common methods for extraction and purification of phycocyanin involve break up of cells using high pressure homogenization (Moraes, 2011), sonication (Furuki, 2003), freeze thawing, or enzymatic treatment; ammonium sulphate precipitation followed by procedures adopting hydrophobic (Bracewell, 2011). ion exchange, size exclusion (Jian-Feng, 2007), hydroxyapatite chromatography (Markov, 1974) and aqueous two phase extraction (Patil, 2007). In spite of the increased purity, these methods, however proven to be expensive and time consuming. In addition, some purification techniques may change the structural and functional properties of proteins, (Bermej, 2008 (Sikarwar, 2017).
Therefore, it is essential to develop a reliable economic process for the separation and purification of phycocyanin. The present work aims at process optimization for increased recovery and purity of phycocyanin using various adsorbent materials like Charcoal, Graphite, Chitosan, Saw dust and Filter aid in a minimum number of operations. The corresponding affinity of the adsorbent towards the protein was studied using Langmuir and Freundlich isotherm constants. (Finette, 1997)Considering the yield and purity index of the phycocyanin obtained after the each adsorption process with different adsorbent material, a suitable method has been developed which is more efficient and economically feasible.
Materials and Methods
Phycocyanin extraction and estimation Phycocyanin was extracted from spray dried Spirulina platensis. About 500mg of biomass was dissolved in 500mL of 50mM phosphate buffer (pH 6.8) or equal volume of distilled water in an Erlenmeyer fl ask (Borosil, India). About 20g of 0.65mm glass beads (Merck, India) and 0.25M CaCl2.2H2O were weighed and pre-mixed with the biomass-solvent mixture. The extraction was done using a rotary shaker (Pharmacon, India) at 150 rpm for 30min. The lysed biomass was centrifuged (10,000 rpm, 10min at 10oC) and the supernatant was estimated for recovery and purity of phycocyanin using a double beam UV-Vis spectrophotomeer (Dynamica, Halo DB20). The peak for C-PC was identified at 620nm while the allophycocyanin was identifi ed at 652 nm. The purity of C-PC was estimated by the absorbance index of A620/ A280.The conc. of pigment (mg mL-1) was estimated using the following equation adopted by (Moraes., 2011)
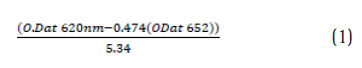
Adsorption Studies
The selected adsorbents for the present study are activated charcoal (Merck, India), Graphite (Ottokemi, India), Chitosan (Ottokemi, India), Hyfl osupercel Filter aid (Ottokemi, India) and Sawdust. The porous sawdust collected was washed with de-ionized water, air dried and sieved to a mesh size of 297 microns.
Batch adsorption experiments were carried out by adding 5% (wt/v) of each adsorbent material to 20mL of crude phycocyanin extract at five different concentrations (obtained by diluting the original crude extract (20%-100% (v/v)). The crude phycocyanin extract was allowed to equilibrate with the adsorbent material for a contact time of 30 minutes on an orbital shaker at 150rpm and then centrifuged at 10,000rpm for 10minutes at 10oC. The corresponding phycocyanin concentration and the recovery after adsorption were calculated. The effect of various concentrations of charcoal (0.5, 1.0, 2.0, 5.0 % w/v) on the recovery and purity of phycocyanin after adsorption were also determined following the same protocol.
The equilibrium concentration (Ce) of the total protein in the supernatant collected was determined by Lowry’s method (farina mujeeb., 2018) referring to the calibration curve of BSA standards. The equilibrium adsorption capacity (qe) is obtained by dividing the amount of total protein adsorbed by the mass of the adsorbent material taken. The adsorption data was fi tted into both Langmuir model (Eq. 2) and Freundlich model (Eq. 3). The maximum adsorption capacity (qm) in Langmuir equation and the Binding constant (KF) in Freundlich equation were determined accordingly. While ‘n’ being the Freundlich isotherm constant.These models were chosen to estimate the adsorption intensity of the sorbent towards the adsorbent
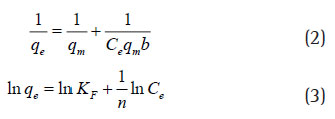
FTIR studies
Structural elucidation of Phycocyanin was made using Fourier transform – Infrared Spectroscopy, Spectrum Two™ was purchased from Perkin Elmer. (USA). The instrument uses LiTa03 (Lithium tantalite) pyroelectric infrared detector for scanning the samples. The CPC samples were applied on to a Calcium fl uoride (CaF2) glass windows for measuring the protein peak after attaining equilibrium during adsorption process. All the spectra during analysis were smoothed and analysed by using Spectrum 10™ version 10.3.06 software.
Results and Discussion
Batch adsorption experiments
The C-PC pigment recovery was made from the S.platensis dried biomass. The recovered extract was designated as 100% crude CPC solution. A series of dilutions was made and five different concentrations of the crude protein extract were generated to study the interaction of the crude CPC with different adsorbent materials- graphite, activated charcoal, filter aid, chitosan and sawdust. Table 1 enlists the adsorbents in the study and their physical properties with various isotherm constants. The methylene blue adsorption studies showed highest specific adsorption by activated charcoal followed by graphite, sawdust, filter aid and chitosan. The specific adsorption of protein in crude phycocyanin solution also revealed a similar order with maximum specific adsorption of protein being 10fold higher than chitosan (Sala, 2014).
Although, Langmuir and Freundlich isotherms follow a similar sequence of ranking for adsorbents, Langmuir ranking had shown significant variation in ‘qm’ (Adsorption capacity) over the ‘KF’ (Binding constant) of Freundlich model,both of which reveal the specific adsorption capacity of an adsorbentNevertheless, irrespective of the variation in the magnitude of constants in both cases, activated charcoal was shown to perform better than any of the adsorbent being adopted in the present study.
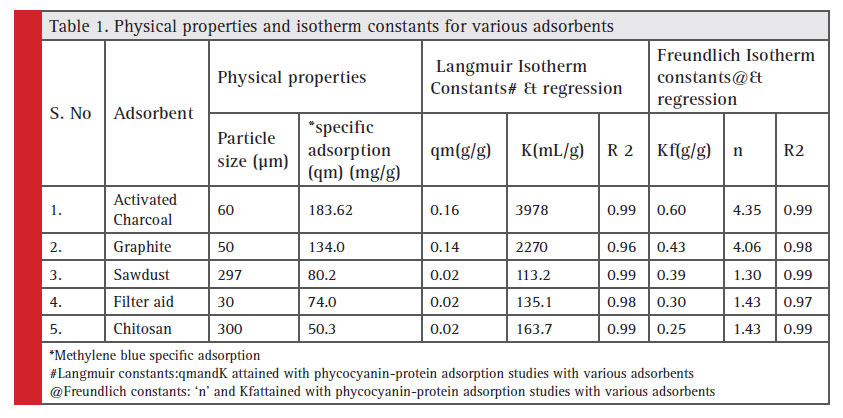 |
Table 1: Physical properties and isotherm constants for various adsorbents |
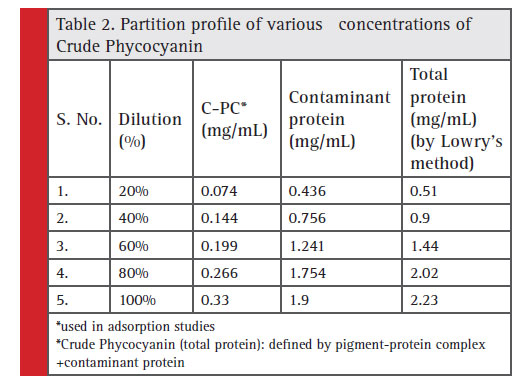 |
Table 2: Partition profile of various concentrations of Crude Phycocyanin |
Comparison of the various adsorbent materials for phycocyanin recovery and yield
Phycocyanin, is essentially a protein –pigment complex. The adsorption of phycocyanin was attributed partly due to the protein complex associated with the pigment chromophore. The algal biomass also was shown to comprise 60% of total protein. Hence, during cell lysis and recovery of intracellular pigment-protein complex, supplemental protein contaminant (protein from biomass) (Rajakumar, 2015) is anticipated along with the crude total protein extract of phycocyanin.
The relative screening of the effective adsorbent material is based on the amount of adsorbed contaminant protein; the CPC recovered after adsorption and the purity index. Table 2 shows the total protein partition profile of the crude protein extract and Table 3 details the adsorption efficiency of the adsorbents during simultaneous CPC recovery and purification. In the initial batch adsorption studies, a higher purity index of 1.87 was observed with activated charcoal at a crude protein concentration of 100% (Table 4). Crude protein adsorption with activated charcoal exhibited a relatively higher purity index at all the five different concentrations when compared to the sorption studies with other adsorbents. Chitosan and Graphite are ranked in the next order for their purity index of the adsorbed phycocyanin (Minkova, 2003)
The importance of the current study lies in highlighting the proportion of the adsorbed contaminant protein. Data (Table 3) shows a significant proportion of the contaminant protein removal (84.95%) was observed with 5 % (w/v) activated charcoal at 60% concentration of the crude protein. Graphite was found to be next in its adsorption capacity of the contaminant protein (68.88%). Filter aid reported 64.46% contaminant protein removal at 80% crude protein concentration while sawdust reported 61.29% removal efficiency. Chitosan reported 42.94% crude protein removal efficiency at 80% crude protein concentration.
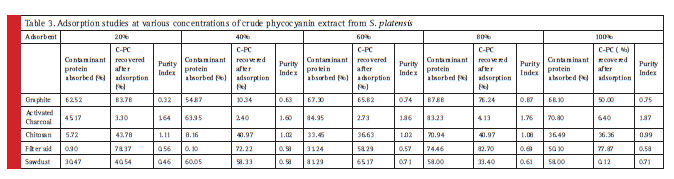 |
Table 3: Adsorption studies at various concentrations of crude phycocyanin extract from S. platensis |
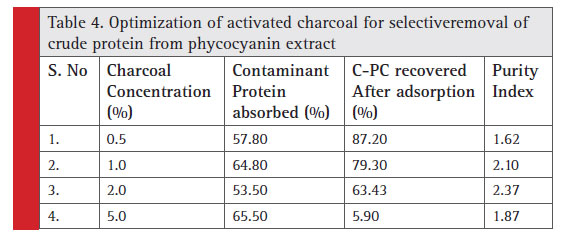 |
Table 4: Optimization of activated charcoal for selective removal of crude protein from phycocyanin extract |
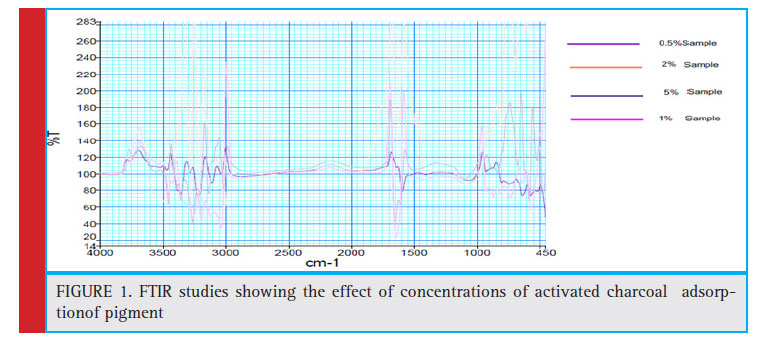 |
Figure 1: FTIR studies showing the effect of concentrations of activated charcoal adsorption of pigment |
The screening of the effective adsorbent for further study lies in its efficiency towards its CPC recovery and the relatively higher purity index after the adsorption experiment. On an average, the CPC recovery after the adsorption with activated charcoal was about 70%. Hence, the activated charcoal though reported lesser recovery of CPC compared to other adsorbents it was screened for further study due to its increased purity index and a higher percentage of contaminant protein removal. (Kethineni, 2018).
Discrete step optimization for Phycocyanin recovery and purification
At high concentration of activated charcoal (5%w/v), indiscriminate adsorption of protein (phycocyanin as well) was observed. However, a supplementary study on optimization of the concentration of activated charcoal for the CPC recovery is performed (Table 4). One percent activated charcoal retained 79.3% of CPC while adsorbing 64.8% of contaminant protein with 2.1 purity index. Nevertheless, a purity index of 2.37 was reported with 2% (w/v) activated charcoal with a CPC recovery of only 63.43%.
FTIR spectra analysis of crude and adsorbed C-PC sample
The structural configuration of the Phycocyanin after adsorption at different concentrations of the charcoal as adsorbent were confirmed by FTIR spectra (Figure1). On the FTIR spectra of crude C-PC, the peaks in the range of 3698.23-3460 cm-1 indicate the presence of O-H stretching vibrations and the peaks at 2932.99-2912.28 cm-1 represent the asymmetrical and symmetrical stretches of aliphatic CH2 groups. The frequency range from 1345.07-1250.18 are assigned to the C-O stretching, O-H bending vibrations and C-O asymmetric C-O-C stretching, which represent the presence of alcohols and esters. Peaks of 1133.11 and 1079.64 cm-1 represent symmetric C-H stretching vibration. The peak values of 677.39 and 640.22 cm-1 represent the stretch of S=O and S-O. The peaks at 537.21, 1650.72 and 3440.91 cm-1 in the FTIR spectra of crude C-PC are characteristic of phycocyanin, which are also present in the spectra of C-PC after adsorption with 1% charcoal. However, it can be seen that some peaks of C-PC crude sample disappear in the 1% charcoal adsorbed C-PC sample explaining the phenomena of increased purity index after adsorption. Thus, all the characteristic peaks of Phycocyanin observed in the crude C-PC sample is evident in the FTIR spectra of C-PC after adsorption with 1% charcoal with a signifi cant reduction in the concentration of other components characteristic of the crude sample. Furthermore, the spectra also provide a fingerprint of the adsorbed Phycocyanin in the sample.
Conclusion
Process intensification approach in the recovery and purification of phycocyanin in the minimum number of operations is proposed. Basing on the scalability and economic feasibility, the activated charcoal (powder) at 1% was chosen to be the material of choice for simultaneous recovery and purification of phycocyanin. Amidst the high value and low volume nature of the targeted product, the recovery and specific yield of cyanobacterial phycocyanin from Spirulina platensis dried biomass showed the suitability of the biomass in various adsorption operations in optimizing the product extraction for maximum pigment recovery. The adsorption phenomena are further confi rmed by the FTIR studies.
References
Gupta, N.K., (2012) Effects of C-Phycocyanin on the representative genes of tumor development in mouse skin exposed to 12-O-tetradecanoyl-phorbol-13-acetate. Environmental toxicology and pharmacology, vol. 34(3), pages 941-948.
Chaiklahan, R., (2011) Separation and purifi cation of phycocyanin from Spirulina sp. using a membrane process. Bioresource technology, vol. 102(14), pages 7159-7164.
Siegbahn, K. (1990). From X-ray to electron spectroscopy and new trends. Journal of Electron Spectroscopy and Related Phenomena, vol. 51, pages 11–36.
Alves, C., Reis, R. and Hunt, J. (2010). The competitive adsorption of human proteins onto natural-based biomaterials. Journal of The Royal Society Interface, vol. 7(50), pages 1367-1377.
Tetsuya yamamoyo., (2017). In-situ adsorption of polymer particles on multiwall. Carbon nanotubes using Colloidal techniques. Colloid and interface ScienceCommunication, vol. 20, pages 1-4.
Sikarwar, V.S., Zhao, M., (2017). Progress in biofuel production from gasification. Progress in Energy and Combustion Science, vol. 61, pages 189–248.
Moraes,C.C., (2011) C-phycocyanin extraction from Spirulina platensis wet biomass. Brazilian Journal of Chemical Engineering, vol. 28(1), pages 45-49.
Furuki (2003) Rapid and selective extraction of phycocyanin from Spirulina platensis adopting with ultrasonic cell disruption. Journal of Applied Phycology, vol. 15(4) pages 319-324.
Bracewell D.G. (2011). Ammonium sulphate precipitation followed by procedures adopting hydrophobic. Dual salt precipitation for the recovery from EscherichiacoliBiotechnical,vol. 27(5), pages 1306-1314.
Jian-Feng, N., (2007). Large-scale recovery of C-phycocyanin from Spirulina platensis using expanded bed adsorption chromatography. Journal of Chromatography B, vol. 850(1), pages 267-276.
Markov, G. G., & (1974). Hydroxyapatite column chromatography in procedures for isolation of purified DNA. Analytical Biochemistry, vol. 59(2), pages 555–563.
Patil, G., Raghavarao, K. (2007). Aqueous two-phase extraction for purification of C-phycocyanin. Biochemical Engineering Journal, vol. 34(2), pages 156-164.
Bermejo, P., (2008). Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulinaplatensis. Food Chemistry, vol. 110(2), pages 436-445.
inette, G., (1997). Comparative studies on the isothermal characteristics of proteins adsorbed under batch equilibrium conditions to ion-exchange, immobilised metal ion affinity and dye affinity matrices with different ionic strength and temperature conditions. Journal of Chromatography, vol. 763(1-2), pages 71–90.
Sala, L., Figueira, F., Cerveira, (2014). Kinetics and adsorption isotherm of C-phycocyanin from Spirulina platensis on ionexchange resins. Brazilian Journal of Chemical Engineering, vol. 31(4), pages 1013-1022
Gangl, D., Zedler, J., Rajakumar, P., Martinez, E., Riseley, A., Włodarczyk, A., Purton, S., Sakuragi, Y., Howe, C., Jensen, P. and Robinson, C. (2015). Biotechnological exploitation of microalgae. Journal of Experimental Botany, vol. 66(22), pages. 6975-6990.
Minkova, K., Tchernov, A., Tchorbadjieva, M., Fournadjieva, S., Antova, R. and Busheva, M. (2003). Purifi cation of C-phycocyanin from Spirulina (Arthrospira) fusiformis. Journal of Biotechnology, vol. 102(1), pages 55-59
Kethineni, C., Choragudi, S., Kokkiligadda, S., Jaswanthi, N. and Ronda, S. (2018). Development of Sequential Processes for Multiple Product Recovery from Microalgae. Industrial Biotechnology, vol 14(2), pages 95-106


