1Department of Botany, Little Flower College, Guruvayoor, Kerala, Pin 680523
2Cell Culture Lab, Department of Botany, Union Christian College, Aluva, Ernakulam, Pin-683 102, Kerala, India.
Article Publishing History
Received: 02/04/2020
Accepted After Revision: 30/05/2020
Endophytes are symbiotic bacteria that inhabit plant tissues and they were recognised as beneficial microorganisms which does not cause any disease symptoms or adverse effect on the host plant. They may be associated with the production of metabolites that either directly or indirectly influence the medicinal properties of plants. The antimicrobial and antioxidant property of the endophytic bacterial species associated with E. sonchifolia can be beneficial for the identification and isolation of valuable bioactive compounds. Six endophytic bacteria were isolated from Emilia sonchifolia (L.) and their ethyl acetate extract was prepared. This extract was then used for the study of antimicrobial and antioxidant properties. The total phenol and flavanoid contents of the bacterial extracts were estimated and antioxidant activity by DPPH, ferric ion reducing, nitric oxide scavenging and cupric ion reducing assays were done. Antioxidant analysis explained the potential antioxidant property of endophytic bacterial isolate from the medicinal plant. The Isolate ES1 indicated the highly efficient antioxidant property. GC-MS and LC-MS analysis were employed for the identification of compounds which imparts the antimicrobial and antioxidant property of the endophytic bacterial isolate ES1.This pointed the presence of bioactive compounds like surfactin, fengycin, iturin from the bacterial extract.
Emilia sonchifolia, Antimicrobial, antioxidant, endophyte, surfactin, iturin
Urumbil S. K, Jesy E. J, Anilkumar M. Antimicrobial and Antioxidant Potential of Endophytic Bacteria Isolated from Emilia sonchifolia. Biosc.Biotech.Res.Comm. 2020;13(2).
Urumbil S. K, Jesy E. J, Anilkumar M. Antimicrobial and Antioxidant Potential of Endophytic Bacteria Isolated from Emilia sonchifolia. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2B0qFZy
Copyright © Urumbil and Jesy This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The rapid emergence of multiple drug resistant strains of pathogens demanded the need for the development of plant based antibiotics. Extensive screening of medicinal plants for unexplored metabolites is fast progressing in this area. Many of them harbour bacterial or fungal endophytes that support the production of metabolites. Endophytes are mutualistic organisms residing inside the plant and produce compounds of pharmacological importance (Pezzuto, 1996). In the recent years, studies on these endophytic microorganisms as an alternative source of various bioactive metabolites resulted in the development of many probiotic and antibiotic compounds. The interest on endophytes associated with medicinal plants led to the identification of different antimicrobial compounds producing strains of microbes. There were many reports on natural products like alkaloids, flavanoids, phenolic compounds, peptides and steroids obtained from medicinal plants that harboured endophytes. In most of the cases the endophytes may either participate or gain some genetic information that led to the production of such metabolites, (Shukla et al., 2015, Ahemad and Kibret, 2014, Gond et al., 2015, Sulistyani et al., 2016, Mohamad et al., 2018, Hazarika et al., 2019).
Along with the production of antimicrobial compounds endophytes are reported to produce potent antioxidants (Zheng et al., 2016, Bintang et al., 2015). Health care and food processing industries demand novel antioxidant compounds that rapidly reduce oxidation and manage reactive oxygen species causing damage to cells. Endophytes play a major role either directly or indirectly by enhancing the growth of the host plant and thus the plant growth promotion. Plants harbouring endophytes shows increased growth rate due to the production of various phytohormones, ameliorating the stress tolerance or by checking pathological conditions (Swarnalatha et al., 2015). Therefore metabolites produced by endophytes gained attention as future prospective antibiotics and antioxidants, (Hazarika et al., 2019, Hanif et al 2019).
The present investigation was carried out in Emilia sonchifolia (Linn.)DC., a member of Asteraceae family of Dicots. In Ayurveda the plant is highly reputed for its medicinal uses and is an important member in Dasapushpa (Raj et al., 2013). This medicinal plant was reported to produce a broad spectrum of therapeutically active metabolites possessing antimicrobial (Latha et al., 2009, Thenmozhi et al., 2013), antioxidant (Sophia et al., 2011) and anti-inflammatory (Essien et al., 2009) activities. It has been used in the treatment of asthma, inflammatory disorders, cuts and wounds. In ethnomedicine, the plant reported for sore throats, eye, ear ailments, malaria, and measles (Kumar et al., 2015). In this regard enquiry of bacterial endophytes in Emilia sonchifolia (Linn.)DC. has been undertaken along with their antioxidant and antimicrobial studies.
MATERIAL AND METHODS
Endophytic bacteria were isolated and identified by 16S rDNA sequencing from Emilia sonchifolia (Urumbil and Anilkumar, 2019). The sequence data were submitted to NCBI Gen Bank. The endophytic bacteria isolated were Bacillus subtilis strain UCCBOT-ES1 (MG692780), Paenibacillus sp strain UCCBOT-ES2 (MG692781), Microbacterium sp. strain UCCBOT-ES3 (MG692782), Bacillus cereus strain UCCBOT-ES4 (MG692783), Bacillus aryabhattai strain UCCBOT-ES5 (MG692784) and Micrococcus sp. strain UCCBOT-ES6 (MH027648).
Preparation of extract:The endophytic bacterial isolates were inoculated into 500ml nutrient broth and incubated at 25±2ºC for 5 days. The cultures were centrifuged at 8000 rpm for 10 minutes and the supernatant was extracted with double the volume of ethyl acetate and concentrated to a powder form. It was further dissolved in methanol and used for the antimicrobial and antioxidant analysis.
Antimicrobial analysis:The antimicrobial activity of endophytic bacterial extracts were tested against human pathogenic bacteria such as Escherichia coli (MTCC 40), Salmonella typhi (MTCC 426), Klebsiella pneumonia (MTCC 109) and Proteus vulgaris (MTCC 3220), and fungi such as Candida albicans (NCIM 3102) and Aspergillus niger (NCIM619) by co culture and disc diffusion methods. The plates were incubated overnight at 37°C and observed for the growth of pathogenic bacteria on either side of the isolates. Further antimicrobial activity of the extracts was studied by disc diffusion method. Streptomycin (10µg/ml) and itraconazole (50µg/ml) was used as antibacterial and antifungal drugs respectively.
Antioxidant analysis: Estimation of total phenol: The total phenol content of the endophytic bacterial extract was calculated by Folin and Ciocalteu method (Swarnalatha et al., 2015). The experiments were repeated thrice and mean value was calculated. The phenolic content was expressed as Gallic acid equivalents in µg/ml.
Estimation of total flavonoid: The total flavanoid content was measured by aluminium chloride colorimetric assay (Kamtekar et al., 2014). The total flavanoid content was expressed as µg/ml of quercetin equivalent.
DPPH free radical scavenging Assay: 50µl of extract in methanol was added to 100µl of DPPH solution and 850µl methanol, so that the final volume was 1ml. Nutrient broth was used as the control and methanol as blank. Percentage of scavenged DPPH radical was calculated using following formula
![]()
Where, Ac is the absorbance of control and A1 is the absorbance of sample. Ascorbic acid was used as standard, (Sulistiyani et al., 2016).
Ferric ion reducing assay: Extracts prepared from the endophytic bacterial isolates were mixed with 2.5ml of phosphate buffer (0.2M, pH 6.6) and 2.5ml potassium ferricyanide (1%w/v). Method reported by Jayanthi and Lalitha, 2011 using trichoro acetic acid and ferric chloride solution was employed for the ferric iron reducing assay. Ascorbic acid at various concentrations was used as standard.
Nitric oxide radical scavenging assay:1ml of different concentrations of the extract was mixed with 0.5ml of 10mM sodium nitropruside in phosphate buffered saline and incubated at 25ºC for 180 minutes. After incubation the extract was mixed with an equal volume of freshly prepared Griess reagent. Control samples without the extract but with an equal volume of buffer were prepared similar to test sample (Boora et al., 2014). Ascorbic acid was used as positive control. The percentage of nitric oxide radical scavenging activity of the endophytic bacterial extract was calculated.
Cupric iron reducing antioxidant capacity assay (CUPRAC Assay): Cupric iron reducing capacity was measured in accordance to the method of Apak et al., (2008). 1ml of crude extract in ethanol was added with 1ml of 7.5×10-3M Neocuproine (Nc) solution, 1ml of 1×10-2M CuCl2 Solution, 1ml of ammonium acetate buffer (pH=7) and 1ml water. Incubate for 30minute at 25ºC and measure the absorbance at 450nm and ascorbic acid was used as standard.
Statistical analysis:All data were expressed as mean ±SD. The mean values were statistically analysed using One-way analysis of variance (ANOVA) using the graph pad instat software package.
GC-MSAnalysis and identification:Ethyl acetate fraction of the endophytic bacterial extract with greater antioxidant property was used for GC-MS analysis (Model Number: QP2010S), Column (Rxi-5Sil MS,30 meter length,0.25 mm ID, 0.25 µm thickness), GCMS Software (GCMS Solutions) and Libraries (NIST 11 & WILEY 8).
LCMS/MS-Q-TOF analysis: The crude ethyl acetate fraction of bacterial extract was subjected to liquid chromatography coupled with mass analyser (Waters Xevo G2 QTOF-MS/MS). The separation was carried out in a Acquity BEH C18 column and the two component solvent system contained 90% water (Acidified with 1% formic acid) and 10% acetonitrile with a flow rate of 0.3 ml/min.
RESULTS AND DISCUSSION
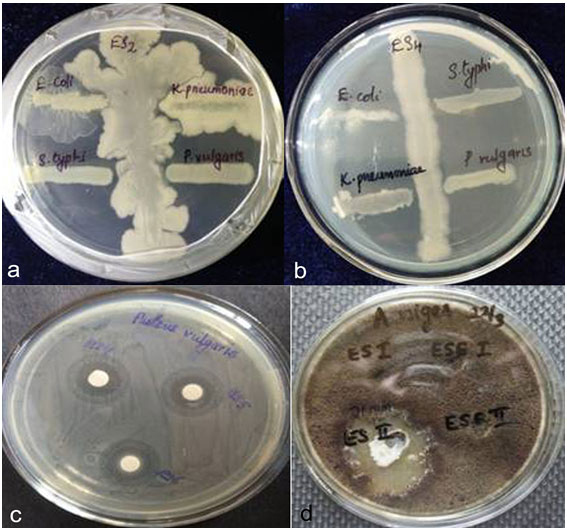
Endophytic bacteria are one of the unexplored, promising and relevant producers of metabolites useful in pharmaceutical, health care and agricultural industries. Endophytic bacteria especially many members from the Bacillus species, are reported to possess antimicrobial activity as an indirect mechanism for plant growth promotion by secretion of compounds to check phytopathological conditions (Ahemad and Kibret, 2014, Gond et al., 2015, Mohamad et al., 2018). In the present study, among the different isolates, ES4, ES5 and ES6 exhibited clear growth inhibition against all tested bacterial strains. At the same time ES1, ES2 and ES3 showed growth inhibition against P.vulgaris and S.typhi only (Fig.1, 2). When disc diffusion method was performed to assess the antibacterial activity, the strain ES4 (Bacillus cereus strain), ES5 (Bacillus aryabhattai strain) and ES6 (Micrococcus sp.strain) produced zone of inhibition against all bacterial strains and maximum zone of inhibition was reported against P.vulgaris and S.typhi (Table 1, Fig.3, 4). On the contrary strain ES1, ES2 and ES3 showed negligible zone of inhibition against the tested pathogens. Sunkar and Nachiyar (2012) reported the use of Bacillus cereus isolated from Garcinia xanthochymus for the synthesis of antibacterial silver nanoparticle indicated its significance as an antibacterial agent .
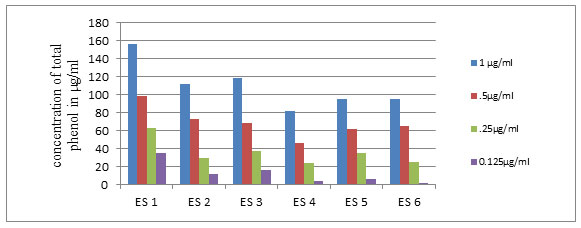
Graph 1-Total phenol content of the isolates at different concentrations of extract
Figure .1 &2: growth inhibition activity of endophytic bacterial isolate in co culture
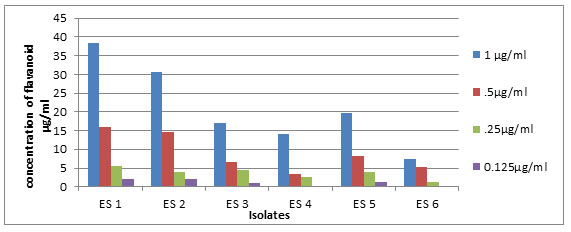
Graph 4-Total flavanoid content of the isolates at different concentrations of extract.
of the six isolates tested for antifungal activity, ES2 (Paenibacillus sp.) produced a zone of inhibition of 15mm which is greater than the standard drug(itraconazole,11mm) (Table 1,Fig.5) Sulistiyani et al., (2016) reported an endophytic Paenibacillus sp. with antifungal property from Curcuma longa rhizome. A particular peptide showing antifungal properties was isolated from Paenibacillus sp. (Alkotaini et al., 2014). Anandaraj et al., (2009) isolated two antibacterial peptides Paenibacillin P and Paenibacillin N from Paenibacillus sp.
Fig.3-Antibacterial activity of isolates against Proteus vulgaris
Antimicrobial activity of endophytic bacterial extract- Fig.1 &2-growth inhibition activity of endophytic bacterial isolate in co culture,Fig.3-Antibacterial activity of isolates against Proteus vulgaris,Fig.4-Antifungal activity of isolate ES2 against A.niger
Table 1. Antimicrobial activity of the isolates
| Inhibitory activity as per co-culture method | Zone of inhibition(in mm)in disc diffusion method | ||||||||||||
| Pathogenic strains | ES1 | ES2 | ES3 | ES4 | ES5 | ES6 | ES1 | ES2 | ES3 | ES4 | ES5 | ES6 | Std. |
| E.coli | – | – | – | – | + | – | 0 | 0 | 0 | 0 | 9 | 0 | 25 |
| S.typhi | + | + | + | + | + | + | 0 | 0 | 0 | 24 | 19 | 17 | 21 |
| K.pneumoniae | – | – | + | + | + | + | 9 | 8 | 0 | 9 | 9 | 9 | 22 |
| P.vulgaris | + | + | + | + | + | + | 8 | 9 | 8 | 20 | 15 | 12 | 20 |
| C.albicans | – | – | – | – | – | – | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| A.niger | – | – | – | – | – | 0 | 15 | 0 | 0 | 0 | 0 | 11 | |
Sophia et al., (2012) reported the antioxidant property of E.sonchifolia the medicinal plant used for the isolation of endophytes in the present study. In the present attempt the antioxidant avtivity of the endophytic bacterial extracts showed positive results indicating that the isolate as well as host plant synthesized some metabolites with common functions that might be due to the plant microbe interactions. Estimation of phenolic content revealed that ES1 produced maximum amount (156µg/ml), while ES4 produced the lowest (Graph 1). Total flavanoid content of the extracts showed that the isolate ES1 contained 37.3µg/ml quercetin equivalent of flavanoid and ES2 and ES5 have comparable flavanoid contents of 29.6µg/ml,18.6µg/ml of quercetin equivalent respectively (Graph 2). Bacillus sp. have been preferred as a probiotic bacteria in the feed industry because of its antioxidant property (Wang et al., 2017).
The most accurate method used for antioxidant analysis was found to be the DPPH assay. Total phenol and flavanoid estimation showed higher amount of these components in Bacillus subtilis strain. Free radical scavenging activity was measured in terms of IC50 value and lowest IC50 indicates a highest antioxidant activity. The highest antioxidant activity by DPPH assay was observed in ES1 (IC50 0.825µg/ml), followed by ES5 (IC50 1.19µg/ml), and ES6 (IC50 1.044µg/ml). Reports by Nongkhlaw and Joshi (2015) specified that L-Asparginase production was directly linked with antioxidant property and the Bacillus subtilis strain cenB associated with Centella asiatica showed positive results for L-Asparginase production and antioxidant property.
The results of each antioxidant assay may be different among the isolates based on the presence of various metabolites produced by the isolates and it cannot be comparable among the assays (Rafat et al., 2012). When ferric ion reducing power analysis was conducted, isolate ES1 (IC50 0.33µg/ml) showed highest reducing power. The extracts of isolates, ES5 (IC50 0.43µg/ml) and ES6 (IC50 0.68µg/ml) also showed higher antioxidant property in accordance with the principles of Ferric reducing power assay. The IC50 value of Nitric oxide radical scavenging assay showed that isolates ES6 (IC50 2.79µg/ml), ES3 (IC50 3.14µg/ml), ES4 (IC50 3.12 µg/ml) had significant antioxidant activity. Among the isolates ES5 showed highest cupric ion reducing property with lowest IC50 value (0.103µg/ml). The statistical analysis was carried out by one way ANOVA followed by Tukey test (Table 2).
Table 2. IC50 values in antioxidant assays
| DPPH Assay | Ferric ion reducing power | CUPRAC Assay | Nitric oxide radical Scavenging assay | |
| Std | 1.654 | 0.84 | 0.138 | 3.68 |
| ES1 | 0.825*** | 0.33*** | 0.66ns | 3.49ns |
| ES2 | 2.23*** | 1.55* | 0.147*** | 5.6 ns |
| ES3 | 2.17*** | 1.25** | 0.266** | 3.14 ns |
| ES4 | 2.9*** | 2.21ns | 0.254** | 3.12 ns |
| ES5 | 1.19*** | 0.43** | 0.103*** | 3.47 ns |
| ES6 | 1.044*** | 0.68** | 0.423ns | 2.79** |
***p < 0.001- highly significant, **p < 0.05-significant, nsp > 0.05-not significant
Antioxidant and antimicrobial studies revealed that the isolate ES1 has significant activity and hence GC-MS Analysis of the ES1 extract was carried out. It revealed nine detectable compounds (Table-3). The major compound detected in the GCMS analysis in accordance with area percentage is Hexadecanoic Acid, 15-Methyl-, Methyl Ester (Common name-Methyl isoheptadecanoate) and it was reported to have antioxidant, nematicide (Imran et al, 2007, Zayed et al., 2014), antifungal and antibacterial properties (Ali et al., 2017). It was for first time we report the presence of this compound from the extract of endophytic bacteria that may contribute in the bioactivity. We conclude that the endophytic bacteria isolated from E. Sonchifolia possess significant antimicrobial and antioxidant properties.
Table 3. GC-MS identification of compounds in extract of isolate ES1
Bioactivity studies revealed that Bacillus subtilis strain UCC BOT ES1 was the most promising among all isolates. LCMS/MS analysis was employed to screen the bioactive compounds produced by this isolate. Bacillus subtilis was already reported as one of the frequently identified bacterial endophytes known to be efficient in plant growth enhancement (Malfanova et al., 2011) and biocontrol activities (Luo et al., 2015, Kim et al., 2017). Liquid chromatography with mass spectral analysis of different Bacillus members revealed high potential for the production of natural bioactive compounds (Hazarika et al., 2019).
Table 4. LC-MS/MS Analysis – Compounds identified in extract of isolate ES1
| Lipopeptide | Molecular Mass | [M+H+] | [M+NH4] | [M+2K+H] | [M+Cl] | [M+CH3OH+H+] | [M+Na-H+] | [M-H+] |
| Surfactin | 992.51 | 993.51 | 1011.53 | 1069.56 | – | – | – | – |
| 1007.52 | 1025.53 | 1083.58 | 1041.5469 | – | – | – | ||
| 1022.52 | 1023.53 | 1041.54 | – | – | – | – | – | |
| 1035.56 | 1036.56 | 1053.56 | – | – | 1069.56 | – | – | |
| Iturin | 1082.57 | 1083.58 | 1101.57 | – | – | – | 1129.56 | – |
| Fengycin | 1414.41 | 1415.82 | 1432.03 | – | – | – | 1058.59 | – |
| 1416.77 | 1417.78 | 1461.77 | 1415.76 |
A cyclic lipopeptide (cLP) biosurfactant ‘surfactin’ was reported from Bacillus species (Haddad et al., 2008). Apparent surfactin peaks were noticed in the current study and were comparable with the earlier reports (Sarwar et al., 2018). Peaks corresponding to the molecular mass 992, 1007, 1022 and 1035 therefore indicated the surfactin biosynthetic potential of Bacillus subtilis strain UCC BOT ES1. Peaks observed at 1083.58 [M+H+] was noted to be that of another non-ribosomal peptide iturin as per the previous reports. Iturin category of compounds was generally considered as antifungal compounds (Arrebola et al., 2010). Fengycin is another category of lipopeptide with wide range of applications as antifungal compounds and they were efficient in preventing the growth of filamentous fungi (Jacques, 2011).
Presence of fengycin was reported from different members of endophytic Bacillus (Hanif et al., 2019).The molecular weight observed in the present study [M+H+] 1417.78 and 1415.82 are in accordance with fengycin A (Table 4). Non-ribosomally synthesised lipopetides showed greater degrees of structural similarity and the difference among them was confined to the fatty acid chain (Roongsawang et al., 2010). However efficient purification and screening methods were required to separate and analyse the different class of lipopetide bioactive compounds from the bacterial extract.
ACKNOWLEDGEMENT
The first author thanks University Grants Commission, New Delhi, for providing financial assistance by Faculty Development Programme.
Conflict of Interest: The authors has no conflict of interest
REFERENCES
Ahemad M Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective Journal of King Saud University Science Vol 26 Pages1-20.
Ali A Javaid A Shoaib A (2017) GCMS analysis and antifungal activity of methanolic root extract of Chenopodium album against Sclerotium rolfsii Planta Daninha Vol35 No e017164713.
Alkotaini B Anuar N Kadhum AAH Sani AAA (2014) Isolation and identification of a new intracellular antimicrobial peptide produced by Paenibacillus alvei AN5. World Journal of Microbiology and Biotechnology Vol 30 Pages 1377-1385.
Anandaraj B Vellaichamy A Kachman M Selvamanikandan A Pegu S Murugan V (2009) Co-production of two new peptide antibiotics by a bacterial isolate Paenibacillus alvei NP75 Biochemical and Biophysical Research Communication Vol 379 No2 Pages 179-85.
Apak R Güçlü K Ozyürek M Celik S E (2008) Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay Microchimica Acta Vol160 Pages 413-419.
Arrebola E Jacobs R Korsten L (2010) Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogen Journal of Applied Microbiology Vol108 Pages 386-395
Bintang M Purwanto UMS Kusumawati DE Yang JJ (2015) Study of endophytic bacteria as a novel source of antioxidant agent based on GC-MS analysis International Journal of chemical, environmental and biological science Vol 3No5 Pages 368-369.
Boora F Chirisa E Mukanganyama S (2014) Evaluation of Nitrite Radical Scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. Journal of Food Processing Vol 2014 Pages 1-8
Essien GE Nwidu LL Nwafor PA (2009) Anti-Inflammatory and Analgesic Potential of Methanolic Extract of Emilia sonchifolia (Compositae) Leaves in Rodents African Journal of Biomedical Research Vol12 No3 Pages199-207
Gond SK Bergen MS Torres MS White JF Jr (2015) Endophytic Bacillus sp. produce antifungal lipopeptide and induce host defence gene expression in Maize Microbiological research Vol172 Pages 79-87
Haddad N Liu X Yang S Mu B (2008) Surfactin isoforms from Bacillus subtilis HSO121: separation and characterization Protein and Peptide Letters Vol15 No 3 Pages 265-269
Hanif A Zhang F Li P Li C Xu Y Zubair M Zhang M Jia D Zhao X Liang J Majid T Yan J Farzand A Wu H Gu Q Gao X (2019) Fengycin produced by Bacillus amyloliquefaciens FZB42 Inhibits Fusarium graminearum growth and mycotoxins biosynthesis Toxins Vol11No295 Pages1-11
Hazarika DJ Goswami G Gautom T Parveen A Das P Barooah M Boro RC (2019) Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens BMC Microbiology Vol 19 No71 Pages 1-13
Imran M Ullah F Ayaz M Sadiq A Shah M R Jan M S Ullah F (2017) Anticholinesterace and antioxidant potentials of Nonea micrantha Bioss &Reut along with GCMS analysis BMC Complementary and Alternative Medicine Vol17 No499 Pages 1-12
Jayanthi P Lalitha P (2011) Reducing power of the solvent extracts of Eichhornia crassipes (mart.) Solms. International Journal of Pharmacy and Pharmaceutical Sciences Vol 3 Pages 126-128
Kamtekar S Keer V Patil V (2014) Estimation of Phenolic content, Flavonoid content, Antioxidant and Alpha amylase Inhibitory Activity of Marketed Polyherbal Formulation Journal of Applied Pharmaceutical Science Vol 4 No 09 Pages 61-65
Kim YT Park BK Kim SE Lee WJ Moon JS Cho MS Park H-Y Hwang I Kim SU (2017) Organization and characterization of genetic regions in Bacillus subtilis subsp. krictiensis ATCC55079 associated with the biosynthesis of iturin and surfactin compounds PLoS ONE Vol12 No e0188179
Kumar DG Syafiq AM Ruhaiyem Y (2015) Traditional uses, phytochemical and pharmacological aspects of Emilia sonchifolia (L.)DC. International Journal of Research in Ayurveda and Pharmacy Vol 6 No 4 Pages 551-556
Latha YL Darah I Sasidharan S Jain K (2009) Antimicrobial activity of Emilia sonchifolia DC., Tridax procumbence L., and Vernonia cineria L., of Asteraceae family, potential as food preservatives Malasian Journal of Nutrition Vol15 No2 Pages 223-231
Luo C Zhou H Zou J Wang X Zhang R Xiang Y Chen Z (2015) Bacillomycin L and surfactin contribute synergistically to the phenotypic features of Bacillus subtilis 916 and the biocontrol of rice sheath blight induced by Rhizoctonia solani. Applied Microbiology and Biotechnology Vol 99 Pages1897-1910
Malfanova N Kamilova F Validov S Shcherbakov A Chebotar V Tikhonovich I Lugtenberg B (2011) Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed Microbial Biotechnology Vol 4 No4 Pages 523-32
Mohamad OAA Li L Ma JB Hatab S Xu L Guo JW Rasulov BA Liu YH Hedlund BP Li WJ (2018) Evaluation of the Antimicrobial Activity of Endophytic Bacterial Populations from Chinese Traditional Medicinal Plant Licorice and Characterization of the Bioactive Secondary Metabolites Produced by Bacillus atrophaeus against Verticillium dahliae. Frontiers in Microbiology Vol 9 No 924 Pages1-14
Nongkhlaw F M W Joshi SR (2015) L-Asparginase and antioxidant activity of endophytic bacteria associated with ethnomedicinal plants Indian Journal of Biotechnology Vol14 Pages 59-64
Pezzuto J (1996) Taxol production in plant cell culture comes of age Nature Biotechnology Vol14 no9 Page1083
Rafat A Philip K Muniandy S (2012) A novel source of bioactive compounds: Endophytic bacteria isolated from Centella asiatica. Journal of Pure and Applied Microbiology Vol6 No1Pages1-10
Raj A G R Shailaja U Prasanna RN Ajayan S (2013) The therapeutic potential of ten sacred plants (Dashapushpa) of Kerala State of Southern India Journal of Ayurveda and Holistic Medicine Vol1No3 Pages1-16
Roongsawang N Washio K Morikawa M (2010) Diversity of non-ribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants International Journal of Molecular Sciences Vol12 No1Pages141-172.
Sarwar A Brader G Corretto E Aleti G Abaidullah M Sessitsch A Hafeez FY (2018) Qualitative analysis of biosurfactants from Bacillus species exhibiting antifungal activity PLoS ONE Vol13 No 6 Pages1-15
Shukla S Naik G Mishra SK (2015) Potential antimicrobial activity of bacterial endophytes isolated from Flacourtia jangomas (Lour.) Raeusch, a less explored medicinal plant Journal of Microbiology Biotechnology Food Science Vol4 No6 Pages 473-477
Sophia D Ragavendran P Arulraj C Gopalakrishnan VK (2011) Invitro antioxidant activity and HPTLC determination of n-hexane extract of Emilia sonchifolia (L.)DC. Vol 2 No 4 Pages179-183
Sulistiyani Ardyati T Winarsih S (2016) Antimicobial and antioxidant activity of endophyte bacteria associated with Curcuma longa Rhizome Journal of Experimental Science Vol6 No1Pages 45-51
Sunkar S Nachiyar CV (2012) Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus Asian Pacific Journal of Tropical Biomedicine Vol2 No12 Pages 953-959
Swarnalatha Y Saha B Choudary L Y (2015) Bioactive compound analysis and antioxidant activity of endophytic bacterial extract from Adhathoda beddomei . Asian Journal of Pharmaceutical and Clinical Research Vol 8 No1 Pages 70-72.
Wang Y Wu Y Wang Y Xu H Mei X Yu D Wang Y Li W (2017) Antioxidant properties of probiotic bacteria Nutrients Vol9 No521Pages1-15
Zayed MZ Ahmad FB Ho WS Pang SH (2014) GCMS analysis of phytochemical constituents in leaf extracts of Neolamarckia cadamba (Rubiaceae) from Malaysia. International Journal of Pharmacy and pharmaceutical Science Vol6 No9 Pages123-127
Zheng LP Zou T Ma YJ Wang JW Zhang YQ (2016) Antioxidant and DNA Damage protecting activity of exopolysaccharides from the endophytic bacterium Bacillus Cereus SZ1 Molecules Vol 21 No174 Pages 1-15