Department of Biological Sciences, College of Science, University
of Jeddah, Jeddah, Saudi Arabia
Corresponding author email: waalsheri@uj.edu.sa
Article Publishing History
Received: 20/10/2021
Accepted After Revision: 28/12/2021
Endophytes are fungi that colonize the internal tissues of plants without causing immediate adverse effects. Saudi Arabia (SA) is rich in Opuntia ficus-indica cacti and the cactus-like plant Aloe vera, which grow in the southern and western areas of SA. This study aimed to isolate and identify endophytic fungi from cacti and cactus-like plants in the Jeddah, Taif, and Al Baha regions KSA and then determine their effects on pathogenic fungal and bacterial growth. The isolates were groupedAloe Vera; Opuntia Ficus-Indica; Endophytic Fungi; Antimicrobial Activities; Pathogenic Bacteria. into 16 distinct operational taxonomic units based on the sequence of the internal transcribed spacer in the rDNA gene with the primers ITS1 and ITS4. Mucor circinelloides was the endophytic fungus found most frequently, with a relative frequency of 20.43%, followed by Talaromyces funiculosus, with a relative frequency of 16.12% when isolated from Opuntia ficus-indica and Aloe vera. Nine out of sixteen endophytic fungi exhibited strong antifungal activity against all the tested pathogens. P. funiculosum, Aspergillus versicolor, Penicillium janthinellum, and Fusarium oxysporum showed vigorous antimicrobial activities against the human pathogenic bacteria Escherichia coli, Shigella sp., and Salmonella typhimurium.
Aloe Vera; Opuntia Ficus-Indica; Endophytic Fungi; Antimicrobial Activities; Pathogenic Bacteria.
Alghamdi A. S, Alshehri W. A, Ashy R. A, Gashgari R, Albejad A. I. Antimicrobial Activities and Molecular Signature of Endophytic Fungi of Opuntia ficus-Indica Cacti and the Cactus-Like Plant Aloe vera. Biosc.Biotech.Res.Comm. 2021;14(4).
Alghamdi A. S, Alshehri W. A, Ashy R. A, Gashgari R, Albejad A. I. Antimicrobial Activities and Molecular Signature of Endophytic Fungi of Opuntia ficus-Indica Cacti and the Cactus-Like Plant Aloe vera. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3n3P3ik“>https://bit.ly/3n3P3ik</a>
Copyright © Alghamdi et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Endophytes are fungi that colonize the internal tissues of plants without causing immediate adverse effects (Khiralla et al. 2017). They are considered a promising source of new natural drug leads with great potential for medicinal and agricultural applications. For instance, many of the products currently used for human or animal therapy are produced by microbial products or derived from them. Furthermore, with the increasing incidence of drug resistance in human, animal, and plant pathogenic bacteria, which are among the major causes of death worldwide, endophytic fungi are considered important biotechnological tools because of the many secondary metabolites that they produce (Bara et al., 2013).
Research on endophytic fungi has demonstrated that they constitute a promising source of biocontrol agents. Fungal endophytes enhance the resistance of their hosts against abiotic stress, disease, insects, and mammalian herbivores by producing a broad range of biologically active fungal metabolites. Indeed, several of the interesting metabolites isolated from endophytic fungi belong to diverse chemical classes, including alkaloids, steroids, flavonoids, terpenoids, quinones, and phenols (Khiralla et al. 2017).
According to Suryanarayanan et al. (2005), various studies have shown that some endophytic fungi are neither artificially residents nor normally latent pathogens of plant hosts. They may protect the plant from insect pests, fungal pathogens, or increase host fitness in harsh environments in addition to possibly playing a role in litter degradation. However, very few plants growing in extreme or harsh habitats have been screened for fungal endophytes. Cacti are a good source of endophytic fungi (Wani and Lone, 2016). Medicinal plants have provided a rich source of novel antimicrobial agents throughout human history, with many infectious diseases traditionally being treated using herbal medicines. A wide range of medicinal plant parts are used to extract raw drugs that possess different medicinal properties, (Suryawanshi et al., 2016). Cactus-like plants are an important food source for wild animals; they are also used in the medicine, food, chemical, spinning, and cosmetic industries; furthermore, they are a cheap source of readily available raw materials (Suryanarayanan et al., 2005; Mauseth, 2021).
The antimicrobial agents of A. vera gel have been reported to effectively eliminate or greatly reduce the growth of a range of wild pathogens (Lawrence et al., 2009; Bashir et al., 2011; Stanley et al., 2014 and Gharibi et al., 2016). A. vera and other cacti plant species have other uses, such as for bactericidal, antibiotic, fungicidal, anti-inflammatory purposes as well as moisturizing tissues and relieving pain associated with joints and muscles (Roşca-Casian et al., 2007, Surjushe et al., 2008, Lawrence et al., 2009, Silva-Hughes et al., 2015 and Ríos and Recio et al., 2005). Several studies have reported the isolation of 44 endophytic fungi species colonizing O. ficus-indica cacti plants in Brazil (Bezerra et al., 2013, 2017). In India, more endophytes were isolated from A. vera and other cacti such as O. ficus-indica (Yadav et al. 2015, Gangurade et al. 2019, and Vyawahare et al. 2019).In the USA, approximately 108 endophytic fungal isolates corresponding to 17 different taxa were obtained and identified as the species most frequently associated with O. humifuse cacti through use of molecular methods (Silva-Hughes et al. 2015). In Arizona, 21 cactus species occurring in various localities were screened for the presence of fungal endophytes (Suryanarayanan et al. 2005). The southern and western areas of Saudi Arabia are rich in A. vera. Endophytic fungi species were previously isolated from A. vera collected from the Asir Desert (Ameen et al., 2021).
This study aimed to isolate and identify and characterize the endophytic fungi from cactus-like plants in Jeddah, KSA, toward defining the endophytic mycobiota of cacti in addition to evaluating the antimicrobial activities of the isolated endophytic fungi and plant extracts on pathogenic microbes. We hypothesized that the endophytic fungi isolated from cactus-like plants have a wide range of therapeutic applications against several diseases.
Material and Methods Collection of Plant Samples:The cacti and cactus-like plants used in this study were fresh, naturally grown stems, leaves, and roots of Aloe vera and Opuntia ficus-indica harvested from the Jeddah, Taif, and Al Baha regions in SA in September 2019 and January 2020. The selected plants, which belonged to different families, are listed in Table 1.
Table 1. List of Plants Utilized in This Study.
| NO | Scientific Name | Family | Common Name | Part of Plant Used | Collection Site |
| 1
|
Aloe vera | Asphodalaceae | Aloe vera | Roots and Leaves | Jeddah |
| 2
|
Opuntia ficus-indica | Cactaceae | Opuntia
Teen Shouki Barshoumi |
Roots and Stems | Albaha and Taif |
Isolation of Fungal Endophytes:Following Schulz et al., (1993), with modifications, samples of Aloe vera and Opuntia ficus-indica plants were selected and washed with running tap water to remove soil particles. The samples were cut into small 1 cm pieces and immersed in 70% ethanol for 2 minutes and 5% sodium hypochlorite solution for 5 minutes for surface sterilization. The samples were washed with distilled water several times and then transferred to a dry sterilized surface.
The sterilized segments were placed in Petri dishes containing potato dextrose agar (PDA) medium (HiMedia, Mumbai, India); these were sealed with parafilm and incubated at 28 ± 1°C for two weeks. Fungi growing out of the plant segments were isolated using the method described in Domsch et al., (1980) and identified based on morphological characteristics with reference to fungi identification manuals (kirk et al., 2011).
DNA Extraction, Amplification, and Sequencing:A 2 μL aliquot of potato dextrose broth (PDB) (HiMedia, Mumbai, India) was poured into PDA tubes and vortexed to disperse the spores. The spore–PDB mixtures were then added into flasks containing 100 mL of PDB. The flasks were kept undisturbed at room temperature for two to three days. The mycelium was harvested by filtration, frozen at −80 °C for 30 minutes, lyophilized, and stored at −80 °C. The mycelium was ground in liquid nitrogen with a sterile mortar to obtain mycelium powder. DNA was extracted from 20 mg of mycelium powder using a DNeasy Plant Mini Kit. The DNA quantity and quality were checked by electrophoresis on 0.8% agarose gel and visualized with ethidium bromide under UV transillumination.
The internal transcribed spacer (ITS) region of ribosomal DNA was amplified by PCR with the ITS1-F (5’-CTTGGTCATT TAGAGGAAGTAA-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) primers (White et al., 1990; Gardes and Bruns 1993). PCR amplifications were carried out in a final volume of 50 µL, containing 2 µL of DNA, 0.5 mM of each primer, 150 mM of dNTP, 1 U of Taq DNA polymerase (Promega), and PCR reaction buffer. Amplification was carried out in a thermal cycler with an initial denaturation of 3 mins at 94 °C, followed by 35 cycles of 1 min at 94 °C, 1 min at 50 °C, 1 min at 72 °C, and a final extension of 10 min at 72 °C.
The amplified products were checked by electrophoresis on 1% agarose gel and visualized with ethidium bromide under UV transillumination based on the manufacturer’s instructions. The PCR products were purified using an Exo SAPIT kit (USB Corporation, Amersham Place, UK, under license from GE Healthcare). The purified products were sequenced in an automated DNA sequencer (ABI PRISM 3700) using the Big Dye Deoxy Terminator cycle-sequencing kit (Applied Biosystems, Darmstadt, Germany). Sequences were submitted to GenBank, NCBI (http://www.ncbi.nl m.nih.gov). Sequences obtained in this study were compared with the previously deposited sequences in the GenBank database, using BLAST, on the NCBI website (http://www.ncbi.nlm.nih.gov/BLAST/).
ITS Sequence and Phylogenetic Analysis:DNA sequences were initially aligned with Clustal Omega (Sievers et al. 2014). TREECON (Van de Peer and Wachter, 1994) for Windows (version 1.3b, 1998) was used to construct a neighbor-joining tree using the Jukes–Cantor model (Jukes and Cantor, 1969).
Antimicrobial Activity of Endophytic Fungi:Three pathogenic fungi, Fusarium oxysporum, Aspergillus terreus, and Penicillium funiculosum, and three human pathogenic bacteria, Escherichia coli, Salmonella typhimurium, and Shigella sp., obtained from King Fahad Researcher Centre in Jeddah, were used as target fungal and bacterial pathogens in this study. Following Balouiri et al. (2016), the cross-streak method was used to detect the antagonistic activity of fungi strains against endophytic fungal strains. The widths of the inhibition zones between the pathogen and the endophytes were grouped as follows: strong inhibition (+++), moderate inhibition (++), weak inhibition (+), and no activity determined (−) (Paul et al., 2007).
Statistical Analysis:The colonization frequency (%CF) and the percentage of the dominant endophytic fungi were calculated (Gherbawy et al., 2014):
![]()
RESULTS AND DISCUSSION
Isolation of Fungal Endophytes:A total of 92 pure isolates of endophytic fungi were obtained from 132 cacti and cactus-like plant samples (leaves, stems, and roots) and were screened for the presence of endophytic fungi. Samples of 16 species and 8 genera were obtained from the leaves, stems, and roots segments of A. vera and O. ficus-indica. O. ficus-indica was found to have a higher endophytic diversity (relative frequency 54.83%) than A. vera (45.16%). The isolates were identified as follows: 4 species of Aspergillus from 14 isolates, 2 species of Curvularia from 2 isolates, 1 species of Epicoccum from 1 isolate, 3 species of Fusarium from 18 isolates, 3 species Penicillium from 15 isolates, 1 species of Talaromyces from 15 isolates, 1 species of Rhizopus from 8 isolates, and 1 species of Mucor from 19 isolates. The most commonly isolated species were Mucor circinelloides, with an overall colonization frequency of 20.43%, and Talaromyces funiculosus, with an overall colonization frequency of 16.12% (Table 2).
Table 2. Colonization Frequency of Endophytic Fungi Isolated from Leaves, Stems and Roots on PDA Medium at 28 ± 1°.
| No | Fungal Endophyte | Isolate Number | CFᵃ | Dominant Fungiᵃ |
| 1 | Aspergillus chevalieri | 2 | 2.15 | 1.51 |
| 2 | Aspergillus niger | 1 | 1.07 | 0.75 |
| 3 | Aspergillus terreus | 4 | 4.3 | 3.03 |
| 4 | Aspergillus versicolor | 7 | 7.52 | 5.30 |
| 5 | Curvularia khuzestanica | 1 | 1.07 | 0.75 |
| 6 | Curvularia sp. MR-2019o strain LC12021 | 1 | 1.07 | 0.75 |
| 7 | Epicoccum sorghinum | 1 | 1.07 | 0.75 |
| 8 | Fusarium falciforme | 8 | 8.6 | 6.06 |
| 9 | Fusarium oxysporum | 4 | 4.3 | 3.03 |
| 10 | Fusarium redolens | 6 | 6.45 | 4.54 |
| 11 | Mucor circinelloides | 19 | 20.43 | 14.39 |
| 12 | Penicillium funiculosum | 3 | 3.22 | 2.27 |
| 13 | Penicillium janthinellum | 9 | 9.67 | 6.81 |
| 14 | Penicillium minioluteum | 3 | 3.22 | 2.27 |
| 15 | Rhizopus oryzae | 8 | 8.6 | 6.06 |
| 16 | Talaromyces funiculosus | 15 | 16.12 | 11.36 |
| Total | 93 | NA | NA |
The sequence results were corroborated by the morphological identification of the isolated fungal endophytes. Most of the isolates were of the Ascomycota (87%) and Mucoromycota (13%) phyla.
Table 3. Identified endophytes related to the species and the identity percentage found in the CBS.
| NO | Isolate Code | Accession Number | The Closet Genebank Taxa | Similarity % |
| 1 | Fung1_ITS1 | MT510010.1 | Penicillium janthinellum | 99.39 |
| 2 | Fung2_ITS1 | MT579855.1 | Fusarium oxysporum | 100 |
| 3 | Fung4_ITS1 | MT279285.1 | Mucor circinelloides | 99.46 |
| 4 | Fung5_ITS1 | MK762588.1 | Epicoccum sorghinum | 100 |
| 5 | Fung6_ITS1 | MT563399.1 | Fusarium redolens | 100 |
| 6 | Fung7_ITS1 | MG437415.1 | Rhizopus oryzae | 95.27 |
| 7 | Fung9_ITS1 | MH688044.1 | Curvularia khuzestanica | 99.27 |
| 8 | Fung10_ITS1 | MT487830.1 | Aspergillus chevalieri | 99.79 |
| 9 | Fung12_ITS1 | KX262973.1 | Talaromyces funiculosus | 99.80 |
| 10 | Fung13_ITS1 | MT558939.1 | Aspergillus terreus | 100 |
| 11 | Fung14_ITS1 | MN215703.1 | Curvularia sp. MR-2019o strain LC12021 | 100 |
| 12 | Fung15_ITS1 | JX500735.1 | Penicillium funiculosum | 99.80 |
| 13 | Fung16_ITS1 | MN555417.1 | Fusarium falciforme | 99.60 |
| 14 | Fung17_ITS1 | JN620402.1 | Penicillium minioluteum | 99.62 |
| 15 | Fung19_ITS1 | MT497452.1 | Aspergillus versicolor | 98.88 |
| 16 | Fung20_ITS1 | MT628904.1 | Aspergillus niger | 99.24 |
Antimicrobial Activity of Endophytic Fungi:Most endophytic fungi exhibit significant inhibition against a wide range of pathogenic plant fungi and pathogenic human bacteria. The P. janthinellum (Fung1_ITS1), F. redolens (Fung6_ITS1), T. funiculosus (Fung12_ITS1), and Curvularia sp. MR-2019o strain LC12021 (Fung14_ITS1) isolates showed strong inhibition toward pathogenic plant fungi. Ten isolates in this work exhibited promising growth-inhibitory activity against at least one of the pathogenic test microbes. Seven endophytic fungi exhibited antimicrobial activity against all three pathogenic bacteria, and four endophytic fungi exhibited antimicrobial activity against all three pathogenic fungi.
The number of fungal isolates displaying antimicrobial activity against F. oxysporum, A. terreus, and P. funiculosum were 1, 5, 9, and 11, respectively. The P. janthinellum (Fung1_ITS1), F. oxysporum (Fung2_ITS1), F. redolens (Fung6_ITS1), C. khuzestanica (Fung9_ITS1), A. chevalieri (Fung10_ITS1), F. falciforme (Fung16_ITS1), and A. versicolor (Fung19_ITS1) isolates displayed the highest level of inhibition against the pathogenic human bacteria Shigella, E. coli, and S. typhimurium. The P. janthinellum (Fung1_ITS1) and F. redolens (Fung6_ITS1) isolates displayed good activity against all pathogenic microbes. However, isolate numbers 1, 7, 8, and 15 displayed strong activity against all pathogenic microbes (Table 4).
Table 4. Antimicrobial Spectra of Endophytic Fungi.
| No | Isolate Code | Fungal Endophyte | Pathogenic Fungi | Pathogenic Bacteria | ||||
| F.
oxysporum |
A.
terreus |
P.
funiculosum |
Shiglla | E.coli | S. tyhpimurium | |||
| 1 | Fung1_ITS1 | Penicillium janthinellum | ++ | + | +++ | ++ | ++ | ++ |
| 2 | Fung2_ITS1 | Fusarium oxysporum | – | + | +++ | ++ | ++ | ++ |
| 3 | Fung4_ITS1 | Mucor circinelloides | + | – | ++ | – | – | – |
| 4 | Fung5_ITS1 | Epicoccum sorghinum | – | – | – | – | – | – |
| 5 | Fung6_ITS1 | Fusarium redolens | + | + | +++ | + | ++ | ++ |
| 6 | Fung7_ITS1 | Rhizopus oryzae | ++ | – | + | – | + | + |
| 7 | Fung9_ITS1 | Curvularia khuzestanica | ++ | – | ++ | + | ++ | ++++ |
| 8 | Fung10_ITS1 | Aspergillus chevalieri | ++ | – | ++++ | +++ | + | + |
| 9 | Fung12_ITS1 | Talaromyces funiculosus | ++ | + | ++++ | – | +++ | – |
| 10 | Fung13_ITS1 | Aspergillus terreus | ++ | – | – | – | – | – |
| 11 | Fung14_ITS1 | Curvularia sp. MR-2019o strain LC12021 | + | ++ | + | – | + | + |
| 12 | Fung15_ITS1 | Penicillium funiculosum | + | ++ | – | – | – | ++ |
| 13 | Fung16_ITS1 | Fusarium falciforme | + | – | + | + | ++ | + |
| 14 | Fung17_ITS1 | Penicillium minioluteum | + | – | +++ | – | – | – |
| 15 | Fung19_ITS1 | Aspergillus versicolor | + | – | +++ | +++ | ++ | +++ |
| 16 | Fung20_ITS1 | Aspergillus niger | + | – | – | – | – | – |
The present study aimed to isolate and identify endophytic fungi in O. ficus-indica and A. vera plants collected from Saudi Arabia, classified at the species level. A total of 93 isolates, representing 16 species and 8 genera, were recovered from plant leaves, stems, and root segments. Most of the fungal genera obtained as endophytes of O. ficus-indica and A. vera were described as endophytes and the eight genera were Aspergillus, Curvularia, Fusarium, Epicoccum, Penicillium, Rhizopus, Mucor, and Talaromyces. M. circinelloides was the species most frequently isolated, with a colonization frequency of 20.43%, followed by T. funiculosus, with a colonization frequency of 16.12%.
The species isolated with the lowest frequency were Curvularia sp., A. niger, and E. sorghinum. In a study conducted by Gangurde et al. 2019 in Sri Lanka, the highest colonization frequency of endophytic Penicillium sp. in A. vera found in India was 60%, followed by Aspergillus sp. at 50%, Nigrospora sp. at 33%, Fusarium sp. at 20%, and Alternaria alternata at 8%. The findings of Ratnaweera et al., 2015 support A. niger as the species showing the highest colonization in the cladodes of O. dillenii. Bezerra et al., 2013, isolated forty-seven species of endophytic fungi from O. ficus-indica from Brazil, and the most commonly isolated species was F. oxysporum. Among all of the endophytic fungi that have been isolated and identified from cacti and cactus-like plants, our study is the first report of these species isolated specifically from O. ficus-indica and A. vera in SA. An explanation for the overall low rate for frequency of colonization noted in this study could be the harsh environmental conditions and dryness in the areas in which the cacti grow.
The molecular analysis of fungal rDNA demonstrated that most of the fungal isolates described in this study belong to Ascomycota (87%) and Mucoromycota (13%). The ITS sequences of the isolated species were compared with the sequences previously deposited in GenBank. In this study, compared with the sequences on GenBank, more fungal isolates were found that belonged to the Ascomycota taxon, which confirms Vyawahare’s findings of 93% of fungal isolates being represented by four endophytic fungal groups, namely Deuteromycetes, Ascomycetes, Zygomycetes, and Basidiomycetes, each with different isolation frequencies (Vyawahare et al., 2019).
Moreover, similar results were also obtained by Mane et al., (2018), who observed Deuteromycetes (55–72%) with high isolation frequencies and Ascomycetes (10–35%) with low isolation frequencies in A. vera and other medicinal plants. According to Silva-Hughes et al., 2015, in the USA, Tremellomycetes and Basidiomycota represent the first reported endophytes associated with Cactaceae. Basidiomycota are rarely isolated as endophytes and are associated with only eight species of cacti (Chlebicki, 2009). Fisher et al., 1994, studied 600 fragments of cacti from Australia and isolated 617 endophytic fungi across 23 taxa within Ascomycota. Suryanarayanan et al., 2005, used 1050 fragments of cacti from Arizona (USA) to isolate 900 endophytes belonging to 22 fungal species (Ascomycota), and Bezerra et al. (2012) used 45 fragments of forage cacti from Brazil to obtain 44 isolates of endophytic fungi belonging to 13 species (Ascomycota).
Some of the fungi in this study (F. oxysporum, A. terreus, and P. funiculosum) are well-known plant pathogens. An endophyte in one plant may act as a pathogen in another plant depending on the balance between the pathogenicity and entophytes of the microorganism in different hosts. The fungi isolated in this study have previously been isolated as endophytes from a different host, such as O. ficus-indica and A. vera. The endophytic fungi were tested for antifungal and antibacterial activity, and the results show that the endophytic fungi have stronger inhibition against plant pathogenic fungi and human pathogenic bacteria. This could be due to the natural existence of the endophytic and plant pathogenic fungi in the same habitat. Ramirez-moreno et al. (2017) tested the antimicrobial activity of the O. ficus-indica seed oil against C. albicans, E. coli, S. aureus, L. monocytogenes, P. aeruginosa, S. cerevisiae, and S. typhimurium. Their results show that the oil extract had high antimicrobial activity against Gram-positive and Gram-negative bacteria. In our study, the endophytic fungi isolated from A. vera constitute the first endophytic fungi apart from A. niger, A. terreus, and F. oxysporum to be identified and tested for antimicrobial activity. O. ficus-indica is a medicinal plant that has been used traditionally for controlling many different pathogenic bacterial infections (Jean et al., 2006). The discovery of novel antimicrobial metabolites in medicinal plants such as O. ficus-indica and A. vera provides an important alternative to conventional drugs and may help to overcome the increasing levels of drug resistance by human pathogens.
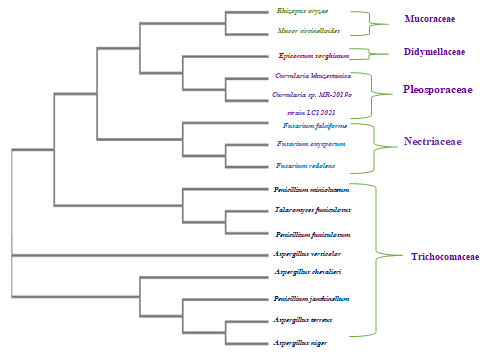
Figure 1: Phylogenetic tree based on neighbor-joining analysis of the rDNA ITS sequences of the endophytic fungal isolates obtained from various tissues of two cacti plants.
CONCLUSION
The assessment of molecular genetics using 18S rDNA gene sequencing is a fast and reliable technique for characterizing fungal taxonomy. The obtained data reveal that Saudi Arabian cacti and cactus-like plants possess an enormous diversity of endophytic fungi. A phylogenetic tree was constructed for each isolated endophytic fungus, and the results confirm that the obtained fingerprints indicate differences within the endophytic fungal community in cacti and cactus-like plants, alongside verifying that the reproducibility of 18S rDNA PCR amplification and its usefulness in such analyses.
Conflict of Interest: None to declare
Authors’ Contribution: All authors have made substantial, direct, and intellectual contribution to the work and approve it for publication
Funding:None
Data availability: All datasets generated or analyzed during this study are included in this manuscript
Ethical approval: This article does not contain any studies with human participants, or animals performed by any of the authors.
REFERENCES
Ameen, F.; Stephenson, S. L.; AlNadhari, S.; and Yassin, M. A. (2021): Isolation, identification and bioactivity analysis of an endophytic fungus isolated from Aloe vera collected from Asir desert, Saudi Arabia. Bioprocess and Biosystems Engineering. DOI: 10.1007/s00449-020-02507-1.
Balouiri, M.; Sadiki, M.; and Koraichi, S. (2016): Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis. (6)2, Pages 71-79.
Bara, R.; Aly, A.; Pretsch, A.; Wray, V.; Wang, B.; Proksch, P.; and Debbab, A. (2013): Antibiotically active metabolites from Talaromyces wortmannii, an endophyte of Aloe vera. Antibiotics J. 66, 491-493.
Bashir, A.; Mujahid, T. Y.; Saeed, B.; and Jehan, N. (2011): Comparative study of antimicrobial activities of Aloe vera extracts and antibiotics against isolates from skin infections. Africa Journal of Biotechnology. 10(19):3835-3840.
Bezerra, J. D. P.; Santos, M. G. S.; Barbosa, R. N.; Svedese, V. M.; Lema, D. M. M.; Fernandes, M. J. S.; Gomes, B. S.; Paiva, L. M.; Almeida-Cortez, J. S.; and Souza-Motta, C. M. (2013): Fungal endophytes from cactus Cereus jamacaru in Brazilian tropical dry forest: a first study. Symbiosis. 60, 53–63. https://doi.org/10.1007/s13199-013-0243-1.
Bezerra, J. D. P.; Oliveira, R. J. V.; Paiva, L. M.; Silva, G. A.; Groenewald, J. Z.; Crous, P. W.; and Souza-Motta, C. M. (2017): Bezerromycetales and Wiesneriomycetales ord. nov. (class Dothideomycetes), with two novel genera to accommodate endophytic fungi from Brazilian cactus. Mycol Progress 16, 297–309. doi.org/10.1007/s11557-016-1254-0.
Domsch, K.H.; Gams, W.; and Anderson, T. (1980): Compendium of soil fungi. Soc General Microbiol. 1. pp.viii + 860pp.
Gardes, M.; and Bruns, T. D. (1993): ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. National Centre of Biotechnology Information. 2(2):113-8. doi: 10.1111/j.1365-294x.1993.tb00005.x.
Gangurde, A. B.; Jagtap, P. R.; Vyawahare, M. A.; Kukreja, G. P.; and Mane, R. S. (2019): Production, purification and evaluation of different functional groups from endophytic Penicillium species derived bioactive compounds isolated from Aloe vera. International Journal of Chemistry Studies. 3(2):35-38. ISSN: 2581-348X.
Gharibi, D.; Hosseini, Z.; Khosravi, M.; and Boroun, F. (2016): Antibacterial Effects of Aloe Vera Extracts on some Human and Animal Bacterial Pathogens. Journal of Medical Microbiology and Infectious Diseases.3 (1-2): 6-10.
Gherbawy, Y.A.; and Gashgari, R. M. (2014): Molecular characterization of fungal endophytes from Calotropis procera plants in Taif region (Saudi Arabia) and their antifungal activities. Plant Biosystems. 148(6):1085–92.
Jean MF, Patricia K, Daming Z, Florian CS, Changping Z (2006). Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits, Frontiers Biosci. 11: 2574- 2589.
Jukes, T. H., and Cantor, C. R. (1969). Evolution of protein molecules. Mammalian protein metabolism, 3, 21-132.
Khiralla, A.; Spina,R.; Yagi, S.; Mohamed. I.; and Laurain-Mattar,D.(2017): Endophytic Fungi: Occurrence, Classification, Function and Natural Products. Nova Science Publishers. ISBN: 978-1-53610-341-0. 1-38.
Kirk, P. Cannon, P, Minter, Stalpers,J. (2011) Dictionary of fungi. Cabi Bioscience . UK
Lawrence, R.; Tripathi, P.; and Jeyakumar, E. (2009): Isolation, Purification and Evaluation of Antibacterial Agents from Aloe vera. Brazilian Journal of Microbiology.40(4).http://dx.doi.org/10.1590/S1517-83822009000400023.
Mane, R. S. and Vedamurthy, A.B. (2018): The fungal endophytes: sources and future prospects. Journal of Medicinal Plants Studies. 6(2) :121-126 .
Mauseth, J.D. (2021): Botany: An Introduction to Plant Biology, seventh edition. World Headquarteres. Burlington, Massachusetts: Jones & Bartlett.
Paul, N.C.; Kim, W.; Woo, S.; Park, M.; and Yu, S. (2007): Fungal Endophytes in Roots of Aralia Species and Their Antifungal Activity. Plant Pathol Journal 23 (4):287–94. doi:10.5423/ppj.2007.23.4.287.
Ramírez-Moreno, E., Cariño-Cortés, R., Cruz-Cansino, N. d. S., Delgado-Olivares, L., Ariza-Ortega, J. A., Montañez-Izquierdo, V. Y., Hernández-Herrero, M. M., & Filardo-Kerstupp, T. (2017): Antioxidant and antimicrobial properties of cactus pear (Opuntia) seed oils. Journal of Food Quality, 2017.
Ratnaweera, P. B.; Silva, E. D. D.; Williams, D. E.; and Andresen, R. (2015): Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opunita dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC complementary & Alternative Medicine. 15:220. Doi: 10.1186/s12906-015-0722-4.
Ríos, J. L.; and Recio, M. C. (2005): Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology. 100(1-2)80-84.
Roşca-Casian, O.; Pârvu, M.; Vlase, L.; and Tămaş, M. (2007): Antifungal activity of Aloë vera leaves. Fitoterapia. 78:219-222.
Schulz, B.; Wanke, U.; Draeger, S.; and Aust, H. J. (1993): Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycological Research. 97(12).1447-1450.
Sievers, F., & Higgins, D. G. (2014): Clustal omega. Current protocols in bioinformatics, 48(1), 3.13. 11-13.13. 16.
Silva-Hughes, A.; Wedge, D.; Cantrell, C.; Carvalho, C.; Pan, Z.; Moraes,R.; Madoxx, V.; and Rosa, L. (2015): Diversity and antifungal activity of the endophytic fungi associated with the native medicinal cactuse Opuntia humifusa ( Cactaceae) from the United States. Microbiological Research. MICRES-25761, NO (11).
Stanley, M. C.; Ifeanyi, O. E.; and Eziokwu, O. G. (2014): Antimicrobial effects of Aloe vera on some human pathogens. International Journal of Current Microbiology and Applied Science. 3 (3) 1022-1028.
Surjushe A, V. R., Saple DG. (2008): Aloe vera: a short review. Indian Journal Dermatol,53(4),163-166. https://doi.org/10.4103/00195154.44785.
Suryanarayanan, T.; Wittlinger, S.; and Faeth, S. (2005): Endophytic Fungi Associated With Cacti in Arizona. The British Mycological Soc. Res. 109(5): 635-639
Suryawanshi, P.; and Vidyasagar, G. M. (2016): Antimicrobial Activity of Opuntia cochenillifera (L.) Mill Fruit and Cladode Extracts. International Journal of Pharmacology, Phytochemistry and Ethnomedicine. ISSN: 2297-6922, Vol. 3, pp 84-89.
Vyawahare,M.; Jagtap,P.; Gangurde, A.; Kukreja,G.; and Mane,R.(2019): Isolation of Endophytes From Roots of Aloe Vera. I JSART. 5(1).273-277.
Wani, S. H.; and Lone, S. A. (2016): Aloe vera a Medicinal Plant: Biotechnology Applications. Educational publishing. Jammu and Kashmir, India.
White, T. J.; Bruns, T. D.; Lee, S. B.; and Taylor, J. W. (1990): Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. Research Gate. In book: PCR – Protocols and Applications – A Laboratory Manual (pp.315-322).
Yadav, R.; Singh, A.; Joshi, S.; and Kumar, M. (2015): antifungal and enzyme activity of endophytic fungi isolated from Ocimum sanctum and Aloe vera African J. Microbiology Res. 9(29), 1783-1788. Doi: 10.5897/AJMR2015.7451


