1Translational Neuroscience, University of Wurburg, Sanderring, Germany.
2Department of Biotechnology, BMS College for Women, Bangalore, Karnataka, India.
3Department of Biotechnology, Ramaiah Institute of Technology, Bangalore, Karnataka, India.
4Department of Biotechnology Bioinformatics, School of Life Sciences, JSS Academy of Higher
Education Research, Mysuru, Karnataka, India.
5Department of Biotechnology, Sri Dharmasthala Manjunatheshwara College, Ujire, India.
6Department of Zoology, University College of Science, Tumkur University, Karnataka, India.
Corresponding author email: krmaruthi@gmail.com
Article Publishing History
Received: 19/08/2021
Accepted After Revision: 25/12/2021
One of the most prevalent malignancies among geriatrics is colorectal cancer (CRC), which starts to develop in the forms of genetic syndromes in young adults. The Piper nigrum is one important common spice used in the household having anticancer activities. The current study aims to evaluate P. nigrum seed extracts potency as anticancer against CRC cell line (COLO205). The extract is used to elucidate the MTT assay, DNA damage studies (COMET assay), Acridine Orange/Ethidium Bromide dual staining, cell death, cell cycle arrest using Flow cytometry, and regulation of Bcl-2, Bax & P53 gene regulation. To check the cell cytotoxicity by MTT assay methanolic extract was used. To evaluate anticancer activity the sample was extracted in methanol. RT-PCR was used to elevate gene expression studies of Bcl-2, Bax, and P53.
In the dose-dependent mode, the extract inhibited the growth of COLO205 cells and the IC50 value was calculated at 48.2 μg/ml. The DNA fragmentation induced by apoptosis was the primary reason for the cell toxicity as observed by DNA damage studies & AO/EB dual staining technique. The extract concentration ranging from 40 & 80 μg/ml remarkably increased the proportion of cells in the S & G2/M phase. Cells at the late-apoptotic stage were found to be in the range of 22% – 57%. The Bax and P53 were upregulated and Bcl-2 was downregulated when treated with the extract. From this investigation underlying the mechanism of CRC was found to be P. nigrum extract caused to induce apoptosis and upregulation of tumor suppressor gene downregulation of apoptosis-suppressing gene bcl-2.
Ao/Eb Staining, Colorectal Cancer, Colo205, Comet Assay, Piper Nigrum.
Giridhar N. J, Vyshali V. M, Gangadharappa B. S, Ramu R, Bajpe S. N, Parimala B, Maruthi K. R. Anticancer and Gene Expression Studies of Piper nigrum Extract on Colon Cancer Cell Line. Biosc.Biotech.Res.Comm. 2021;14(4).
Giridhar N.J, Vyshali V.M, Gangadharappa B.S, Ramu R, Bajpe S.N, Parimala B, Maruthi K.R. Anticancer and Gene Expression Studies of Piper nigrum Extract on Colon Cancer Cell Line. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3J51P9M“>https://bit.ly/3J51P9M</a>
Copyright © Giridhar et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
In the digestive system of the body colon is an important role in the absorption of food, water & minerals, and aids in the removal of de-nutritional food materials. It is bowel in the large intestine and measures approximately 1.5 mt (Azzouz and Sharma 2019). Cancer is the world’s leading cause of mortality with roughly 10 million fatalities recorded in 2020 worldwide (Ferlay et al. 2020). About 2.0 million deaths related to cancer was reported to be Colorectal cancer (CRC) is prevalent cancer followed by breast and lung cancer.
The change in the lifestyle such as consumption of canned foods, low intake of dietary fibers and increase in sedentary activities, smoking, obesity, and aging are leading causes of CRC (Kuipers et al. 2015; Valderrama-Trevino et al. 2017). Furthermore changing in lifestyle has affected the gut microbiota. Normal microflora is disturbed leading to increase in the pathogenic microbiota further aggravating the incidence of occurrence of CRC (Siegel et al. 2020).
The imbalanced gut microbiota leads to a release of toxins which causes DNA damage in turn leading to cell transformation (Jahani-Sherafat et al. 2018). Conventional methods of treating CRC are operations, chemotherapy, and radiotherapy, it depends upon the cancer pathology stage. Minimal invasive surgeries like Endoscopic Mucosal Resection, Polypectomy, and Laparoscopic Surgery to excise affected CRC in initial stages.
In the advanced phase, fractional colectomy and elimination of lymph nodes are recommended procedures along with immuno-therapy, hormone-therapy, and photodynamic therapy are found to be effective. These methods have an invasive and effect on the quality of lifestyle of affected individuals. Therefore, the new methods of cancer treatment are alternative options to reduce the toxicity of drugs on healthy tissues (Arruebo et al. 2011; Singh and Chaturvedi 2015; Ferlay et al. 2020).
The traditional system of medicine (TSM) Ayurveda, Unani and Amchi system have become alternatives to the conventional method of treatment has shown resistance to cancer, TSM gives better quality of life since they have minimal or no side effects. In TSM utilize ingredients are food & spices as well as having medicinal & therapeutic properties (Greenwell and Rahman 2015). Members of the Piperaceae family are native Indian and many rare species, and have been part of a diet for a long time.
Black pepper (P nigrum) contains Piperine, which is an abundant bioactive alkaloid and has a pungent flavor of pepper (Gorgani et al. 2017; Dong et al. 2019). Piperine is a Vaso Modulator & antihypertensive activity, antiplatelet, antioxidant, antitumor, antiasthmatics, antiarthritic and anti-inflammatory, antidepressants, immunomodulatory, anticonvulsant, antimicrobial. In this study the molecular basis of the P. nigrum extract as an anticancer agent CRC cell line (COLO205) was investigated (Damanhouri 2014; Zou et al. 2015; Chung 2019; Yu et al. 2020; Haq et al. 2021).
MATERIAL AND METHODS
Freshly harvested Piper nigrum seeds were purchased from farmers in Ujire, Dakshina Kannada, India. The seeds were coarsely powdered, passed through a 20 μm stainless steel sieve. The sieved P. nigrum seed powder was used for the solvent extraction by decoction method. The extraction was carried out with 400 ml methanol by steam heating at 400 C for 6 hours. (Takooree et al. 2019). The methanolic decoction was filtered using Whatmann filter paper No.42, concentrated using wet heat on a 400 C water bath, and stored in a glass vial at 40 C (Zhang et al. 2018, Patil et al. 2021).
Using Dulbecco’s Modified Eagle Medium (DMEM) fortified with inactivated fetal bovine serum (FSB – 10%), 100 IU/ml penicillin, 100 µg/ml streptomycin at 370 C in a carbon dioxide incubator (5% CO2, humidified) cultured CRC cell lines COLO 205 (ATCC® CCL-222™), procured from ATCC (Takashi et al. 2015). The cell dissociation solution containing trypsin (0.2%), EDTA (0.02%), glucose (0.05%) in phosphate basal salt solution. The cells were collected by subjecting to the cell sedimentation at ~1000g (1000 rpm) for a couple of minutes and viability was checked. Additionally, in a 96 well plate, 5 X 104 cells/well were seeded & incubated at 370 C for 24 hours, in a carbon dioxide incubator (5% CO2).
To investigate cytotoxicity using the MTT test, the cultured cells were trypsinized. The monolayer culture was counted and adjusted to 1 X 105 cells per ml. 100 µl of diluted cell suspension (5 X 104 cells/well) was put to each well of microtiter plates and allowed to develop a partial monolayer (after 24 hours), P. nigrum extracts concentrations of 40 & 80µg/ml of 100 µl each was added and incubated at 370 C for 24 hours. To dissolve the formed Formosan, add 0.1 ml of DMSO to the supernatant and shake.
To calculate the cytotoxicity (IC50 values) microtiter plates were read at 590 nm (Thermo Fisher Scientific, Varioskan™ multimode microplate reader) and cytotoxicity IC50 values calculated by standard protocol (Graph pad, SanDiego, CA, USA) (Ref 2021). In a 96-wells plate with 2 ml media, 106 cells were seeded and cultured for 24 hours. The cells were then treated with 40µg/ml & 80µg/ml ethanolic extract of P. nigrum and incubated for another 24 hours in a 370 C in carbon dioxide incubator with 5%.
After the stipulated period of incubation extract-treated cells were aggregated by centrifugation at 3000 rpm for 5 minutes at 40 C. discard the supernatant. The cells were splashed with PBS, air dried, and the pellet was dissolved in 0.5 ml PBS. Following cell isolation, 0.02 ml of cell suspension (1×105 cells/ml), 0.08 ml of 0.5 percent low melting point agarose (LMPA) were blended, layered over the wells, containing Normal Melting Agarose (NMA) in the slide.
In a coplin jar containing lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Trizma Base, 1 percent SDS) place a cell fixed slide for 2 hours at 40. In the electrophoretic buffer (0.3N NaOH and 100mM EDTA) cells were separated by electrophoresis at 0.74 V/cm for 30 minutes. After electrophoresis, the slides were rinsed in chilled distilled water and air-dried followed by a wash with 70% chilled ethanol and air dry. Stain the slides with 80 µl Ethidium Bromide (EtBr) for visualizing. Immediately after staining with EtBr to score the slides, it was observed under a 40X objective lens of the fluorescent microscope and recorded.
To estimate the extent of DNA damage 50 to 100 cells are randomly chosen for each analysis. “The cells damage is determined by measuring the length of DNA migration & the percentage of migration of DNA with ImageJ software with the open comet plug-in (www.rsbweb.nih.gov/ij/)”. The tail moment, the amount of migration per cell, the number of cells with amplified migration, the level of migration among damaged cells, and viability were analyzed using the program (Lan et al. 2014).
Using “5 µl AO-EtBr (Acridine orange & Ethidium Bromide) to a 25 µl (~1×105 cells) of untreated & treated cells stained separate Eppendorf tubes, and after a couple of minutes mixed gently. The AO-EtBr stained cell suspension about 10 μl of was mounted on a glass slide with a coverslip, visualized under a fluorescence microscope with fluorescein filter” (Kasibhatla et al. 2006; Liu et al. 2015). To record apoptosis, “1 X 106 cells per well were placed in a 6-well plate containing the DMEM and incubated”. Subsequently floating cells detached by a pipette, make up the volume after 18 hours. Incubated in carbon dioxide incubator for 24 hours at 370 C of 40 & 80 µg/ml P. nigrum extract treated samples.
“Incubated cell culture medium transferred into fresh 15 ml centrifuge tubes and centrifuged at 2000 rpm for 5 minutes in 40 C. The cells pellet was rinsed with ice-cold PBS, then suspended in 1 ml 1X binding buffer”. After aliquoting 500µL of cell suspension, 10µl of PI and 5µL of Annexin V were added. “The suspension was incubated for 15 minutes at room temperature in dark and was analyzed by FACS Caliber (BD Biosciences, San Jose, CA), as per the guidelines” (Wang et al. 2003).
Two ml of medium were dispensed in a 96-well plate seeded 1 x 106 cells and grown for 24 hours. Subsequently treated with 40 & 80 µg/ml of P. nigrum extract, incubate in a carbon dioxide incubator for the next 24 hours at 370 C. Harvesting the cells by centrifuging at lower rpm for a couple of minutes at room temperature. Discard the supernatant with care to retain the cell pellets were retained. “Cell pellets were washed by resuspending them in 2 ml of PBS (1X) and repeating this process twice. Discarded the supernatant and the cell pellets preserved”. “Cells were fixed by re-suspending them in 0.3 ml of sheath fluid, then adding 1 ml of chilled 70% ethanol drop by drop with continuous & gentle shaking, repeat the procedure couple of times” (Jackman and O’Connor 2001).
The cells were then stored at a temperature 40 C overnight, centrifuged at lower rpm for a couple of minutes to collect a cells pellet. Then the cell pellets were rinsed in 2 ml of ice-cold 1X PBS and centrifuge. “Re-suspended in 0.45 ml of sheath fluid containing 50 µg/ml PI & 50 µg/ml RNase A & incubated in the dark for 15 minutes. The populations of treated and untreated cells in various phases of the cell cycle were analyzed by FACS Caliber” (BD Biosciences, San Jose, CA) (Magadi et al. 2015; Patil et al. 2020a; Patil et al. 2020b).
Using TRizol Reagent (Invitrogen) total RNA was isolated from COLO205. “The isolated RNA was dissolved in 25 µl of DEPC treated water. A semi-quantitative reverse transcriptase-polymerase chain reaction was used to determine the sequence of mRNA of expressed sequence tags of Bax, Bcl-2, P53, and β-Actin (Applied Biosystems, VERITI 96 well thermal cycler). The cDNA was generated by 2 μg of RNA with the Verso cDNA synthesis kit (Thermo Fisher Scientific), with oligo dT primers”. The final reaction volume was 20 μl, and cDNA synthesis was carried out for an hour at 420 C and reverse transcriptase inactivated at 850 C for 5 minutes.
RESULTS AND DISCUSSION
Cancer is the second leading cause of death globally, Colorectal cancer (CRC) third most common cancer followed by breast and lung cancer (Siegel et al. 2020; WHO 2018). The conventional approach of cancer therapy is chemo & radiotherapy has side effects, recovery chances shrink with immune compromised patients. Traditional medicine systems have become a promising method of treatment with minimal or no side effects. Plants are the treasure of secondary metabolites such as alkaloids, flavonoids, lignans, saponins, terpenes, and taxanes, synthesized due to biotic or abiotic stress. These phytochemicals are the treasures of therapeutic molecules and it has been used in medicinal practices since ancient times (Upreti 2021).
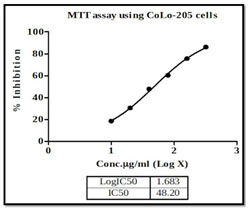
There are currently many therapeutic approaches that are considered to persuade cytotoxicity, which hinder tumor growth. The goal of any therapeutic approach is to affect the wide number of tumor cells while having no adverse effects on healthy tissue or cells; this can be achieved by inducing apoptosis (Rundqvist et al. 2020). The current research looked at the cytotoxic effect of different concentrations of methanolic extract of P. nigrum seeds on Colon cancer cell line COLO205 with increasing dosage (0 – 320 μg/ml), the number of viable cancer cells decreased significantly. In the current study IC50 value of P. nigrum extract after 24 hours intervals is at 48.2 μg/ml, likewise, crude extract of P. nigrum at 13.70 μg/ml was observed in the previous studies (Tedasen et al. 2020) (Figure 1).
Bioactive compounds Kusunokinin and piperloguminine isolated from P. nigram were effective against breast cancer, and P. nigram also effectively inhibited the growth of HeLa cells (Priya and Kumari 2017; Sriwiriyajan et al. 2017). The Ekowati et al. (2019) combined ginger and pepper extract which triggered apoptotic mechanism due to cytotoxicity in HeLa cell line had IC50 at 33.81µg/ml which is significantly lower than the current study. This study implicates that it is P. nigrum has a synergistic effect when combined with other natural food ingredients such as Citrus limon, P. nigrum, and Melaleuca alternifolia (Nikolić et al. 2017). Also, P. nigrum in combination with cardamom has a cytotoxic effect against YAC-1 tumor cells (Majdalawieh and Carr 2010; Rundqvist et al. 2020).
Figure 1: CRC COLO205 Cell proliferation inhibition effect of P. nigrum extract. Cell viability positively
correlated with the maximum concentration of 320 μg/ml extract, using the MTT assay.
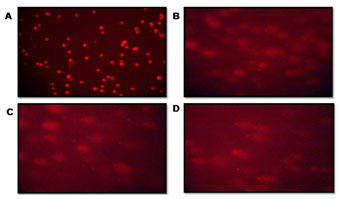
The crude extract potency as a cytotoxic agent was confirmed from the morphological study using COMET assay and AO/EB staining. The extent of DNA damage in CRC cells was measured by DNA migration. Randomly selected cells were analyzed by calculated tail moment. The comet assay images of the Fluorescence microscope were visualized after treatment (Figure 2 and Table 1). The olive movements at 40 & 80 µg/ml were 8.8±11.0 and 11.4±10.3 respectively while the control had 4.1±11.5 was observed. Maximum DNA damage was recorded with 80 µg/ml (p<0.05) of P. nigrum extract-treated cells (Figure 2 C). A comparison of the extent of DNA damage with and without oxidant pretreatment was also reported, with the comet assay being declared the best option among all other methods for studying DNA damage (Jahangir et al. 2020).
Table 1. The Effect of P. nigrum methanolic extract on CRC cell lines showing
mean Olive moments against control and standard.
| Variables | Olive tail moments
Mean ± SD |
| Positive Control | 4.1 ±11.5 |
| P. nigrum methanolic extract 40 µg/ml | 8.8 ± 11.0 |
| P. nigrum methonic extract 80 µg/ml | 11.4 ± 10.3 |
| Negative Control H2O2 (150 µM) | 12.7 ± 26.2 |
Figure 2: The patterns of DNA fragment migration determined by the comet assay were examined using a fluorescence microscope. (A) Control cells, (B) 40 μg/ml extract-treated Cell lines (C) 80 μg/ml extract-treated Cell lines (D) Positive Control: 150µM of H2O2 treated Cell lines.
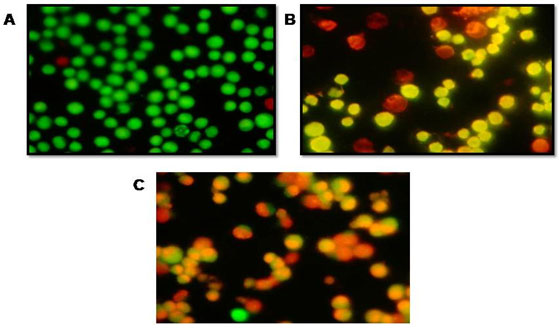
On observation under fluorescence microscope of AO/EtBr dual stained COLO205 cells treated & untreated cells with P. nigrum extract, normal cells had a circular nucleus depicts early-stage apoptotic cells (Figure 3A, 3B), with the nucleus displaying yellow-green fluorescence due to AO staining & condensed into a semicircle in a corner of the cell. The late apoptotic phase in necrotic condition, evidenced by the EtBr intercalated to the damaged cell’s DNA (Figure 3C). Similar results have been reported in Piper betle leaf extract and Syzygium aromaticum flower extract (Azhagumeena et al. 2021).
Figure 3: A fluorescence microscopic image showing CRC Cell lines treated with AO/EB dual staining. (A) Control CRC cells, (B) P. nigrum extract (40 μg/ml) treated Cell lines (C) P. nigrum extract (80 μg/ml) treated Cell lines.
Staining cells with FITC-Annexin V (green fluorescence) simultaneously with dye exclusion of propidium iodide (negative for red fluorescence) at the same time allows for the differentiation of intact cells, early apoptotic & late apoptotic or necrotic cells. In control G2M was observed to be 8.5%. When treated with 40 & 80 µg/ml of P. nigrum extract G2M & S phase arrest was observed to be 2.7%, 16.6% and 17.1%, 21.0% respectively as illustrated in Figure – 6 & 7.When compared to control cells, the 40 & 80 µg/ml treated were induced both early and also late apoptosis in CRC cell line (COLO-205) with 5.7%, 2.2%, & 22.4% (Figure 4 and Figure 5,) apoptotic cells, respectively, and 14.9% and 26.2% necrotic cells, therefore the MTT assay tends to overestimate the efficacy of drugs that alter cell metabolism; it also fails to differentiate cell death from growth inhibition (Gomez-Guitieerz et al. 2021).
Figure 4: AV-FTIC/PI dual-staining assay. After treating with methanolic extract of P. nigrum
cells stained with AV-FTIC/PI & analyzed by fluorescence microscopy.
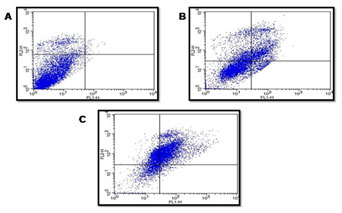
Flow cytometric analysis of AV-FTIC/PI dual-staining: CRC COLO205 cells left untreated or treated for 24 h 40 & 80 μg/ml extract. A) Untreated, B) After 24 hours treatment with 40 μg/ml of sample, C) After 24-hour treatment with 80 μg/ml of sample. The bottom left quadrants in untreated cells were primarily AV-FITC/PI negative, live cells. The upper left quadrant shows non-viable cells, undergoing apoptosis (AV-FITC positive and PI negative). The bottom right quadrants indicate the early apoptotic cells. The upper right quadrants indicate that they were in the late apoptosis phase, positive for both AV-FTIC and PI.
Figure 5: Apoptosis detection: fraction of cells CRC COLO-205 cells
present in each stage expressed in percentage.
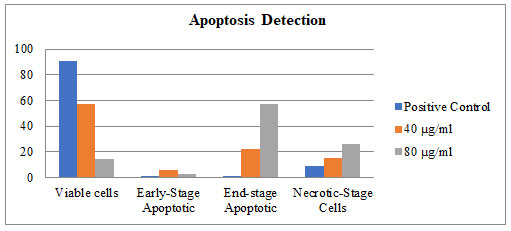
Figure 6: Data indicating the proportion of cells in each stage of the cell cycle, for the of
P. nigrum methanolic extract of treated with 40 and 80 µg/ml.
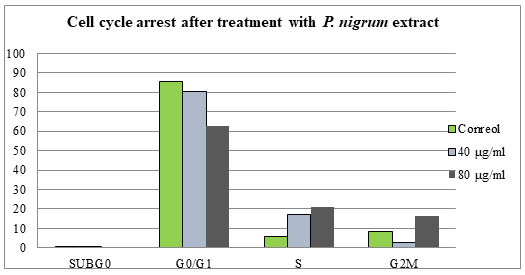
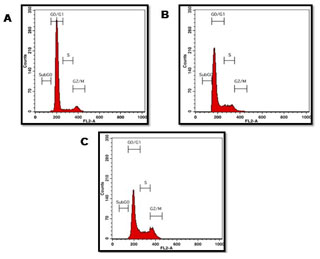
Figure 7: Analysis of CRC COLO205 cell cycle after being treated for 24 hours with 40 & 80 μg/ml extract. A) Positive Control Untreated cells, B) Treated with 40 μg/ml of P. nigrum ethanolic extract, C) Treated with 80 μg/ml of P. nigrum ethanolic extract.
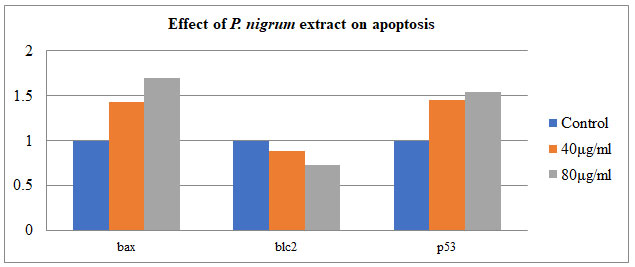
Semi-quantitative PCR was used to investigate the effect of P. nigrum on gene tumorigenic and oncogene – BAX, Bcl-2, and P53 in COLO205. The housekeeping gene β-Actin was taken as control. The treatment of P. nigrum extract led to upregulation of BAX & P53 by 0.4 & 0.5 folds while anti-apoptotic Bcl-2 were down-regulated by 0.7 & 0.5 fold (Figure – 8 & 9). Apoptosis occurs in cells accumulated in the G2/M phase via the BAX-initiated mitochondrial pathway (Celentano et al. 2019).
Figure 8: Expression of BAX, Bcl-2 genes. P53-independent on gene expression in
DNA damage-induced apoptosis in COLO205 cells.
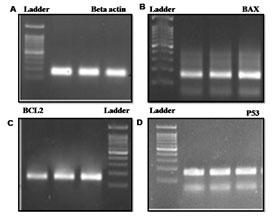
Figure 9: Effect of P. nigrum extract on apoptosis-related gene expression: BCL2-associated X protein
(BAX), tumor protein P53 & B-cellCLL/lymphoma 2 (bcl2) in COLO 205
Our findings are consistent with previous research, indicating that P. nigrum is effective on cell death in the colorectal cancer cell line COLO-205 to a significant extent.
CONCLUSION
The results of this study indicate that Piper nigrum seed extract is an effective anticancer agent. While awareness on the biological activities of P. nigrum has improved, the scientific basis for the conventional use of P. nigrum in the treatment of colorectal cancer does not provide adequate information on the efficacy of the drug, the molecular mechanisms. Therefore, the data produced in this study are important to demonstrate evidence of potential pharmacological action. Our results correlate which mainstream research, which suggests that Piper nigrum is effective in inducing cell death in colorectal cancer cell line COLO-205 to a considerable extent.
Conflict of Interests: Authors have no conflict of interests to disclose.
REFERENCES
Azzouz L L and Sharma S (2020). Physiology, Large Intestine In: StatPearls. StatPearls Publishing, Treasure Island (FL). PMID: 29939634..
Chung H Y (2019). Anti-inflammatory and antioxidant activities of piperine on t-BHP-induced in Ac2F cells. SDRP Journal of Food Science & Technology, 4(4).
Collins, A R (2004). The comet assay for DNA damage and repair: principles, applications, and limitations. Molecular Biotechnologyl, 26, 249-61. https://doi.org/10.1385/MB:26:3:249
Ćurčić, M G, Stanković, M S , Mrkalić, E M., et al. (2012). Antiproliferative and proapoptotic activities of methanolic extracts from Ligustrum vulgare L. as an individual treatment and in combination with palladium complex. International journal of molecular sciences, 13(2), pp.2521-2534
Damanhouri, Z A and Ahmad, A (2014). A review on therapeutic potential of Piper nigrum L. Black Pepper): The King of Spices. Medicinal & Aromatic Plants, 3, p.161.
Denizot, F and Lang, R (1986). Rapid colorimetric assay for cell growth and survival:Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of Immunological Methods, 89, 271-7.
Elmore, S. (2007). Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35, 495-516.
Florento, L, Matias, R, Santiago, TE et al. (2012). Comparison of Cytotoxic Activity of Anticancer Drugs against Various Human Tumor Cell Lines Using In Vitro Cell-Based Approach. International Journal of Biomedical Science, 8, 76-80.
Gonzalez, R J and Tarloff, J B (2001). Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicol In Vitro, 15, 257-9.
Gorgani, L, Mohammadi, M, Najafpour, G D et al. (2017). Piperine—the bioactive compound of black pepper: from isolation to medicinal formulations. Comprehensive Reviews in Food Science and Food Safety, 16, 124-140.
Greenwell, M. and Rahman, P K (2015). Medicinal Plants: Their Use in Anticancer Treatment. International Journal of Pharmaceutical Sciences and Research, 6, 4103-4112.
Jackman, J and O’connor, P M (2001). Methods for synchronizing cells at specific stages of the cell cycle. Current Protocols in Cell Biology, Chapter 8(1), Unit 8.3.
Jahani-Sherafat, S, Alebouyeh, M, Moghim, S, et al. (2018). Role of gut microbiota in the pathogenesis of colorectal cancer; a review article. Gastroenterology and Hepatology from Bed to Bench, 11, 101-109.
Kasibhatla, S, Amarante-Mendes, G P, Finucane, D, et al. (2006). Acridine Orange/Ethidium Bromide (AO/EB) Staining to Detect Apoptosis. Cold Spring Harbor Protocols (3), pp.pdb-prot4493.
Kasibhatla, S and Tseng, B (2003). Why target apoptosis in cancer treatment?. Molecular cancer therapeutics, 2(6), pp.573-580.
Kuipers, E J, Grady, W M, Lieberman, D, et al. (2015). Colorectal cancer. Nature Reviews Disease Primers, 1, 15065.
Lai, L H, Fu, Q H, Liu, Y, et al. (2012). Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacologica Sinica, 33, 523-30.
Lan, J, Gou, N, Gao, C, et al. (2014). Comparative and mechanistic genotoxicity assessment of nanomaterials via a quantitative toxicogenomics approach across multiple species. Environmental Science & Technology, 48, 12937-45.
Liu, K., Liu, P C., Liu, R. et al. (2015). Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Medical Science Monitor Basic Research, 21, 15-20.
Magadi, V P, Ravi, V, Anantharaju A L, et al. (2015). Evaluation of cytotoxicity of aqueous extract of Graviola leaves on squamous cell carcinoma cell-25 cell lines by 3-(4, 5-dimethylthiazol-2-Yl)-2, 5-diphenyltetrazolium bromide assay and determination of percentage of cell inhibition at G2M phase of cell cycle by flow cytometry: An in vitro study. Contemporary Clinical Dentistry, 6, 529.
McCauley, J, Zivanovic A and Skropeta, D (2013). Bioassays for Anticancer Activities. In: Roessner U., Dias D. (eds) Metabolomics Tools for Natural Product Discovery. Methods in Molecular Biology (Methods and Protocols), vol 1055. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-577-4_14.
Arora, M., (2013). Cell culture media: a review. Mater methods, 3(175), p.24.
Mosmann, T (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods, 65(1-2), pp.55-63.Prives, Prives, C and Hall, P A, (1999). The p53 pathway. The Journal of pathology, 187(1), pp.112-126.
Siegel, R L, Miller, K D and Jemal, A, (2016). Cancer statistics. CA: a cancer journal for clinicians, 66(1), pp.7-30.
Singh, P and Chaturvedi, A, (2015). Complementary and alternative medicine in cancer pain management: a systematic review. Indian journal of palliative care, 21(1), p.105.
Srinivasan, K, (2009). Black pepper (Piper nigrum) and its bioactive compound, piperine. In Molecular targets and therapeutic uses of spices: Modern uses for ancient medicine (pp. 25-64).
Takooree, H, Aumeeruddy, M Z, Rengasamy, K R, et al. (2019). A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Critical reviews in food science and nutrition, 59 (sup1), pp.S210-S243.
Taqvi, S I H , Shah, A J and Gilani, A H, (2008). Blood pressure lowering and vasomodulator effects of piperine. Journal of cardiovascular pharmacology, 52(5), pp.452-458.
Tsujimoto, Y. (1998). Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells, 3, 697-707.
Valderrama-Trevino, A I, Barrera-Mera, B, Ceballos-Villalva, J C et al. (2017). Hepatic Metastasis from Colorectal Cancer. Euroasian Journal of Hepato-Gastroenterology, 7, 166-175.
Vignon, C, Debeissat, C, Georget, M T, et al. (2013). Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS One, 8, e68425.
Wang, J, Chang, Y and Chern, Y (2003). Study of in vitro and in vivo effects of 1, 6-Bis [4-(4-amino-3-hydroxyphenoxy) phenyl] diamantane (DPD), a novel cytostatic and differentiation inducing agent, on human colon cancer cells. British Journal of Cancer, 89, 1995-2003.
Yu, Y, Hu, S, Fu, D et al. (2020). Surfactant-assisted enzymatic extraction of piperine from Piper nigrum L. International Journal of Food Properties, 23, 52-62.
Zhang, Q W, Lin, L G and Ye, W C. (2018). Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medical, 13, 20.
Zou, L, Hu, Y Y and Chen, W X (2015). Antibacterial mechanism and activities of black pepper chloroform extract. Journal of Food Science and Technology, 52, 8196-8203.


