1Department of Botany, MMV, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India
2Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, Uttar Pradesh, India
Corresponding author Email: kumaridrnishi@yahoo.co.in
Article Publishing History
Received: 22/07/2019
Accepted After Revision: 27/09/2019
Antibacterial efficacy of both dry and green leaf extracts of Spondias mangifera was observed by using their methanol, ethanol, and aqueous extracts. Six human pathogenic bacterial strains were selected as test organisms and antibacterial activities were assessed by using disc diffusion method. Maximum inhibition of Enterococcus faecalis was observed by ethanolic dry leaf extract (25.00 ± 0.58).Similarly, methanolic dry leaf extract was very effective against Shigella boydii (25.17±0.44). Higher antibacterial activity was observed by green leaf extracts for other test organisms. Aqueous extract of green leaf showed maximum inhibition (11.50± 0.76) against Staphylococcus aureus. Ethanolic extract of green leaf showed maximum activity (10.17±0.44) against Escherichia coli. Similarly, methanolic extract of green leaf against Klebsiella pneumoniae and Proteus vulgaris showed maximum antibacterial activities, i.e. (15.50±0.29) and (12.50±0.29) respectively.
Antibacterial, Bacteria, Extract, Inhibition, Solvent
Jaiswal P, Yadav A, Nath G, Kumari N. Antibacterial Activity of Leaf Extracts of Spondias mangifera Willd:A Future Alternative of Antibiotics. Biosc.Biotech.Res.Comm. 2019;12(3).
Jaiswal P, Yadav A, Nath G, Kumari N. Antibacterial Activity of Leaf Extracts of Spondias mangifera Willd:A Future Alternative of Antibiotics. Biosc.Biotech.Res.Comm. 2019;12(3). Available from: https://bit.ly/2kMTW2u
Copyright © Jaiswal et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Since ancient times, we depend on plants or plant products for medicines. Plants serve as source of many chemicals and many of them have been identified as pharmaceutically important. Presence of secondary metabolites such as alkaloids, tannins, flavonoids, terpenoids, etc. contribute significant role in developing antimicrobial properties. After the discovery of antibiotics, our dependence on antibiotics had minimized the use of such plants. Many microbes have developed resistance against several antibiotics and the treatment of patients infected with such microbes has become a big concern for medical practitioners. Improper use of antibiotics is also one of major cause for rising number of patients with resistance of antibiotics and for their treatment an alternative of antibiotics is urgently required. The use of plant products as antimicrobial is an efficient way to combat above problem. There is another advantage that plant products show almost negligible side effects,( Li and Webster, 2018).
Spondias mangifera Willd. is also called as Spondias pinnata (Linn. F.) and belongs to Anacardiaceae. The plant is cultivated for its edible fruits and it is known by different names in different localities and languages such as hog plum, wild mango, amra, etc. Its leaf, bark and fruits are used for the treatments of various ailments (Tripathi and Kumari, 2010). Exocarp of the fruit has shown the presence of various activities such as antioxidant, antimicrobial and thermolytic (Manik et al, 2013). Similarly, its resin also showed antimicrobial activity (Gupta et al, 2010). The present investigation was done to evaluate antibacterial activities of fresh and dried leaf extracts in different solvents.
Material and Methods
Material Collection and Preparation of Extracts
For green leaves
Green young leaves of Spondias mangifera were collected from the campus of Banaras Hindu University (BHU), Varanasi, India. The surface of leaves was cleaned by running tap water and then blotted by blotting papers. 1g of leaves was crushed in mortar and pestle in 5 ml of different solvents like ethanol, methanol and double distilled water separately. Solutions were centrifuged at 3000 rpm for 10 minutes to remove all the cell debris. Supernatant was separated out and final volume was maintained up to10 ml. The extract solutions were stored at 4 ◦C.
For dry leaves
Leaves were shade dried for 6-7 days, oven dried at 45-50 ◦C for 2-3 hrs and then grinded in mechanical grinder to make coarse powder. For the preparation of extract, 20 g of powdered leaf were mixed in 200 ml of solvent by using a Soxhlet apparatus. Ethanol, methanol and Double distilled water (DDW) were used as solvents for extraction. Extracts were then filtered and dried at 45 ◦C on rotary evaporator. Extracts were stored at -20 ◦C for further use.
Preparation of samples
Stock samples of leaf extracts were prepared in dimethyl sulphoxide (DMSO) and the concentration of stocks was 100 mg ml-1. About 5 µl extracts were dispensed on sterile disc for susceptibility test.
Test Microorganisms
Six human pathogenic bacterial strains were selected for screening of antibacterial activity. Two Gram positive bacteria (Staphylococcus aureus and Enterococcus faecalis) and four Gram positive bacteria (Klebsiella pneumoniae, Escherichia coli, Shigella boydii and Proteus vulgaris) were taken for the investigation. Microbial cultures were obtained from Department of Microbiology, Institute of Medical Sciences, BHU, Varanasi, India.
Medium preparation
For the preparation of medium, 38 g Muller Hinton agar (MHA) and 10 g bacteriological agar were dissolved in 1 litre double distilled water. Saline was prepared by dissolving 8.5 g NaCl in 1 litre double distilled water. The medium was autoclaved for 15 min at 1.1 kg/cm2 and 121°C. Approximate 20 ml autoclaved molten medium was poured in autoclaved Petri dishes in laminar flow.
Preparation of Inoculums
Bacterial inoculums were prepared by growing cells on MHA (Himedia, Mumbai) for 24 h at 37°C. The turbidity of the bacterial suspension was adjusted to about 0.5 McFarland turbidity standard (~1 x 107 CFU/ml).
Antibacterial Sensitivity
The disc diffusion method (Zaidan et al, 2005 and Singh et al, 2016) was used to screen antibacterial activity. The test cultures were swabbed on the top of the solidified media and dried for 5 min. About 5 µl of extract was loaded to each disc. The loaded discs were placed on the surface of the medium. Dimethyl sulphoxide (DMSO) was used as negative control. Specific standard drugs Streptomycin was used against all Gram positive and Gram negative bacteria. The plates were incubated for 24 h at 37 °C for bacteria in BOD incubator (REMI). Zone of inhibition (diameter) was recorded in millimeters.
Results and Discussion
Frequent use of antibiotics and development of resistant varieties of microbes have become a major concern for medical practitioners (Kourkouta et al, 2017). Many patients show resistance for several antibiotics and their treatments pose a major challenge for physicians. Antimicrobial activities have been reported by many plants, but their use as alternative to antibiotics are not in practice. There is utmost need to identify the plants with such activities, so that it could replace the use of antibiotics effectively. Use of antibiotics shows many side effects, but plant products have less or negligible side effects. Present work used Mueller Hinton Agar medium and disc diffusion method (Fig 1). Mueller Hinton Agar is considered best medium for reactive antibiotic susceptibility test. In the present study, both dried and fresh leaf extracts have shown antimicrobial activity (Table 1 & 2). Previous reports also showed antimicrobial activities of fruit and leaves of S. mangifera (Tripathi and Kumari, 2010 and Jain et al, 2014). Here antimicrobial properties of both fresh and dried leaf extracts in different solvents have been tested for different test organisms. The antimicrobial activity depends upon the type of extract, concentration of the extract, type of solvents and type of test organisms. Maximum inhibition for E. faecalis (25.00±0.58) was seen in ethanolic extract of dried leaves, whereas aqueous extract also showed high inhibition activity (23.88± 0.60) (Table 1 & 2). Inhibition of K. pneumoniae was maximum (11.50±0.29) by methanolic extract of dried leaves. Poor inhibition was observed for S. aureus and E. coli by different extracts of dry leaves (Table 1). However, hexane extract of leaves was reported highly effective against S. aureus (Jain et al, 2014). Maximum inhibition of P. vulgaris (10.67± 0.33) was seen by the methanolic extract of dried leaves. High antibacterial activities were seen against S. boydii by methanolic (25.17±0.44) and ethanolic (23.50±0.29) extracts of dried leaves. Maximum antimicrobial response was seen in hexane extract of leaf but present report shows methanolic extract of dry leaf more effective. The solvents dissolve phytochemicals of similar polarity and dissolved chemicals play significant role in showing antimicrobial activities (Altemimi et al, 2017 and Ngo et al, 2017).
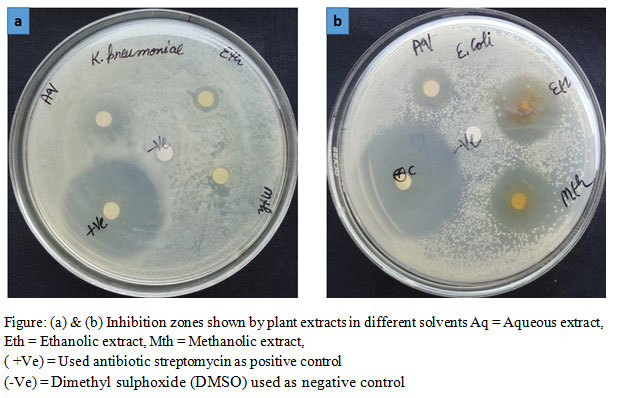 |
Figure: (a) & (b) Inhibition zones shown by plant extracts in different solvents Aq = Aqueous extract, Eth = Ethanolic extract, Mth = Methanolic extract, |
Table 1: Antimicrobial activity of dry leaf extract of Spondias mangifera
| Test organisms | Inhibition zone diameter (mm) | ||||
| Ethanolic extract | Methanolic extract | Aqueous extract | Control | Standard drugs (5μl/disc) | |
| S. aureus | 8.83 ± 0.17 | 6.83 ± 0.17 | 5.67 ± 0.67 | 0.00 ± 0.00 | 35.50 ± 0.29 |
| E. faecalis | 25.00± 0.58 | 8.83 ± 0.44 | 23.88± 0.60 | 0.00 ± 0.00 | 42.67 ± 1.20 |
| E. coli | 7.83 ±0.17 | 8.67± 0.33 | 6.67 ± 0.33 | 0.00 ± 0.00 | 39.17 ± 0.44 |
| K. pneumoniae | 8.67 ± 0.33 | 11.50± 0.29 | 5.83 ± 0.17 | 0.00 ± 0.00 | 38.67 ± 0.33 |
| P.vulgaris | 10.67± 0.33 | 10.5±0.29 | 4.5 ± 0.29 | 0.00 ± 0.00 | 23.00±0.58 |
| S. boydii | 23.50±0.29 | 25.17±0.44 | 11.50±0.29 | 0.00 ± 0.00 | 36.17±0.60 |
Table 2: Antimicrobial activity of green leaf extract of Spondias mangifera
| Test organisms | Inhibition zone diameter (mm) | ||||
| Ethanolic extract | Methanolic extract | Aqueous extract | Control | Standard drugs (5μl/disc) | |
| S. aureus | 8.50 ± 0.29 | 4.67 ± 0.33 | 11.50± 0.76 | 0.00 ± 0.00 | 35.50 ± 0.29 |
| E. faecalis | 9.33± 0.33 | 11.50± 0.29 | 24.17± 0.44 | 0.00 ± 0.00 | 42.67 ± 1.20 |
| E. coli | 10.17±0.44 | 8.17± 0.73 | 9.17 ± 0.60 | 0.00 ± 0.00 | 39.17 ± 0.44 |
| K. pneumoniae | 7.83 ± 0.17 | 15.50± 0.29 | 7.00± 0.58 | 0.00 ± 0.00 | 38.67 ± 0.33 |
| P.vulgaris | 6.00± 0.58 | 12.50±0.29 | 5.17±0.60 | 0.00 ± 0.00 | 23.00±0.58 |
| S. boydii | 6.50±0.76 | 13.8±0.44 | 11.16±0.73 | 0.00 ± 0.00 | 36.17±0.60 |
( ± ) = showing for standard error
Therefore, a wide range of solvents should be tried to get the best response. In general, extracts prepared from green leaves showed less antibacterial potential compared to the extracts of dried leaves. But, aqueous extract of green leaf showed significantly high antibacterial activity against E faecalis (24.17±0.44). Similarly, higher antibacterial activities against E coli were seen by both ethanolic (10.17± 0.44) and aqueous (9.17±0.60) extracts of green leaves. Methanolic extract of green leaf showed maximum inhibition (15.50±0.29) for K. pneumoniae. Thus, present work shows significant antibacterial activity of both green and dried leaf extracts of Spondias mangifera.
Acknowledgements
Financial assistance by University Grants Commission, New Delhi, India is highly acknowledged by first and corresponding author (UGC Major Project:F.No.41-457/2012 (SR) sanctioned to Dr Nishi Kumari)
Conflicts of Interest
The authors declare no conflicts of interest.
Reference
Altemimi A., Lakhssassi N., Baharlouei A., Watson D. G. and Lightfoot D. A. (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants Vol.6 No 42: Pages 1-23
Gupta V. K., Roy A., Nigam V. K. and Mukherjee K. (2010) Antimicrobial activity of Spondias pinnata Resin. Journal of Medicinal Plants Research Vol. 4 No 16: Pages 1656-1661
Jain P., Hossain K. R., Mishu T. R. and Reza H. M. (2014) Antioxidant and antibacterial activities of Spondias pinnata Kurz. leaves. European Journal of Medicinal Plants Vol. 4 No 2: Pages 183-195
Kourkouta L., Kotsiftopoulos C. H., Papageorgiou M., Iliadis C. H. and Monios A. (2017) The rational use of antibiotics medicine, Journal of Healthcare Communications Vol. 2 No 3: Pages 1-4
Li B., Webster T. J. (2018) Bacteria antibiotic resistance: new challenges and opportunities for implant- associated orthopaedic infections. Journal of Orthopaedic Research Vol. 36 No 1: Pages 22-32
Manik M. K., Islam S. M., Wahid M. A., Morshed M. M., Kamal S., Islam M. S. and Ahmed K. T. (2013) Investigation of in vitro antioxidant, antimicrobial and thrombolytic activity of the exocarp of Spondias pinnata (Anacardiaceae). Canadian Chemical Transactions Vol.1 No 3: Pages 191-201
Ngo T. V., Scarlett C. J., Bowyer M. C., Ngo P.D. and Vuong Q.V. (2017) Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. Journal of Food Quality Vol. 2017: Pages 1-8
Singh R., Kumari N. and Nath G. (2016) Free radicals scavenging activity and antimicrobial potential of leaf and fruit extracts of Sapindus mukorossi Gaertn. against clinical pathogen. International Journal of Phytomedicine Vol. 8: Pages 22-28
Tripathi M. And Kumari N. (2010) Micropropagation of a tropical fruit tree Spondias mangifera Willd. through direct organogenesis. Acta Physiologiae Plantarum, Vol. 32 No 5: Pages 1011–1015
Zaidan M. R. S., Noor R. A., Badrul A. R., Adlin A., Norazah A. and Zakiah I. (2005) In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Tropical Biomedicine. Vol. 22 No 2: Pages 165–170


