Division of Microbial Technology, PG and Research Department of Zoology, Chikkanna Government Arts College, Tirupur, Tamilnadu, India
Corresponding author Email: nanochithira@gmail.com
Article Publishing History
Received: 21/10/2017
Accepted After Revision: 19/12/2017
Recurrently typhoid fever, caused by Salmonella typhi, remains a significant cause of mortality and morbidity in many regions of the world. So predominant pathogen S.typhi is one of the major causes of food and water borne gastroenteritis in human and remains an important health problem. So fecal samples were collected from the poultry retail shop in tirupur city. Totally 50 multidrug resistant Salmonella spp were isolated from 75 fecal samples and confirmed by using routine laboratory techniques. Later, the antimicrobial pattern of this isolates were studied by using 11 antibiotic discs which include Amikacin (10mcg), Co-trimoxazole (25mcg), Ciprofloxacin (30mcg), Tetracycline (30mcg), Cephalothin (30mcg), Ceftriaxone (30mcg), Entrofloxacin (10mcg), Gentamicin (10mcg), Ampicillin (10mcg), Trimethoprim (10mcg), Cefoxitin (30mcg). Among these strains (12%), (62%), (28%), (80%), (12%), (4%), (6%), (26%), (36%), (100%), and (8%) were found to be exhibit a significance degree of resistance to different groups of antibiotics. Further, plasmid profile were performed for the five multidrug resistance isolates and observed the molecular weight was 1500bp and 700bp respectively. Recurrently, the metal oxide nanoparticles are currently the most promising tools applied as antimicrobial agents for diagnosis of diseases. Nanoparticle Zirconium oxide was used to against Salmonellaspp. Different concentration of Zirconium oxide 50μl, 100μl and 150μl were used against Salmonella spp. Among the three concentration of nanoparticle, maximum zone of inhibition 16mm was observed against the isolate CH36 at 150μl concentration of nanoparticle. Minimum zone of inhibition 13mm was observed against the isolate CH37. So hence the present study Zirconium oxide was used and it shows prominent antibacterial activity against typhoid causing organism.
Typhoid Fever, Salmonella Typhi, Antibiotic Resistance, Plasmid Profile And Zro2
Chithira A, Farheena M. I, Mohankumar A. Antagonistic Activity of Zro2 Against Typhoid Fever Causing Salmonella Typhi, Isolated from Retail Poultry Shops in and Around Tirupur District. Biosc.Biotech.Res.Comm. 2017;10(4).
Chithira A, Farheena M. I, Mohankumar A. Antagonistic Activity of Zro2 Against Typhoid Fever Causing Salmonella Typhi, Isolated from Retail Poultry Shops in and Around Tirupur District. Biosc.Biotech.Res.Comm. 2017;10(4). Available from: https://bit.ly/2XLCRUS
Introduction
Typhoid fever is a major health problem in developing countries, for thousands of years, thriving in conditions of poor sanitation, crowding and social chaos, contaminated water, milk, food or fruits vegetables or via convalescent or chronic carrier (Harish Menezes, 2011). Typhoid fever is the most serious form of enteric fever and in 2000 it was estimated that the global number of typhoid causes exceeded 21,00,000 with more than 2,00,000 death. Globally, up to 27 million infections occur per year, with over 2×105 attributable deaths annually, predominantly among children under the age of five years (Clark et al., 2010). The predominant Salmonella species are Gram-negative rod shaped bacteria that are members of the family Entero bacteriaceae and are considered threatened food borne pathogens facing food safety and public health. Salmonella is a serious threat facing poultry industries as it has the ability to infect chickens causing diarrhoea. In the last few years, consumption of contaminated poultry, eggs, and their products become the most common sources of foodborne human salmonellosis (Mahmoud et al., 2015; Park et al., 2015; Hsu et al., 2016). Arena et al., 2017; Pashazadeh et al., 2017 and Kalupahana et al., 2017).
A variety of food products, especially poultry and other types of meat products, are the most important sources of human Salmonella spp. infection, but water borne outbreaks have also occurred birds are mainly infected through feed, drinking water or environmental sources. The organisms route of infection is the faecal – oral route via food or water contaminated with faeces or urine of previously infected persons or animals. Most environmental concerns over land application of animal manure have focussed on either the effect of applied nutrients, especially N and b.
Although poultry production is considered as secondary agricultural production systems and it has an important role in high quality protein. Poultry provide globally important sources of animal protein and are amongst the most intensively reared of all livestock species several microbial diseases have been affecting the poultry and it is a major concern, both locally and international levels. The low productivity is mainly due to high mortality, which is caused particularly by bacterial disease and the mortality has been estimated in the range of 80-90% (Debnam and Jackson, 2005).
Food borne diseases are main problems, particularly in developing countries and cause the majority of illnesses and death around the world. Food is the most important vehicle that transmits the microorganisms to human (Varnam, 1991) among microorganisms Salmonella still a major cause of food – borne human disease in most parts of the world (Soultose et al., 2003 and Carraminana et al., 2004). Poultry and poultry products are frequently contaminated with Salmonella that can be transmitted to humans through the handling of raw poultry carcasses and products, or through consumption of undercooked poultry meat (Kimura et al., 2004). Young chick, mortality up to 100%, week chicks, loss of appetite, diarrhoea, and adult birds: no sings depression, diarrhoea, and drop in egg production, low mortality. The poultry farms bird flu has become a lethal condition that is occurring around the world more frequently (Julie et al., 2004).
| Table 1: Antibiotic Resistant Percentage of Salmonella typhi | ||
| S: No | Antibiotics | Percentage of resistant |
| 1 | Amikacin | 12% |
| 2 | Ceftriaxone | 4% |
| 3 | Ciprofloxacin | 28% |
| 4 | Cephalothin | 12% |
| 5 | Entrofloxacin | 6% |
| 6 | Gentamicin | 26% |
| 7 | Ampicillin | 36% |
| 8 | Cefoxitin | 8% |
| 9 | Tetracycline | 80% |
| 10 | Trimethoprim | 100% |
| 11 | Co trimoxazole | 62% |
The sub therapeutic use of antibiotics in poultry has become a popular practice and these is a growing body of scientific evidence to the effect that the increasing incidence of antibiotic resistant bacteria is closely associated with the heavy use of these antibiotics in poultry and other related agricultural practices. Despite the great progress in antimicrobial development, many infectious diseases, especially intracellular infections, remain difficult to treat. One major reason is that many antimicrobials are difficult to transport to cell membranes and have low activity inside the cell, there by imposing negligible inhibitory or bactericidal effects on the intracellular bacteria (Zhang et al., 2010).
In 2013 many techniques were used by several researchers to Salmonella spp. but these techniques could not completely cure Salmonella spp. Pathogenic bacteria still remain a major health concern, which are responsible for causing a large number of deaths and hospitalizations each year. Although we have current treatments such as antibiotics, bacteria are gaining resistance to these therapeutics at an alarming rate. That is why new therapeutic and diagnostic treatments are necessary if we want to be prepared against known and unknown pathogenic bacterial infections. A large group of these studies includes the implementation of nanotechnologies and nanomaterials to create new antibacterial nano-medicines that increased effectiveness and efficiency.
 |
Plate 1: Isolated colonies of Salmonella typhi |
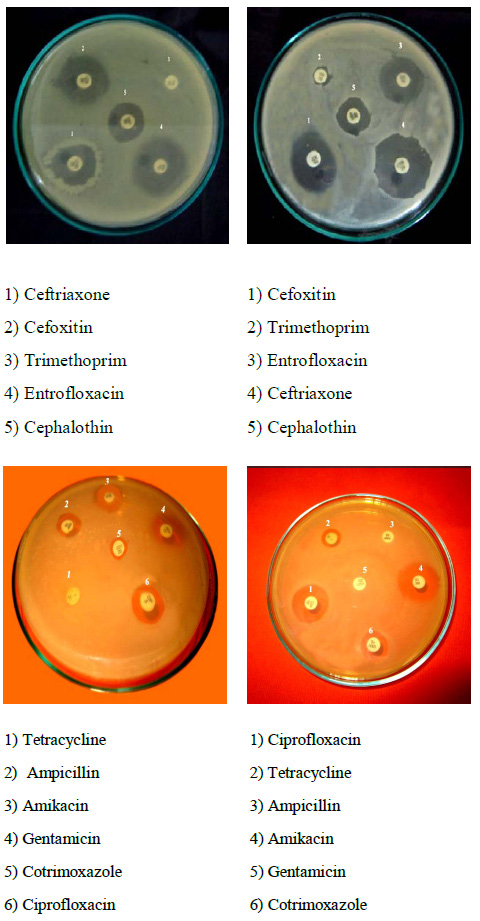 |
Plate 2: Antibiotic susceptibility test of Salmonella typhi |
Moreover, nanotechnology is through to be technology of the future with several opportunities for applications one of the most important nanotechnology applications areas that hold the expectations of providing create benefits for humanity in the future is medicine (Neuberger et al., 2005). Therefore, it is important to find another efficient treatment for Salmonella infection instead of antibiotic. In the last few years, there has been a growing interest in nanotechnology. Indeed, nanoparticles have been gaining importance in recent years and became an effective revolution therapy against pathogenic bacteria due to their bactericidal properties. The nanoparticles size and surface area are significant agents to which their bactericidal mechanism of action attributed to several pathogens (Devi et al., 2017).
ZrO2 nanoparticles with antimicrobial activity when embedded and coated and the surface can find immense applications in water treatment, synthetic textiles, biomedical and surgical device, food processing and packaging. Moreover, the composites prepare using ZrO2 and polymers can find better utilization due to the enhanced antimicrobial activity.The multi drug resistant pathogens due to antigenic shift are ineffectively managed with current medications. This resistance to medication by pathogens has become a serious problem in public health and therefore mandating the need to develop new bactericides and virucides. Zirconium oxide nanoparticle (ZrO2), having a long history of general use as an antiseptic and disinfectant, are able to interact with disulphide bonds of the glycoprotein / protein contents of microorganisms, viruses, bacteria, fungi. The ZrO2 nanoparticle change the three dimensional structure of proteins by interfering with S- bonds and block the functional operation of the microorganisms. ZrO2 nanoparticles with antimicrobial activity when embedded and coated and the surface can find immense applications in water treatment, synthetic textiles, biomedical and surgical device, food processing and packaging. Moreover, the composites prepare using ZrO2 and polymers can find better utilization due to the enhanced antimicrobial activity.
Recently the antibiotics such as tetracycline, amikacin, co-trimoxazole ciprofloxacin, cephalothin, ceftriaxone, entrofloxacin, gentamicin, ampicillin, trimethoprim, cefoxitin are used for the poultry bacterial disease. One of the earliest nanomedicine applications particularly, an antimicrobial agent from ZrO2 nanoparticle for the treatment of various microbial diseases is being emerged. However, studies related the ZrO2 nanoparticle against S.typhi is too limited. Hence, the present study has been made an attempt to point out the bioactive medical properties of metal oxide nanoparticle (ZrO2) against Salmonella typhi isolated from retail poultry shop in and around Tirupur District.
Materials and Methods
Sterile spatulas were used to collect samples of freshly passed poultry droppings in sterile universal sampling bottles. 75 samples were collected from different poultry retail shop in Tirupur city. The droppings were collected from litter at random points and transported to the laboratory where they were analyzed within one hour from the time of collection. Pre-isolation enrichment of the faecal samples were carried out by inoculating each sample directly in to tryptone soy broth (TSB) and incubated at 35oC for 18-24 hrs. Immediately after enrichment, the organisms were serially diluted from 10-1to 10-9 and the dilutions 10-4 to 10-6 were plated on to Xylose lysine decorboxylase media (XLD) agar it was inoculated at 35oC for 24 hours onto XLD agar plate for the isolation of strains of Salmonella spp. respectively. The individual colonies with different morphology were picked using sterile tooth pick and grown in Tryptone soy broth and it was incubated at 37oC for 24 hours. Further it was plated to check for purity.
The isolated bacteria were primarily identified on the basis of the Gram staining, IMViC, Citrate Utilization, Triple sugar Iron agar, Nitrate reduction, Motility, Catalase, Oxidative. All Salmonella typhi. Strains used in this study were grown in TSB broth at 37oC for 24 hours. The following antibiotic discs: Amikacin, Co-trimoxazole, Ciprofloxacin, Tetracycline, Cephalothin, Ceftriaxone, Entrofloxacin, Gentamicin, Ampicillin, Trimethoprim, Cefoxitin were used for antibiotic sensitivity assay.
Selected colonies were inoculated into nutrient broth then incubated at 37 oC for 12 hrs. These cultures were used for further experiment.In this present study antibiotic susceptibility of Salmonella typhi was performed using Kirby Bauer disc diffusion method (1979). Plasmid were isolated from Salmonella typhi using the method of alkaline lysis (Niels, 1994) and the presence of plasmid was checked by 0.7% agarose gel was with visualized under UV light on transilluminator and photographed. Size of the plasmids was determined with the help of the standard molecular marker.
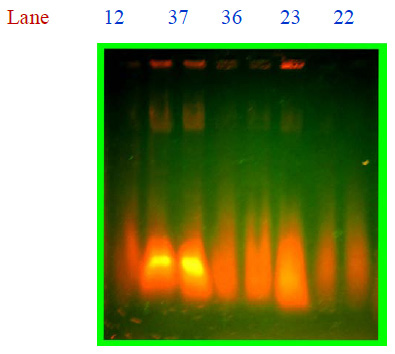 |
Plate 3: Isolation of plasmid from Salmonella typhi |
The antibacterial activity of the ZrO2 nanoparticles was performed by using well diffusion method. About 20 ml of sterile molten Mueller Hinton agar was poured into the sterile petriplates. Triplicate plates were swabbed with the overnight culture (108cells /ml) of pathogenic bacteria Salmonella spp. Different concentration of nanoparticles (50μl, 100μl, 150μl) was prepared with DMSO. The different concentrations of nanoparticles were screened against fifty isolates of Salmonella spp. The isolates were selected on the basis of Salmonella spp. should above 50% antibacterial activity against the antibiotics tested: Amikacin, Co-trimoxazole, Ciprofloxacin, Tetracycline, Cephalothin, Ceftriaxone, Entrofloxacin, Gentamicin, Ampicillin, Trimethoprim, Cefoxitin. The solid medium was gently punctured with the help of cork borer to make a well. Finally the nanoparticle samples with the concentration: 50μl, 100μl, 150μl were added from the stock into each well and incubated for 24h at 37±2oC. After 24 hrs of incubation, the zone of inhibition was measured and expressed as millimetre in diameter.
 |
Plate 4: Activity of Zro2 Nanoparticle against of Salmonella typhi |
Results and Discussion
Totally 75 samples were collected from different poultry retail shops in Tirupur city. 50 isolates of Salmonella typhi were isolated from the samples. The Salmonella typhi strains were confirmed by comparing the results with standard biochemical test of Salmonella typhi such as gram negative in rod shape as result of gram staining. Indole negative, MR-VP- positive, Voges Proskauer – negative, Citrate positive, positive results were observed in case of Catalase, Nitrate reduction, Motility, Triple sugar iron agar, Oxidase. Selective media like XLD (Plate: 1) and Mac-Conkey agar media were used to isolate the Salmonella typhi. It showed black centre colony and white colony respectively. These colonies were isolated and stored for further experiment.
Antimicrobial susceptibility patterns were determined by using commercial antimicrobial disc (HIMEDIA, Mumbai): Amikacin (10mcg), Cotrimoxazole (25mcg), Ciprofloxacin (10mcg), Tetracycline (30mcg), Cephalothin (30mcg), Ceftriaxone (30mcg), Entrofloxacin (10mcg), Gentamicin (10mcg), Ampicillin (10mcg), Trimethoprim (10mcg), and Cefoxitin (30mcg). Antimicrobial susceptibility testing was performed in accordance with the standard guidelines of Kirby – Bauer (1979) disc diffusion method.
Totally 11 antibiotic discs were used for this assay, among that strain CH12 showed maximum resistance of 72.72% and the antibiogram AK- TET- COT- CTR- CEP- GEN- AMP-TR was recorded. Strain CH32 showed minimum resistance of 9.09% and the antibiogram TR was recorded (Plate: 2). The isolates were analyzed for antibiogram as described to determine the antibiotic susceptibility pattern along with the tendency of current resistance against widely used drugs. Among 50 isolates, 30 different antibiogram were found in this study and the resistance was found against Amikacin (12%), Cotrimoxazole (62%), Ciprofloxacin (28%), Tetracycline (80%), Cephalothin (12%), Ceftriaxone (4%), Entrofloxacin (6%), Gentamicin (26%), Ampicillin (36%), Trimethoprim (100%), and Cefoxitin (8%) (Table: 1).
Five strains CH12, CH22, CH23, CH36 and CH37 showed more than 50% percentage frequency among the fifty isolates of Salmonella typhi.
Multiple Antibiotic Resistance (MAR) index was calculated according to the formula
Maximum MAR index 0.7272 was showed by CH12 and minimum MAR index 0.0909 was showed by CH32. Strains which showed more than 50% resistance was taken for isolation of plasmid, by the following method of Niels (1994), two fragments were obtained from the strains: CH12, CH22, CH23, CH36, and CH37, but all the strains were plasmid born Salmonella spp. 100bp DNA ladder (MEDOX, Chennai), was used to know the molecular weight of the strain, it showed 1500 bp and 700 bp (Plate:3). The medical application of nanoparticles is gaining popularity with an increasing number of nanoparticle based therapeutics currently in clinical development. We expect that with the introduction of safer nanomaterials together with novel engineering approaches that result in optimally designed nanoparticles, enter the clinic in future.
Different concentration of nanoparticle Zirconium oxide 50μl, 100μl, and 150μl were prepared with Dimethyl sulphoxide (DMSO), well diffusion method was used; Different concentration of nanoparticle were impregnated into well on the seeded Mueller Hinton Agar (MHA) media. The plates were incubated at 37oC for 24hrs. Zone of inhibition was recorded (Plate 4). The strains CH12, CH22, CH23, CH36 and CH37 which showed more than 50% resistant against 11 antibiotics were used to test against Zirconium oxide Nanoparticle. Salmonella spp.
Among the three concentrations of nanoparticles tested against five strains, maximum zone of inhibition (16mm) was observed against CH36 at 150μl, followed by 15mm against CH23 at 150μl. The minimum zone (13mm) was recorded in the strain CH37. Although in many areas of endemic city in Asia and the Indian subcontinent typhoid outbreaks in Sub-Sharan Africa are rarely documented, and data on incidence and antimicrobial susceptibility patterns are scarce. The observed rise in MDR S.typhi in Kenya is particularly alarming. For example during the period of their study, most of the S.typhi isolates from blood culture of patients prior to 1993 were fully sensitive to all antimicrobials (Bhay et al. 2005). But in the present study the strain CH12 showed maximum resistance 72.72% against all the antibiotics tested. Salmonella typhi recorded 100% resistance to tetracycline, 66.7% resistance to gentamicin and ampicillin respectively. These antibiotics are very common and are readily available as over the counter drugs to consumers in Nigeria (Funso Omojaasola and Folakemi Omojaasola, 2001). 80% of resistance showed by tetracycline, 26% resistance to gentamicin, and ampicillin showed 36% of resistance in the present study.
The resistance pattern of 101 strains of S.typhi to 11 drugs was determined by using the plate dilution method. All 101 strains tested were inhibited by Cephalothin; Gentamicin, (Olarte and Galindo, 1973), similar method was followed in the present investigation, 50 strains of S.typhi isolated were inhibited by Cephalothin (12%) and Gentamicin (26%).A total of 323 S.typhi isolates from three hospitals covering the Nairobi region of Kenya, 54 (16.7%) isolates were fully susceptible to all eight antibiotics tested, (Kariuki et al. 2010), 50 strains of S.typhi isolated from retail poultry shop in around Tirupur, in all isolates (2%) was fully susceptible to all eleven antibiotics tested. Further the authors were reported that a total of 74 (22.9%) isolates were resistant to ampicillin or tetracycline, similar results was recorded in the study but total of fifty isolates showed 36% and 80% resistant to Ampicillin and tetracycline respectively.
One hundred and thirty two (132) bacteria were isolated from 1000 cow dung samples, among that 18 isolates of Salmonella typhi were cultured. 100% resistance to tetracycline by all the isolates, ampicillin showed 85.6% resistance (Omojowo and omojasola, 2013), in the present research work 75 faecal samples of retail poultry shop, were collected among them, 50 isolates of Salmonella typhi were isolates, ampicillin showed 36% resistance against all tested antibiotics and tetracycline showed 80% resistance.Six strains of Salmonella typhi were resistant to ampicillin, trimethoprim, tetracycline and gentamicin, these strains were isolated from the blood with typhoid patients. These strains showed multiple antibiotics resistance (MDR) and all the six strains were harbored plasmid about 50 kb (Pai et al. 2003). Similar results was obtained, fifty strains of Salmonella typhi were showed resistant to all tested antibiotics expect trimethoprim and all strains showed multiple antibiotic resistant. These strains were harboured plasmid about 1500 bp and 700 bp.
Hirose et al. (2001) focused the antibiotics susceptibilities of 62 strains of S.enterica serovar typhi of S.enterica serovar paratyphi were investigated with 18 antibiotics. Eighteen S.enterica serovar typhi isolates and five S.enterica serovar paratyphi A isolates were resistant to one or more antimicrobial agents among which 10 S.enterica serovar typhiisolates were susceptibility against ciprofloxacin. But our findings showed the isolates Salmonella typhi were showed antibiotics susceptibility against all tested antibiotics except trimethoprim, and these strains were resistant to one or more antibiotics.
Sisak et al. (2006) isolated 126 Salmonella spp from pigs were tested against 14 antibiotics. They found that the isolates showed resistance 1-8 antibiotics, S.typhimurium strains were found to the most resistant to streptomycin (91.5%), sulphonamides (88.1%), ampicillin (86.4%), and chloramphenicol (83.0%), in the present study, eleven antibiotics were tested against the isolates, among that ampicillin showed lesser percentage of inhibition while compare with work done by Sisak
et al. (2006), but it showed 36% resistance only. Ashok et al. (2010) reported that they collected data for 2007 and 2008, ampicillin showed 18.2% and 52.8% of resistance against Salmonella typhi, there as tetracycline showed resistance of 9% and 33.4%, none of the antibiotics resistance against ciprofloxacin and ceftriaxone antibiotics, there uses controversy while compare the results with present study, tetracycline showed second highest resistance (80%), ciprofloxacin 28%, ceftriaxone 4% showed resistance.
The antibacterial potential of metal oxide nanoparticles viz, Al2O, Fe3O4, CeO2, ZrO2 and MgO against poultry pathogens: Klebsiella spp., E.coli, Staphylococcus spp. and Salmonella spp. The ZrO2 showed maximum antibacterial activity against Salmonella spp. followed by E.coli respectively. The author reported in their study that, the ZrO2nanoparticles could be used as effective antibacterial agent against poultry pathogens. In the present investigation, ZrO2 was used as a nanoparticles against the Salmonella spp isolates which is isolated from retail poultry shop. Totally five isolates CH12, CH22, CH23, CH36, and CH37 of Salmonella typhi were tested against different ZrO2 concentration (50μl, 100μl, and 150μl), as the zone of inhibition were rapidly increased from 50μl – 150μl concentration of nanoparticles. Among five isolates tested, CH36 showed maximum zone of inhibition (16mm).
Mrithunjai singh et al. (2008) found that the nanoparticles increase chemical activity due to crystallographic surface structure with their large surface to volume ratio. They used silver ions and silver based compounds including silver nanoparticles, there has promoted research and this effect was size and dose dependent and was more pronounced against Gram – negative bacteria than Gram – positive organisms, in the present investigation, ZrO2 was used as a nanoparticles, this showed maximum activity, the activity was dependent on dose.
Conclusion
Development of resistance to antibiotics by bacteria is inevitable, not only because of their rates in mutation and transferability of drug resistant genes. This constitutes a significant public health risk due to possible cross-contamination with antibiotic resistant bacteria of food and drinking water meant for public consumption, which always culminates in human illnesses, mostly typhoid fever. The growing incidence of multi-drug resistant Salmonella typhi has become a global phenomenon and antibiotic resistant bacteria are increasingly isolated from a wide array of sources, in the clinical environments, poultry. There is some scientific evidence of the growing rate of recovery of antibiotic resistant S. typhi from poultry products. So It is concluded from the present study that species of Salmonella typhi isolated from retail poultry shop in around Tirupur Dt. The rapid emergence of drug resistant strains of microbial Salmonella typhi pathogen especially those with multi drug resistance characteristics and the organism link with a plasmid. Due to the development of drug resistant urgently need new therapeutic among drug to combat the infectious disease. So in this study the biomedical properties of metal oxide nanoparticles was used in different concentration to treat against multidrug resistant Salmonella typhi.
References
- Arena, E.T., Tinevez J, Nigro G, Sansonetti P.J, Marteyn B.S, (2017). The infectious hypoxia: occurrence and causes during Shigella Microbes Infect, 19(3): 157-165.
- Ashok, R., K. Peter, N. Joselyne, and N. Emma, (2010). Antimicrobial susceptibility patterns of Salmonella typhifrom Kigali, Rwanda. Shiraz E- Medical Journal, 11(3): 117 – 121.
- Carraminana, J. J., C. Rota, I. Agustin, and A. Herrera, (2004). High Prevalence of multiple resistance to antibiotics in Salmonellae serovarsisolated from a poultry slaughter house in Spain. Vet. Microbia., l 104: 133-139.
- Clark, T. W., C. Daneshvar, M. Pareek, N. Perera, and I. Stephenson, (2010). Enteric fever in a UK regional infectious diseases unit: A10 year retrospective review. J Infect., 60(2): 91-98.
- Crump, J. A., S. P. Luby, and E. D. Mintz,(2004). The global burden of typhoidal fever. Bull World Health Organ, 82: 346-353.
- Debnam, A. L., and C. R. Jackson, (2005). Effect of growth promotant usage on enterococci species on a poultry farm. Avian Dis., 49: 361-365.
- Devi, G.K., Kumar K.S, Parthiban R, Kalishwaralal K, (2017). An insight study on HPTLC fingerprinting of Mukia maderaspatna:Mechanism of bioactive constituents in metal nanoparticle synthesis and its activity against human pathogens. Microb. Pathog, 102: 120-132.
- Harish, B. N., and G. A. Menezes, (2011). Antimicrobial resistance in typhoidal Salmonellae. Indian Journal of Medical Microbiology, 29(3): 223-9.
- Hsu, Y. M., Yu B, Tseng C. S, Chang C. H, Chen D.S., Su C.H, Chen Y.S, (2016). Preventive activities of Scutellariae radix, Gardeniae fructus, and probiotics in Salmonella entericaserovar typhimurium infection in chickens. Anim. Feed Sci. Technol, 214: 121–129.
- Hyunjoo Pai., Jeong Hum Byeon, Sunmi Yu, Bok kwon Lee, and Shukho Kim, (2003). Salmonella enterica serovar typhi strains isolated in Korea containing a multidrug resistance class 1 integron. Antimicrobial Agents and Chemotherapy, 47(6): 2006 – 2008.
- Kalupahana, R.S., Rajapaksa D.I.G, Fernando P.S, Thilakarathne D.S, Abeynayake P,(2017). Occurrence and characterization of nontyphoidal Salmonellain retail table eggs in Kandy district of Sri Lanka. Food Control, 72: 244-248.
- Kenji Hirose., Kazumichi Tamura, Hiroko Sagara and Haruo watannabe, (2001). Antibiotic susceptibility of Salmonella enteric serovar paratyphiA isolated from patients in Japan. Antimicrobial Agents and Chemotherapy, 45(3): 956 -958.
- Kimura, A. C., V. Reddy, and R. Marcus, (2004). Chicken consumption is a newly identified risk factor for sporadic Salmonellae enteric serotype enteritidisinfections in the United States. Clin. Infect. Dis., 38: 244-252.
- Mahmoud, B.S.M., Chang S, Yuwei W, Nannapaneni R, Sharma C.S, Coker R, (2015). Effect of X-ray treatments on Salmonella entericaand spoilage bacteria on skin-on chicken breast fillets and shell eggs. Food Control, 57: 110-114.
- Mritunjai Singh., Shinjini singh, S. Prasad, and I. S. Gambhir, (2008). Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Digest Journal of Nanomaterials and Biostructures, 3(3): 115 – 122.
- Namagirilakshmi, S., Selvaraj P, Nanjappan k, Jayachandran S, Visha P, (2016). Turmeric (Curcuma Longa) as an alternative to in-feed antibiotic on thegut health of broiler chickens. Tamilnadu. J. Vet. Anim. Sci, 6: 148–150.
- Neuberger, T., B. Schopf, H. Hofmann, M. Hofmann, B. Von Rechenberg, (2005). Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. Magn. Mater., 293: 483-496.
- Niels Bohr., (1998). An improved method for the isolation of supercoiled plasmid DNA. Current Science, 74: 572 – 573.
- Park, S., Choi S, Kim H, Kim Y, Kim B, Beuchat L.R, Ryu J, (2015). Fate of mesophilic aerobic bacteria and Salmonella entericaon the surface of eggs as affected by chicken feces, storage temperature, and relative humidity. Food Microbiol, 48: 200-205.
- Samuel Kariuki., Gunturu Revathi, John Kiiru, M. Doris mengo, Joyce Mwituria, Jane Muyodi, Agnes Munyalo, Y. Yik Teo, E. Kathryn Holt, A. Robert Kingsley, and Gordon Dougan, (2010). Typhoid in Kenya is associated with a dominant multidrug resistant Salmonella enterica serovar typhihaplotype that is also widespread in Southeast Asia. Journal of clinical Microbiology, 48(6): 2171-2176.
- Shi, R., Yang X, Chen L, Chang H, Liu H, Zhao J, Wang X, Wang C, (2014). Pathogenicity of Shigellain Chickens. PLoS ONE, 9(6): e100264.
- Sisak, F., H. Havlickova, H. Hradecka, I. Rychlik, I. Kolackova, and R. Karpiskova, (2006). Antibiotic resistance of Salmonella isolates from pigs in the Czech Republic. Veterinarni Medicina., 51(5): 303- 310.
- Soultose, N., P. koidis, and R. H. Madden, (2003). Prevalence of listeriaand Salmonellae in retail chicken in Northern Ireland. Appl. Microbiol., 37: 421-423.
- Tseng, C., Yen Y, Chang C, Hsu Y, (2014). Polymorphism of gene cassette promoter variants of class 1 integron harbored in choleraesuisand typhimurium isolated from Taiwan. Biomedicine, 4: 1–6.
- Varnam, A. H., (1991). Food borne pathogens 1st Edn. Wolfe publication Ltd, 71-76.
- Xiangqian, Li., X. U. Huizhong, Zhe-sheng Chen, and Guofang chen, (2011). Biosynthesis of nanoparticles by microorganisms and their applications. Journal of Nanomaterials, 10: 1155.
- Xu, L.J, Wang C.Q, Hu G.Z, Kang X.T, Ren J, et al (2004). Discovery on shigellosis of flock in china and studies on the pathogenic specialty. Chin J Prev Vet Med, 26: 281–286.
- Zhang, L., D. Pornpattananangkul, C. M. J. Hu, and C. M. Huang, (2010). Development of nanoparticles for antimicrobial drug delivery. Current Medicinal Chemistry, 17: 585-594.


