1Department of Advance Zoology & Biotechnology, Guru Nanak College, Velachery, Chennai, Tamilnadu – 600042.
2Bioinformatics & Entomoinformatics Lab, Department of Genetic Engineering, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur – Tamilnadu – 603203.
Corresponding author email: habeeb_skm@yahoo.co.in & habeebm@srmist.edu.in
Article Publishing History
Received: 17/04/2020
Accepted After Revision: 15/06/2020
Plant secondary metabolites are being extensively screened for various therapeutic purposes with the help of virtual screening. Annonaceous acetogenins are a large class of secondary metabolites known to possess various therapeutic activities. These have proven to efficiently regulate cancer in brine shrimp. Molecular modeling plays a pivotal role in computer aided drug design. In this study, based on literature survey, three major acetogenins were tested for their binding affinity to the target Homo sapiens mitochondrial NADH Dehydrogenase subunit-I. The i-Tasser 3D model was energy minimized. Acetogenins Annonin-I, Squamocin-C & Squamocin-D were docked with target using Schrodinger’s Glide and the analysis was done based on docking score and hydrogen bonding between the molecules. The acetogenin annonin-I had the best binding affinity and a molecular dynamics simulation using Desmond package was done for 30 ns (NPT ensemble). Trajectories were plotted against time to assess potential energy, RMSD and RMSF between apo and NADH1-annonin-I complex. Hydrogen bonding involving residues Thr108 and Ser159 facilitating the binding of NADH1 and annonin-I were maintained throughout the simulation and the system was nicely equilibrated. The ADME properties were also predicted. Annonin-I was tested against HCT-116 cancer cells for its efficacy with the help of MTT assay and the in-vitro results showed anticancer activity in favour of annonin-I. At the 100 µM concentration of the annonin-I, only 46% growth of the HCT-116 cells was observed.
MTT assay, Acetogenins, Annonin-I (Squamocin A), Molecular Modeling & Docking, NADH Dehydrogenase Subunit-I (ND1), HCT-116
Ghosh S, Mohideen H. S. Annonaceous Acetogenin Annonin – I Inhibits Hct116 Colorectal Cancer Cells Proliferation by Acting on Mitochondrial Nadh Dehydrogenase-1. Biosc.Biotech.Res.Comm. 2020;13(2).
Ghosh S, Mohideen H. S. Annonaceous Acetogenin Annonin – I Inhibits Hct116 Colorectal Cancer Cells Proliferation by Acting on Mitochondrial Nadh Dehydrogenase-1. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2WhFu29
Copyright © Ghosh and Mohideen This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Computer aided drug design (CADD) is one of the tools which can be used to increase the efficiency of the drug discovery process (Ooms, 2012). Molecular modeling describes the generation, manipulation or representation of three-dimensional structures of molecules and associated physico-chemical properties (Nadendla, 2004; Wade and Salo-Ahen, 2019). The orientation of a ligand and its affinity to the receptor helps to predict the different binding modes of a ligand – receptor complex; this complex could further be assessed for its behavioral changes by molecular dynamics simulation (Andrew, 2001). Application of CADD has already resulted in discovery of novel therapeutic compounds in the treatment of variety of diseases (Baig et al., 2017; Yang et al., 2018; Dar et al., 2019).
Cancer is a fatal disease attributed to life style and environmental factors which are responsible for the mutation in vital genes related to cell regulatory proteins. This leads to abnormal cell growth surrounding the normal tissues and destroys vital organs(Ruddon W, 2007; Hassanpour and Dehghani, 2017). With an estimation of over 1 million new diagnosed cases; colorectal cancer is the second most familiar malignancies in the United States and clocks approximately 608700 deaths worldwide every year (Jemal et al., 2011) and it is forecast approximately 147,000 may die in 2020 (Siegel et al., 2020). Diagnosis of cancer has proven to be a daunting task for the scientific community over the years. Chemotherapy and anti-hormone therapies have shown good results in treating cancer (Huang et al., 2017; Stevanato Filho et al., 2017; Falzone, Salomone and Libra, 2018; Ji, Sundquist and Sundquist, 2018).
Present studies focus on gene manipulating and molecular genetic analysis. Nowadays, a group of biologically active plant secondary metabolites termed as phytochemicals are known to reduce the carcinogenicity (Wang et al., 2012; Seca and Pinto, 2018; Shin et al., 2018). Phytochemical substances such as Resveratrol (Feng et al., 2016) and Bufalin have been proven to be inducing apoptosis in human colorectal cancer (Wang et al., 2015).
Similarly, the custard apple family Annonaceae, consists of various biologically active acetogenins. Structurally, they belong to a series of C-35/C-37 natural products, which possess a terminal g-lactone ring and a terminal aliphatic side chain connected with some oxygen-bearing moieties and several hydroxyl groups (Alali, Liu and McLaughlin, 2010; Liaw et al., 2010, 2016). They are known to inhibit mitochondrial NADH Dehydrogenase family of enzymes leading to ATP synthesis (Pomper et al., 2009; Alali, Liu and McLaughlin, 2010). Structure activity relationship (SAR) studies for acetogenin compounds have been found out on cancer cell cultures, brine shrimp, mosquito larvae, isolated mitochondria and mitochondrial fragments, MDR human mammary MCF7/Adr cells (Oberlies, Chang and McLaughlin, 1997) and their positioning in the cell membranes are predicted by nuclear magnetic spectroscopy (Shimada et al., 1998). Specifically, squamocin-C, D and annonin-I are proven to possess significant cytotoxic activity when tested upon brine shrimp and five in-vitro cell lines (Abdel-lateff et al., 2009; Md Roduan et al., 2019).
Studies have also reported the selective action of these acetogenins on the target NADH Dehydrogenase subunit I (ND1) between the normal and tumor cells (James Morré et al., 1994; González-Coloma et al., 2002; McLaughlin, Benson and Forsythe, 2010). As the mitochondria of tumor cells are not involved in the cellular ATP production; tumor cells which rely hugely on glycolysis for energy production and consume approximately 19 times more glucose than the normal cell, therefore, normal cells may not get affected due to acetogenins (González-Coloma et al., 2002).
In this study, the efficacy of squamocin-C, squamocin-D and annonin-I are tested against the target Homo sapiens NADH Dehydrogenase Subunit-I (ND1) for anti-cancerous activity by molecular docking studies. The ADMET properties of the selected compounds was predicted and compared with that of the available drugs in the market. The best performing docked complex was subjected to molecular dynamics simulation to further understand the behavioral changes in the docked complex. This compound was further tested for its efficacy by performing MTT cell line assay against HCT-116 cell lines in-vitro.
MATERIAL AND METHODS
Sequence and structure retrieval: The sequence data for Homo sapiens mitochondrial NADH Dehydrogenase subunit1 (ND1) was obtained from the NCBI database (Accession #: ABH03920.1) consisting of 318 amino acids. Acetogenins Annonin-I (CID 441612), Squamocin C (CID 44307147) and Squamocin D (CID 164503) (Fig 1a) were downloaded from the pubchem database (Kim et al., 2019) and were converted to the required format by using Ligprep module of Schrodinger.
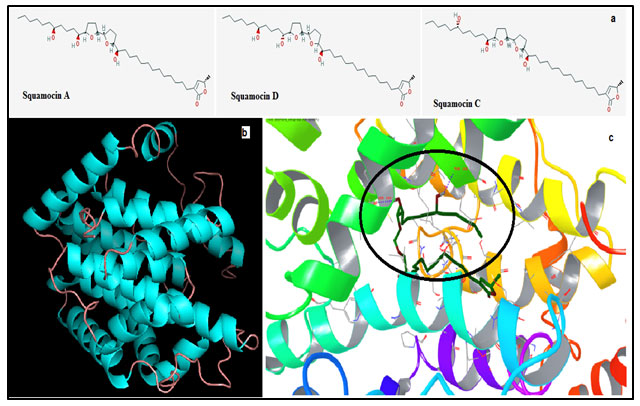
Figure 1: (a) Structure of the three acetogenins Squamocin A (Annonin – I), C & D. (b) Modeled structure of ND1. (c) Shows interactions between the best docked pose ND1 – Annonin – I complex. Circle emphasizes the region of hydrogen bond formation and the residues involved.
Molecular Modeling, Docking & Dynamics
The 3D structure of the target sequence was modeled by threading approach using a web server iTasser (Yang et al., 2014). This structure was used as a target for our study and the binding site of the target was predicted with the help of the Sitemap. Molecular docking of the target structure and the three acetogenins selected for the study was performed using Glide. ADME properties were predicted using the Qikprop module of Schrodinger.
Molecular dynamics simulations for the human ND1-Annonin-I docked complex was performed using Desmond (version 3.2, D. E. Shaw Research, NY, 2011), implemented in Schrodinger package with 30ns simulation time. OPLS2005 force field was used to perform initial steps of the simulation. Water molecules were added to ND1- Annonin-I complex using the SPC and system was neutralized with ions and periodic boundary conditions were used.
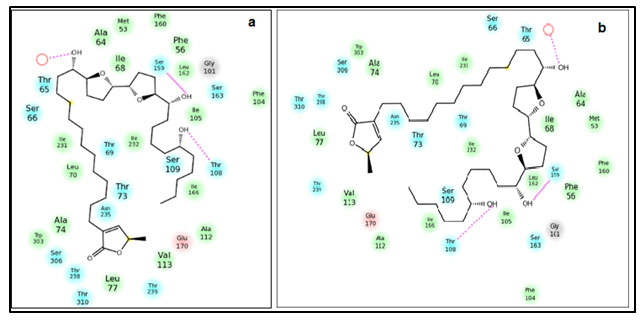
Figure 2: Position of residues and the interaction between the ND1-Annonin-I complex. (a) Before 30ns simulation of the docked complex (b) after 30ns simulation of the docked complex.
The full system of 43089 atoms was simulated through the multistep MD protocols of Maestro. The relaxed system was simulated for a simulation time of 30000ps, NPT ensemble using a Berendsen thermostat at 310K and velocity resampling for every 1ps. Trajectories after every 0.300ps were recorded. Energy fluctuation and RMSD of the complex in each trajectory were analyzed with respect to simulation time. The intermolecular interactions of ND1-Annonin-I complex were assessed for stability of the docked complex.
MTT assay: The growth inhibition property of Annonin-I was assessed against the HCT-116 (Human Colon Adenocarcinoma) cell lines by the MTT assay (Carmichael et al., 1987). The HCT-116 cell lines purchased from NCCS, Pune, India; were cultured in 25 t-flasks in DMEM medium containing, 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2, 95% air and complete humidity. When the cell density in a culture flask reached 70-80% confluence, they were trypsinized and seeded into 96-well plates at a density of 2500 cells per well in 100μl and incubated for 24 hours in CO2 incubator.
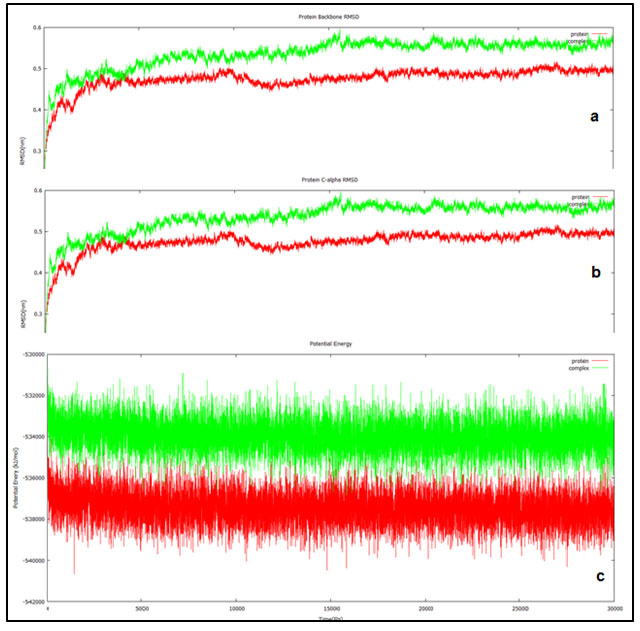
Figure 3: Trajectories of the 30ns simulation done for the ND1 alone (red) and ND1 – Annonin-I complex (red). (a) Shows the protein backbone RMSD plotted against the simulation duration. (b) Shows the C- alpha RMSD plotted against the time (ps). (c) Shows the behavior of ND1 & ND1-Annonin- I complex with respect to potential energy plotted against time (ps).
The cultivated cells were treated with Annonin-I, the test compound, which was dissolved directly in to the RPMI media at 100, 10, 1.0 and 0.01 μM/ml concentrations. Untreated wells were kept as controls. The plates were further incubated for 48 hours in the CO2 incubator. MTT solution was added to each well and the plates were further incubated for 2.5 hours. The medium was carefully decanted and the formazan crystals were air dried in dark place and dissolved in 100μL DMSO; the plates were mildly shaken at room temperature and the OD was measured using Synergy H4 microplate reader at 570nm.
From the optical densities the percentage growths were calculated using the following formula:
Percentage growth= 100× [(T-T0)/(C-T0)]
if T is greater than or equal to T0, and if T is less than T0,
Percentage growth = 100× [(T-T0)/T0)],
Where T is optical density of test; C is the optical density of control; T0 is the optical density at time zero.
From the percentage growth a dose response curve was generated and GI50 values were interpolated from the growth curves. After 48 hours of incubation the images were captured prior to the adding of MTT. Cells treated at different concentrations were observed under the microscope and images of each concentration were captured and recorded.
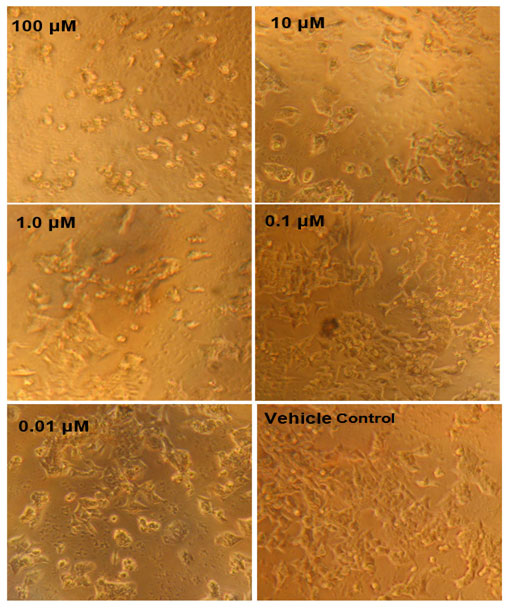
Figure 4: MTT assay results against HCT116 cells. Concentration dependent cell inhibition ranging from 0.01 to 10 µM.
RESULTS AND DISCUSSION
Amongst five models predicted by the iTasser server; one with the best C-score (Confidence Score) of -3.92 was chosen. More negative the Z score for a particular model better will be its overall quality. The modeled structure mainly consists of alpha helices and an odd beta strand connected by intermittent loops (fig 1b). The stereochemistry of this model was verified by submitting to Procheck validation server and Ramachandran plot showed 96% of the total residues to be aggregating in the most favorable and allowed regions. Minor corrections which were reported by the procheck server were corrected by energy minimization technique before the model could be considered for further study.
Sitemap helped us to find the binding cavity in the ND1 and therefore docking of Annonin1 was performed with Glide module and the best docked pose with respect to glide score of ND1 with Annonin-I is shown in fig 1c. The mode of interaction of Annonin-I with ND1 was due to H-bond formation with Thr65 through a water molecule intermediate, and directly by Thr108 & Ser159 of the ND1. Table 1 shows the energy contribution of various parameters for the formation of receptor – ligand complex. It can be seen from the table that Annonin-I to possess the lowest glide score -10.108KJ/mol in comparison to the other two compounds, marginally higher than squamocin C. The glide score involving Squamocin C was -10.103KJ/mol, whereas -10.108 in the case of Annonin-I.
Table 1. Glide docking results of Squamocin A, C & D with ND1.
| Compound | Gscorea | Gevdwb | Gecoulc | Genergyd | Geinternale | Gemodelf |
| Squamocin A | -10.108 | -54.653 | -9.656 | -64.309 | 10.490 | -87.835 |
| Squamocin C | -10.103 | -50.513 | -9.282 | -59.795 | 5.1160 | -74.590 |
| Squamocin D | -6.4154 | -54.878 | -5.194 | -60.073 | 8.0975 | -78.714 |
All terms are expressed in Kcal/mol. a – Glide Score; b – Van der Waals energy; c – Coulomb energy; d – Modified Coulombic – Van der Waals interaction energy; e – Internal torsional energy; f – Model energy (Emodel combines GlideScore, the non-bonded interaction energy, and, for flexible docking, the excess internal energy of the generated ligand conformation).
Annonin-I – ND1 complex was predicted to have overall non bonded glide energy of -64.309 KJ/mol. This was relatively better than the other two acetogenins by approximately -4.00KJ/mol (squamocin C) and -5.00KJ/mol (squamocin D). Model energy which combines glide score, the non-bonded interaction energy and the excess internal energy was better in case of Annonin-I at -87.835 Kj/mol much higher than squamocin C (-74.590 Kj/mol) and squamocin D (-78.714 Kj/mol). These results were an indication for Annonin-I possessing the best binding affinity among the three acetogenins.
Molecular Dynamics Simulation of ND1- Annonin-I complex: Though molecular docking resulted in Annonin-I having best affinity to ND1, it was necessary to understand major conformational changes, if any, between the unbound state and bound state of Annonin-I with ND1. Molecular dynamics simulation was performed for 30 ns for the apo ND1 and ND1-Annonin-I complex to study the thermodynamics fluctuations between the two states. The stability of the complex was assessed and the RMSD values for the protein Cα atoms calculated by aligning the MD production phase trajectories to their initial structures. Examination of the data presented in the plots (fig 2) revealed the exact binding interaction of the docking complex with system embedded with water molecules, temperature and pressure. Interactions responsible for holding the ligand and the receptor complex together were found to be constantly present over the period of dynamics study as approved by the molecular docking results. No major change in the interactions before and after dynamics study of the complex was witnessed (fig 2a & b).
The ND1 – Annonin-I docking interactions were reproduced during the entire simulation period. The two hydrogen bond interactions involving the residues Thr108 and Ser159 responsible for the complex formation were examined during the entire simulation and were observed to be intact. Interestingly, at few steps these interactions were found to be competing with the water molecule for the hydrogen bond formation. And it was during these instances, that the RMSD of the Ser159 for hydrogen bond was higher, otherwise a very stable interaction. In case of Thr108, similar conclusion could not be reached, because of the increasing hydrogen bond RMSD with the increasing timestep (fig 3a).
RMSD which is measured relative to the reference structure for the backbone atoms was found to be stable from 18000 ps attaining equilibrium for both the protein and the docked complex. Initial randomness in the system during the simulations generally results in higher fluctuations however; they tend to get equilibrated over the simulation period. In our study both the trajectories reported equilibration from the 18000 ps and the behavior was maintained until the end of the simulation up to 30000 ps. Final RMSD found to be aggregating at 0.5 to 0.6 nm and 0.4 to 0.5 nm for the protein and ligand complex respectively. Similar pattern was observed in the RMSD analysis involving C alpha atoms as well (fig 3b).
Potential energy of the system as a function of time was plotted to study the behavior of the complex and the apo (ND1 only) protein, indicated equilibration over the entire simulation. Potential energy of the docked complex was calculated to be -533954 kJ/mol (fig 3c) and the total energy at -434583 kJ/mol maintained at a temperature of 300 (K) and pressure at -0.7689 bar. This when compared with the apo protein was -537455 kJ/mol, -437518 kJ/mol, 299.999 (K) and -0.6038 bar of potential energy, total energy, temperature and pressure respectively.
ADME Properties: ADME – toxicity and lipinski assessment for Annonin-I, squamocin C & D and that of the most commonly prescribed drugs in the treatment of cancer such as Temsirolimus, Vinorelbine, Cabazitaxel and Goserelin was predicted. This was done to find the relative efficacy of acetogenins with that of the existing market drugs. Table 2 lists the results of this exercise and it could be seen none of the 7 compounds including the market drugs seem to obey the lipinski’s criteria. When analyzed at the level of molecular weight acetogenins (622.924 g/mol) were found to have lesser molecular weight than others in the range of 778 to 1269 g/mol.
Table 2. Comparative Qikprop properties of acetogenins with the market cancer drugs.
| Molecule | MWa | dHBb | aHBc | QPlogPo/wd | PHOAe | QPlogHERGf |
| Temsirolimus | 1030.3 | 0 | 20.7 | 7.286 | 58.368 | -6.74 |
| Vinorelbine | 778.944 | 1 | 11.5 | 6.184 | 50.754 | -8.076 |
| Cabazitaxel | 835.944 | 2 | 17.35 | 5.312 | 66.323 | -6.704 |
| Goserelin | 1269.43 | 11.5 | 25.7 | -2.267 | 0 | 5.003 |
| Squamocin C | 622.924 | 3 | 11.5 | 7.386 | 89.543 | -7.056 |
| Squamocin A | 622.924 | 3 | 11.5 | 7.386 | 89.543 | -7.056 |
| Squamocin D | 622.924 | 3 | 11.5 | 7.386 | 89.543 | -7.056 |
a – Molecular Weight (g/mol); b – Hydrogen Bond Donors; c – Hydrogen Bond Acceptors; d – Predicted octanol/water partition coefficient; e – Predicted human oral absorption on 0 to 100% scale (acceptable range: <25% is poor, >80% is high); f – Predicted IC50 value for blockage of HERG K+ channels (concern below −7).
Similar conclusion could be made with respect to other parameters such as partition coefficient and acceptor hydrogen bonds. With an exception to Goserelin all the compounds had the donor hydrogen bonds to be well in the acceptable limits as was enunciated by lipinski’s criteria. Poor human oral absorption was recorded for Goserelin and higher for acetogenins which was in the range of 89%. In general, the pharmacokinetics of the drugs must be taken into consideration, as it determines the concentration of drug reaching the heart and therefore the hERG channel, which implicates cardiac functioning. These acetogenins were calculated to have QPlogHERG as -7.056, negligibly higher by 0.056 units. All these results of Qikprop study therefore, point to the fact that these acetogenins are better in comparison to other commonly used drugs.
Table 3. MTT assay growth inhibition results
| Compound | Percentage Growth | Growth Inhibiton µM | ||||||
| 100 µM | 10 µM | 1.0 µM | 0.1 µM | 0.01 µM | GI50 | TGI | LC50 | |
| Annonin-1 | 46 | 78 | 89 | 85 | 90 | 76.13 | 100.0 | 100.0 |
Goserelin which is classified as a leutinizing hormone releasing hormone (LHRH) agonist, used to cure prostate and breast cancer is known to result in testesterone imbalance coupled with 5% chances of heart failure (Goserelin – Drug Information – Chemocare, 2019). Cabazitaxel used in the treatment of prostate cancer has side effects such as fatigue, respiratory issues, low blood count, and diarrhea in more than 30% of population (Cabazitaxel – Drug Information – Chemocare, 2019). Temsirolimus (Temsirolimus – Chemotherapy Drugs – Chemocare, 2019) and Vinorelbine (Vinorelbine – Chemotherapy Drugs – Chemocare, 2019) are other common drugs used to treat renal cell cancer and other types of cancer also reported to have some side effects. Annonin-I is found to have better and satisfactory predictions relative to the drugs earlier. Annonin-I is already reported to be one of the potent compounds that has the pesticidal properties by targeting the ND1 (Habeeb and Sanjayan, 2015).
The drugs we took here for reference and comparison are to support our findings that annonin-I is almost having properties similar to few of the known and existing drugs though they might have their own side effects or the existence of better ones than these. Based on the observations made from the results obtained, we proceeded further for assessing the growth inhibition property by MTT studies.
MTT Assay: Table 3 shows the MTT assay growth inhibition results. 46% growth inhibition was witnessed when the concentration of the Annonin-I was 100 µM. The next concentration chosen for the study was 10 µM, 1.0µM, 0.1µM and 0.01µM and the corresponding growth inhibition was 78%, 89%, 85% and 90% respectively (Fig 4). From figure 4 differences in the cellular growth could be clearly seen as the control was highly packed with a greater number of cells than the 100 µM treated. These results therefore indicate that there was a good growth inhibition property of the compound Annonin-I in HCT-116 cell line.
CONCLUSION
Annonaceous acetogenins are proven to have the potential to become anti-cancer drugs. The acotogenins selected in this study were positively tested to hold anticancer activity when tested upon brine shrimp cell line. The molecular mode of action of these acetogenins is known to be mitochondrial NADH dehydrogenase enzyme, playing a key role in the electron transport chain leading to energy synthesis. Molecular modeling results reported Annonin – I to have a favorable affinity towards ND1 and the molecular dynamics simulation studies also proved that ND1 upon binding to Annonin-I maintained its structural behavior as that of the ND1. From the in-silico studies it was decided to test annonin-I on cancer cell lines HCT-116. An MTT assay was performed on this cell line, at various concentrations and the growth inhibition was predicted to be ranging from 46% the least to 90% the most. The GI50 was predicted to be at 76.13µM, and these indicate that the compound to having a resounding activity against the HCT-116 cell lines. These findings could further be supported by more wet lab experiments and clinical assays to confirm the potential of annonin-I to be a candidate for cancer treatment. Due to financial consideration we could not perform further experiments where we could have tested the effect of annonin-I on different caspases which could be great idea to take this study forward in future.
DECLARATION
The corresponding author Habeeb Shaik Mohideen has been publishing articles earlier in the name of S. K. M. Habeeb. Authors thank M/s Agri Life, Hyderabad for giving us annonin-I to execute this work.
REFERENCES
Abdel-lateff A., El-Menshawi B.S., Haggag M.Y. and Nawwar M.A. (2009) Cytotoxic acetogenins from Annona glabra cultivated in Egypt, Pharmacognosy Research, 1(3), pp. 130–135. Available at: http://www.phcogres.com/article.asp?issn=0974-8490.
Alali F.Q., Liu X. and McLaughlin J.L. (2010) ChemInform Abstract: Annonaceous Acetogenins: Recent Progress, ChemInform. Wiley, 30(28), p. no-no. doi: 10.1002/chin.199928305.
Andrew R.L. (2001) Molecular Modelling: Principles and Applications. 1st edn. Prentice Hall. USA.
Baig M.H., Ahmad K., Rabbani G. et al. (2017) Computer Aided Drug Design and its Application to the Development of Potential Drugs for Neurodegenerative Disorders, Current Neuropharmacology. Bentham Science Publishers Ltd., 16(6), pp. 740–748. doi: 10.2174/1570159×15666171016163510.
Cabazitaxel – Drug Information – Chemocare (2019) Chemocare. Available at: http://chemocare.com/chemotherapy/drug-info/cabazitaxel.aspx (Accessed: 6 May 2020).
Carmichael J., DeGraff W.G., Gazdar A.F. et al. (1987) Evaluation of a Tetrazolium-based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing, Cancer Research. American Association for Cancer Research, 47(4), pp. 936–942.
Dar K.B., Bhat A.H., Amin S. et al. (2019) Modern Computational Strategies for Designing Drugs to Curb Human Diseases: A Prospect, Current Topics in Medicinal Chemistry. Bentham Science Publishers Ltd., 18(31), pp. 2702–2719. doi: 10.2174/1568026619666190119150741.
Falzone L., Salomone S. and Libra M. (2018) Evolution of cancer pharmacological treatments at the turn of the third millennium, Frontiers in Pharmacology. Frontiers Media S.A., p. 1300. doi: 10.3389/fphar.2018.01300.
Feng M., Zhong L., Zhan Z. et al. (2016) Resveratrol treatment inhibits proliferation of and induces apoptosis in human colon cancer cells, Medical Science Monitor. International Scientific Literature Inc., 22, pp. 1101–1108. doi: 10.12659/MSM.897905.
González-Coloma A., Guadan˜o A., Ine´s C.d. et al. (2002) Selective action of acetogenin mitochondrial complex I inhibitors, Zeitschrift fur Naturforschung – Section C Journal of Biosciences, 57(11–12), pp. 1028–1034. doi: 10.1515/znc-2002-11-1213.
Goserelin – Drug Information – Chemocare (2019) Chemocare. Available at: http://chemocare.com/chemotherapy/drug-info/goserelin.aspx (Accessed: 6 May 2020).
Habeeb S.K.M. and Sanjayan K. P. (2015) Molecular Docking and Enzymatic Analysis of Annonin-I, Against the Dusky Cotton Bug Oxycarenus laetus Kirby, Current Bioinformatics. Bentham Science Publishers Ltd., 10(1), pp. 91–96. doi: 10.2174/1574893609666140815204522.
Hassanpour S.H. and Dehghani M. (2017) Review of cancer from perspective of molecular, Journal of Cancer Research and Practice. Medknow, 4(4), pp. 127–129. doi: 10.1016/j.jcrpr.2017.07.001.
Huang C-Y., Ju D-T., Chang C-F. et al. (2017) A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer, BioMedicine. EDP Sciences, 7(4), p. 23. doi: 10.1051/BMDCN/2017070423.
James Morré D., Cabo R.d., Farley C. et al. (1994) Mode of action of bullatacin, a potent antitumor acetogenin: Inhibition of NADH oxidase activity of HELA and HL-60, but not liver, plasma membranes, Life Sciences. Life Sci, 56(5), pp. 343–348. doi: 10.1016/0024-3205(94)00957-0.
Jemal A., Bray F., Center M.M. et al. (2011) Global cancer statistics, CA: A Cancer Journal for Clinicians. Wiley-Blackwell, 61(2), pp. 69–90. doi: 10.3322/caac.20107.
Ji J., Sundquist J. and Sundquist K. (2018) Use of hormone replacement therapy improves the prognosis in patients with colorectal cancer: A population-based study in Sweden, International Journal of Cancer. Wiley-Liss Inc., 142(10), pp. 2003–2010. doi: 10.1002/ijc.31228.
Kim S., Chen J., Cheng T. et al. (2019) PubChem 2019 update: improved access to chemical data, Nucleic Acids Research, 47. doi: 10.1093/nar/gky1033.
Liaw C.C., Wu T-Y., Chang F-R. et al. (2010) Historic perspectives on Annonaceous acetogenins from the chemical bench to preclinical trials, Planta Medica. Planta Med, pp. 1390–1404. doi: 10.1055/s-0030-1250006.
Liaw C.C., Liou J-R., Wu T-Y. et al. (2016) Acetogenins from Annonaceae, Progress in the chemistry of organic natural products, pp. 113–230. doi: 10.1007/978-3-319-22692-7_2.
McLaughlin J., Benson G. and Forsythe J. (2010) A Novel Mechanism for the Control of Clinical Cancer: Inhibition of the Production of Adenosine Triphosphate (ATP) with a Standardized Extract of Pawpaw (Asimincz triloba, Annonaceae), Pawpawworldwide.Com. Available at: http://pawpawworldwide.com/pawpaw-trials1.pdfnhttps://www.joyfullivingservices.com/uploads/1/3/7/9/13798116/pawpawtrials.pdf (Accessed: 6 May 2020).
Md Roduan M.R., Hamid R.A., Cheah Y.K. et al. (2019) Cytotoxicity, antitumor-promoting and antioxidant activities of Annona muricata in vitro, Journal of Herbal Medicine. Elsevier
GmbH, 15, p. 100219. doi: 10.1016/j.hermed.2018.04.004.
Nadendla R.R. (2004) Molecular modeling: A powerful tool for drug design and molecular docking, Resonance. Springer Science and Business Media LLC, 9(5), pp. 51–60. doi: 10.1007/bf02834015.
Oberlies N.H., Chang C.J. and McLaughlin J.L. (1997) Structure-activity relationships of diverse annonaceous acetogenins against multidrug resistant human mammary adenocarcinoma (MCF-7/Adr) cells, Journal of Medicinal Chemistry. J Med Chem, 40(13), pp. 2102–2106. doi: 10.1021/jm9700169.
Ooms F. (2012) Molecular Modeling and Computer Aided Drug Design. Examples of their Applications in Medicinal Chemistry, Current Medicinal Chemistry. Bentham Science Publishers Ltd., 7(2), pp. 141–158. doi: 10.2174/0929867003375317.
Pomper K.W., Lowe J.D., Crabtree S.B. et al. (2009) Identification of annonaceous acetogenins in the ripe fruit of the north american pawpaw (Asimina triloba), Journal of Agricultural and Food Chemistry. J Agric Food Chem, 57(18), pp. 8339–8343. doi: 10.1021/jf9018239.
Ruddon W.R. (2007) Cancer Biology. Oxford University Press.
Seca A.M.L. and Pinto D.C.G.A. (2018) Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application, International Journal of Molecular Sciences. MDPI AG. doi: 10.3390/ijms19010263.
Shimada H., Grutzner J.B., Kozlowski J.F. et al. (1998) Membrane conformations and their relation to cytotoxicity of asimicin and its analogues, Biochemistry. American Chemical Society , 37(3), pp. 854–866. doi: 10.1021/bi972348l.
Shin S.A., Moon S.Y., Kim W-Y. et al. (2018) Structure-based classification and anti-cancer effects of plant metabolites, International Journal of Molecular Sciences. MDPI AG. doi: 10.3390/ijms19092651.
Siegel R.L., Miller K.D., Sauer A.G. et al. (2020) Colorectal cancer statistics, 2020, CA: A Cancer Journal for Clinicians. Wiley, p. caac.21601. doi: 10.3322/caac.21601.
Stevanato Filho P.R., Junior S.A., Begnami M.D. et al. (2017) Oestrogen receptor beta isoform expression in sporadic colorectal cancer, familial adenomatous polyposis and progressive stages of colorectal cancer, BMC Cancer. BioMed Central Ltd., 17(1), p. 754. doi: 10.1186/s12885-017-3688-4.
Temsirolimus – Chemotherapy Drugs – Chemocare (2019) Chemocare. Available at: http://chemocare.com/chemotherapy/drug-info/Temsirolimus.aspx (Accessed: 6 May 2020).
Vinorelbine – Chemotherapy Drugs – Chemocare (2019) Chemocare. Available at: http://chemocare.com/chemotherapy/drug-info/Vinorelbine.aspx (Accessed: 6 May 2020).
Wade R.C. and Salo-Ahen O.M.H. (2019) Molecular modeling in drug design, Molecules. MDPI AG. doi: 10.3390/molecules24020321.
Wang H., Khor T.O., Shu L. et al. (2012) Plants vs. Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability, Anti-Cancer Agents in Medicinal Chemistry. Bentham Science Publishers Ltd., 12(10), pp. 1281–1305. doi: 10.2174/187152012803833026.
Wang J., Chen C., Wang S. et al. (2015) Bufalin Inhibits HCT116 Colon Cancer Cells and Its Orthotopic Xenograft Tumor in Mice Model through Genes Related to Apoptotic and PTEN/AKT Pathways. doi: 10.1155/2015/457193.
Yang B., Li X., He L. et al. (2018) Computer-aided design of temozolomide derivatives based on alkylglycerone phosphate synthase structure with isothiocyanate and their pharmacokinetic/toxicity prediction and anti-tumor activity in vitro, Biomedical Reports. Spandidos Publications, 8(3), pp. 235–240. doi: 10.3892/br.2018.1051.
Yang J., Yan R., Roy A. et al. (2014) The I-TASSER suite: Protein structure and function prediction, Nature Methods. Nature Publishing Group, pp. 7–8. doi: 10.1038/nmeth.3213.