1Department of Biochemistry, Navarasam Arts and Science College for women, Arachalur, India.
2Department of Biochemistry, PSG College of Arts and Science, Coimbatore, India.
Corresponding author email: chitraratheesh@gmail.com
Article Publishing History
Received: 19/04/2021
Accepted After Revision: 27/06/2021
Diabetes is a leading cause of the death in the world’s growing population and the occurrence of diabetes in adults worldwide is estimated to rise up to 592 million in the year 2035. A number of modern drugs are available in controlling diabetes, but their long term utilization may lead to side effects, therefore interest has been focused to the consumption of medicinal plants as an alternative or complimentary treatment for diabetic medication to avoid the side effects caused by the synthetic drugs. Hence, this study was designed to evaluate the effect of methanolic extract of Bougainvillea spectabilis leaves (MEBS) on carbohydrate metabolism and oxidative stress in streptozotocin (STZ)-induced diabetic rats. Administration of STZ to rats demonstrated that hyperglycemia, increased lipid profile, altered carbohydrate metabolism and decreased antioxidant status in liver and kidney. On the other hand, treatment with MEBS (200 and 400mg/kg BW) to diabetic rats for 28 days suppressed the blood glucose and increased the insulin levels and thereby attenuated hyperlipidemia and lipid peroxidation.
At the same time, MEBS increased the activities of glucokinase, pyruvatekinase, glucose-6-phosphate dehydrogenase and glycogen synthase and concomitantly increased the glycogen content in the liver and reduced the activities of glucose-6-phosphatase, fructose-1,6-bisphosphatase and glycogen phosphorylase. MEBS also attenuated the oxidative stress by maintaining the enzymatic and non-enzymatic antioxidants homeostasis as well as protected from the damage of pancreas, liver and kidney due to glucose toxicity which was established by the histopathological analysis. Hence, the current study recommended that MEBS contribute to preventing hepatic and renal complications associated with diabetes mellitus.
Bougainvillea Spectabilis, Carbohydrate Metabolism, Diabetes Mellitus, Medicinal Plants, Oxidative Stress
Devi R. C, Ramesh B. Amelioration of Streptozotocin-Induced Diabetes by Bougainvillea spectabilis Leaf Extract Through Modulation of Carbohydrate Metabolic Enzymes and Oxidative Stress in Rats. Biosc.Biotech.Res.Comm. 2021;14(2).
Devi R.C, Ramesh B. Amelioration of Streptozotocin-Induced Diabetes by Bougainvillea spectabilis Leaf Extract Through Modulation of Carbohydrate Metabolic Enzymes and Oxidative Stress in Rats. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <ahref=”https://bit.ly/2TdCKUF“>https://bit.ly/2TdCKUF</a>
Copyright © Devi and Ramesh This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Diabetes mellitus (DM) is a persistent multifactorial syndrome of disordered metabolism related with the metabolism of carbohydrates, proteins and fats due to insulin resistance (IR) or insulin deficiency (Kleinaki et al. 2020; Uddandrao et al. 2020a; Gokçay and Şahin 2021). The global occurrence of DM in 2019 is accounted to be 9.3% (463 million people), mounting to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045 (Saeedi et al. 2019). Uncontrolled hyperglycemia can leads to macro and micro-vascular complications such as nephropathy, retinopathy and cardiomyopathy (Parim et al. 2019). The metabolic dysregulations of glucose homeostasis are the main cause for the progress of DM and the principal reason of diabetic morbidity and death (Jiang et al. 2020).
During glucose homeostasis, the liver serves an imperative role in carbohydrate production, storage and redistribution (Jiang et al. 2020). The liver executes conflicting functions during hyperglycemic and hypoglycemic states; therefore, the physiological regulation of hepatic glucose production is a multifaceted procedure (Jiang et al. 2020). Patients with type 2 DM (T2DM) and type 1 DM (T1DM) exhibit increased hepatic glucose synthesis, of which numerous extra hepatic mechanisms add to the physiological regulation of hepatic glucose production (Kalidhindi et al. 2020). Conversely, evidences have been distinguished that sustain the role of oxidative stress in the pathogenesis of both T1DM and T2DM. Free radical formation in DM by glucose oxidation, increased lipid peroxidation and non-enzymatic glycation of proteins leads to damage of enzymes, cellular mechanism and also increased IR due to oxidative stress (Sathibabu et al. 2019). A number of experimental studies have recommended that the increased production of reactive oxygen species causes damage to cells and tissues like liver, pancreas and kidney throw in to diabetic complications (Uddandrao et al. 2020a).
Diet, exercise and numerous pharmacological agents are treatment strategies for DM. Taking into account the results associated with the treatment by synthetic drugs and insulin which are accessible at present, screening for safer and effective antidiabetic plant drugs are going on all over the planet. Herbal medicines play an imperative role in this part to put off adverse effects (Sathibabu et al. 2018; Parim et al. 2019). The systematic investigation of plants documented for their therapeutic value is an efficient and alternate approach for the invention of new remedial agents. Several contemporary medicines are available in controlling DM, but prolonged utilization may lead to adverse effects, therefore interest has been shifted towards medicinal plants as an alternative therapy for diabetic medication to avoid the side effects caused by the synthetic drugs (Win 2020). Bougainvillea spectabilis (Family: Nyctaginaceae), is one of the conventional herbal plants (Ghogar and Jiraungkoorskul 2017).
B. spectabilis is documented to have therapeutic effects which include anti-hepatotoxic, anti-inflammatory, antioxidant, antimicrobial, antihyperlipidemic, anticancer and antiulcer properties (Anisa et al. 2016). Our previous study reported that preliminary antidiabetic activity of methanolic extract of B. spectabilis leaves (MEBS) against streptozotocin (STZ)-induced DM in rats (Chitra Devi and Ramesh 2018). Conversely, to the best of our knowledge, the effect of MEBS as prospective antidiabetic ingredient and its effect on carbohydrate metabolic enzymes and oxidative stress are yet to be studied (Chitra Devi and Ramesh 2018). Therefore, the current study was intended to assess the impacts of MEBS on gluconeogenic and glycogenesis enzymes activities along with oxidative stress in liver and kidney of STZ-induced diabetic rats.
MATERIAL AND METHODS
For the sample collection, the leaves of B. spectabilis were collected from surrounding area of Kangayam, Tirupur district, Tamilnadu, India. The plant was identified by expert of Dr. K. Madhava Chetty, Plant Taxonomist, Department of Botany, Sri Venkateswara University, Tirupati. For the preparation of MEBS, the leaves of B. spectabilis were shade dried at room temperature. The dried plant leaves were subjected to size reduction to a coarse powder (1000 grams) by using dry grinder and passed through sieve. The powder was packed into soxhlet apparatus and extracted consecutively with petroleum ether (60-80ºC) and 90% methanol. The extraction was carried out until the extract becomes colorless. The solvent was removed by distillation under reduced pressure and stored in desiccators for further experiment. For the GC-MS (Gas Chromatography-Mass Spectrophotometry) analysis, the MEBS was subjected to identify bioactive compounds by GC-MS as described by Uddandrao et al. (2020b). Interpretation of mass spectrum GC-MS was conducted by using the database of NIST (National Institute Standard and Technology) and the compounds were identified (Uddandrao et al. 2020b).
For the High-performance thin-layer chromatography (HPTLC) analysis for flavonoid profile, the HPTLC analysis was done with B. spectabilis leaves sample (20mg) using mobile phase for flavonoids, i.e., 90:10 ratios of chloroform and methanol solvents. The HPTLC analysis was carried out as per the protocol previously described by the Pallavi and Hemalatha (2018). For the experimental animals, male Wister albino rats (weight: 170-230gm; age: 8-12 weeks) were procured from Sri Venkateswara College of Pharmacy, Chittoor, Andhra Pradesh, India (Pallavi and Hemalatha 2018).
The animals were maintained in a well-ventilated room with at 12:12 h light, dark cycle in polypropylene cages and maintained at 22±1ºC with humidity at 55±5% (Pallavi and Hemalatha 2018). They were fed balanced rodent pellet diet and tap water ad libitum throughout the experimental period. The protocol of this study was approved by the Institutional Animal Ethics Committee of Sri Venkateswara College of Pharmacy (Reference No: SVCOP/IAEC/002/2016-17). Induction of DM was persuaded in rats by a single intraperitoneal injection of STZ (40mg/kg body weight) in 0.1M citrate buffer (pH 4.5). DM was confirmed by the lofty glucose level in plasma which was identified after 48 hrs of STZ administration. The rats with successful elevated plasma glucose levels (above 250 mg/dL) were chosen for further treatment (Pallavi and Hemalatha 2018). For the experimental design, the rats were divided in to five groups and each comprising with 6 animals. The doses of MEBS (200 and 400mg/kg BW) were fixed based on the toxicological evaluation done (Chitra Devi and Ramesh 2018) as per Organization for Economic Cooperation and Development (OECD) guideline 423, fixed dose procedure. All the respective drugs were administered orally by using intragastric tube in a vehicle solution (normal saline) for a period of 28 days. Rats in group 1 and 2 received normal saline (2 mL/kg BW) as placebo treatment.
Group 1: Normal control rats Group 2: Diabetic control rats Group 3: Diabetic + MEBS (200mg/kg BW) Group 4: Diabetic + MEBS (400mg/kg BW) Group 5: Diabetic + Glibenclamide (600μg/kg BW) After completion of treatment duration, the animals were anaesthetised using low doses of phenobarbitone and sacrificed by cervical decapitation. Blood samples were collected by retro orbital sinus puncture method. On the other hand, organs (pancreas, liver and kidney) were straight away dissected out, washed in ice cold saline, dried, weighed, and used for biochemical estimation.
For the estimation of body weight, blood glucose, insulin and glycated hemoglobin, at the end of the experimental period, body weights of the animals were recorded. On the other hand, plasma glucose (Sigma-aldrich Kits, India), insulin (Bio-Merieux, France), and glycated haemoglobin (Hitachi 912, Germany) was evaluated by using the respective kits. To begin with oral glucose tolerance test (OGTT), overnight fasted rats were received a dose of 2.0g/kg BW glucose orogastrically and blood samples were collected from supra orbital sinuses at various intervals such as 0, 30, 60, 90, and 120 min, and the glucose levels (Sigma-aldrich Kits, India) were estimated for all time intervals.
For the biochemical analysis, liver biochemical markers such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were estimated as described by Gella et al. (1985). Lactate dehydrogenase (LDH) was measured as per commercially available kit (MAK066-1KT, Sigma-Aldrich, USA). For the estimation of serum lipid profile, the serum lipid profile markers such as total cholesterol (TC), high density lipoprotein (HDL-C), very low-density lipoprotein (VLDL-C), low density lipoprotein (LDL-C) and triacylglycerides (TG) were assessed by enzymatic colorimetric methods using kits (Nicholas Piramal India Ltd., Mumbai, India). For the determination of liver carbohydrate metabolizing enzymes activity, the carbohydrate metabolic marker enzymes namely glucokinase, pyruvatekinase, glucose-6-phosphatase, glucose-6-phosphate dehydrogenase, fructose-1,6-bisphosphatase were estimated with respective protocols (Koida and Oda 1959; Walker and Rao 1964; Gancedo and Gancedo 1971; Rudack et al. 1971; Ingeborg and Erich 1974). For the assessment of liver glycogen metabolizing enzymes activity, the liver glycogen content, glycogen synthase and glycogen phosphorylase were assessed with respective protocols (Ong and Khoo 2000; Nielsen et al. 2001; Poucher et al. 2007).
For the determination of lipid peroxidation, the level of Thiobarbituric acid reactive substances (TBARS) in serum, liver and kidney was estimated by the method of Fraga et al. (1988). For the estimation of oxidative stress markers, the oxidative stress markers in serum, liver and kidney namely reduced glutathione (GSH), vitamin A, vitamin C, vitamin E, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) were determined as described by Saravanan and Ponmurugan (2013).
For the histopathological analysis of pancreas, liver and kidney, the pancreas, liver and kidney tissues were obtained from the sacrificed rats, immediately washed by using saline solution and fixed in 10% formalin. Then, the tissues were embedded in paraffin and 5𝜇m thick sections were prepared from the paraffinized samples and stained using hematoxylin and eosin stain. The histopathological alterations in the prepared sections were determined under light microscope. For the statistical analysis, all the results were expressed as the mean ± SEM (n=6). All the grouped data was statistically assessed with SPSS\10.0 software. Hypothesis testing methods included one way analysis of variance (ANOVA) followed by the least significant difference (LSD) test, significance level at and P<0.05, 0.01, were considered to point out statistical significance.
RESULTS AND DISCUSSION
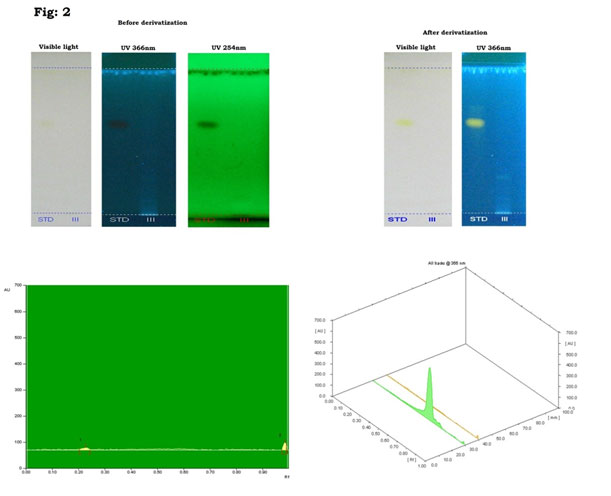
Natural products of plant origin are extensively documented in the pharmaceutical manufacturing for their broad structural multiplicity as well as their wide range of pharmacological actions (Thomford et al. 2018). In the current study, GC-MS chromatogram of MEBS showed 20 peaks represented the presence of twenty compounds (Fig 1). GC-MS investigation uncovered the presence of twenty unique phytochemical compounds as described in table 1 and it was revealed that the MEBS is mainly composed of oxygenated hydrocarbons and predominantly phenolic hydrocarbons. At the same time, figure 2 and table 2 represents the HPTLC analysis of flavonoids present in the B. spectabilis (Thomford et al. 2018).
These phytochemicals may responsible for various pharmacological actions like antimicrobial, antibacterial, antidiabetic and other biological activities. GC-MS analysis of phytoconstituents in plants gives a clear depiction of the pharmaceutical value of that plant. Therefore, this type of GC-MS analysis is the initial move towards indulgent the nature of medicinal properties in this therapeutic plant and this type of explore will be supportive for further comprehensive study (Uddandrao et al. 2020b). HPTLC analysis revealed that the yellowish blue colored fluorescent zone at UV 366nm mode were present in the tracks, and it was observed from the chromatogram after derivatization, which confirmed the presence of flavonoid in the given standard and as well as in the B. spectabilis sample (Jakimiuk et al. 2021).
HPTLC is realistic for expansion of chromatographic fingerprints to find out major active constituents of therapeutic plants. The separation and resolution are greatly superior, and the results are much more consistent and reproducible than thin layer chromatography. Flavonoids, the most prevalent group of innate compounds, principally consist of a fused aromatic ring (A-ring) and a heterocyclic ring (C-ring) associated through a carbon-carbon bridge to an aromatic B-ring (Jakimiuk et al. 2021).
Flavonoids are fitting the subject of medical research as they have been documented to own many medicinal properties, like anti-inflammatory, enzyme inhibition, antimicrobial, anticancer, antiallergy, and antioxidant properties (Jamuna and Paulsamy 2016). Other therapeutic effects include enhanced blood flow, the reticence of cholesterol absorption and guard from damage by ultraviolet B radiation. On the other hand, we also found significant amount of flavonoids by HPTLC in the B. Spectabilis, which indicates that this medicinal plant may have therapeutic properties as like reported earlier (Jakimiuk et al. 2021).
Figure 1: GC-MS chromatogram of the MEBS.
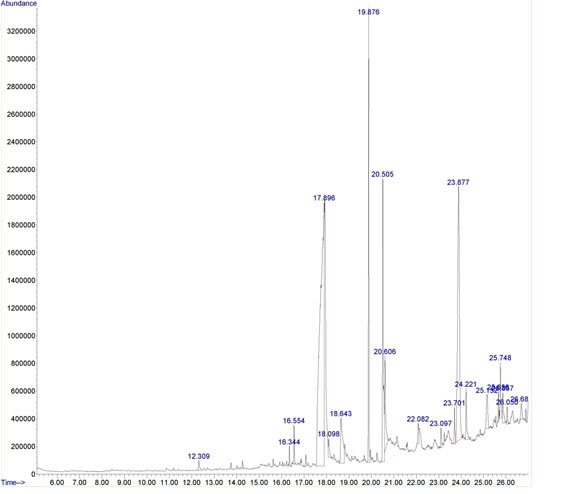
Table 1. Bio active compounds identified in the methanolic extract of B. spectabilis with GC-MS
| Peak | RT | Area % | Compound name | Molecular formula |
| 1 | 12.309 | 0.45 | 4-Nitrophenylglyoxylic acid | C8H5NO5 |
| 2 | 16.344 | 0.48 | Megastigmatrienone | C13H18O |
| 3 | 16.554 | 0.80 | Phytol acetate | C22H42O2 |
| 4 | 17.896 | 42.02 | Cyclobutanecarboxylic acid | C5H8O2 |
| 5 | 18.098 | 0.72 | Hexadecanoic acid, methyl ester | C16H32O2 |
| 6 | 18.643 | 3.78 | Pentadecanoic acid | C15H30O2 |
| 7 | 19.876 | 8.82 | Butyric acid, 3-tetradecyl ester | C18H36O2 |
| 8 | 20.505 | 9.33 | Oleic Acid | C18H34O2 |
| 9 | 20.606 | 3.56 | Z-1,9-Hexadecadiene | C16H30 |
| 10 | 22.082 | 0.75 | Triacontyl acetate | C32H64O2 |
| 11 | 23.097 | 0.63 | Pyrazole, 5-methyl-3-(5-nitro-2-furyl)- | C8H7N3O3 |
| 12 | 23.701 | 0.99 | 7-Pentadecyne | C15H30 |
| 13 | 23.877 | 19.29 | Cholestan-6-one, (5.alpha.)- | C27H46O |
| 14 | 24.221 | 1.69 | Glycerol 1-palmitate | C19H38O4 |
| 15 | 25.152 | 1.83 | Octacosanol | C28H58O |
| 16 | 25.656 | 0.59 | Squalene | C₃₀H₅₀ |
| 17 | 25.748 | 1.91 | Oleoyl chloride | C18H33ClO |
| 18 | 25.857 | 1.12 | 1,3,12-Nonadecatriene | C19H34 |
| 19 | 26.050 | 0.44 | Cyclohexanone, 2-(2-propenyl)- | C9H14O |
| 20 | 26.688 | 0.78 | Fumaric acid, 3-fluorophenyl undecyl ester | C16H10F2O4 |
Table 2. Peak table with Rf values, height and area of flavonoids
| Track | Peak | Rf | Height | Area | Assigned substance |
| STD | 1 | 0.62 | 483.9 | 22949.6 | Flavonoid standard |
| Sample III | 1 | 0.25 | 8.7 | 199.6 | Flavonoid 1 |
Figure 2: HPTLC chromatogram of MEBS showing presence of flvonoids.
The development of safer medicine for DM is still a question for researchers working in this region. The research data on herbal medicine can suggest new functional leads to decrease toxicity, money and time are the three main challenges in drug detection. Hence, in the current study we made an attempt to evaluate the effect of MEBS against STZ-induced DM in rats. STZ is used as a mediator to produce DM by particular cytotoxicity effect on pancreatic b-cells. Accordingly, it affects endogenous insulin ejection and as a result increases blood glucose level (Kotb et al. 2020).
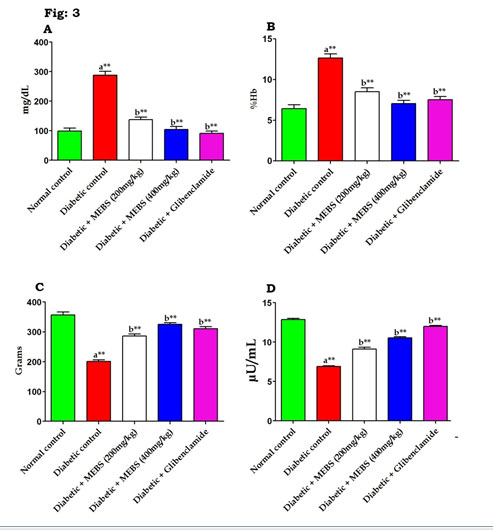
Similarly, figure 3 revealed that there was a significant (P<0.01) increase in the blood glucose (Fig. 3A), glycated hemoglobin content (Fig. 3B) and concomitant decrease in the body weight (Fig. 3C) and insulin levels (Fig. 3D) in the STZ-induced diabetic rats. On the other hand, supplementation with MEBS to diabetic rats notably tended to bring the above parameters towards near normal levels that might be due to the occurrence of various phytochemicals in the MEBS (Chitra Devi and Ramesh 2018; Uddandrao et al. 2020b).
Figure 3: Effect of MEBS on (A) blood glucose, (B) glycated hemoglobin, (C) body weight and (D) insulin in control and experimental animals. All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, **P<0.01.
Several studies have reported that phytochemicals extracted from medicinal plants display an exciting prospect for the development of new types of medicines for DM. Most extensive among phytochemical groups are the alkaloids, glycosides and phenolics such as terpenoids, flavonoids and steroids and our results are in line with this statement (Uddandrao et al. 2020b).
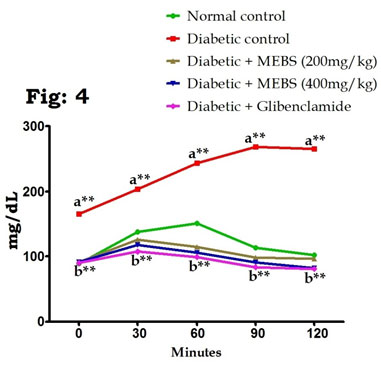
On the other hand, OGTT in control and experimental rats established that the blood glucose levels in the control rats increased to a maximum value after 30 min of glucose load and decreased to nearly basal levels after 120 min, whereas in rats with STZ-induced DM, peak increases in blood glucose levels were noticed even after 60 min and remained high over the next 60 min. Oral supplementation of MEBS (200 and 400mg/kg) in rats with DM caused a significant decrease in the blood glucose levels after 60 min compared with those of the untreated rats with DM (Fig. 4). These results are in line with the previous studies reported by Sathibabu et al. (2019) and Uddandrao et al. (2020c).
Figure 4: Effect of MEBS on OGTT in control and experimental animals. All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, **P<0.01.
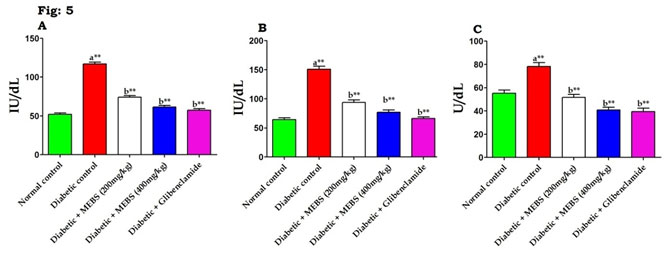
In DM, several studies have demonstrated that increases in AST and ALT activities as well as changes in lipid levels (Saravanan et al. 2009; Sathibabu Uddandrao et al. 2019). An increase in these enzyme activities disclose active liver damage and rise in the activities of plasma ALT, AST, and LDH indicates liver malfunction and moreover liver was necrotized due to STZ (Saravanan et al. 2009). Similarly, figure 5 depicts that there was noteworthy (P<0.01) increase in the liver marker enzymes such as AST (Fig. 5A), ALT (Fig. 5B) and LDH (Fig. 5C) in STZ-induced diabetic rats when compared to normal control. At the same time remarkable (P<0.01) restoration of these liver marker enzymes activities to near normal noticed when diabetic rats treated with MEBS contrasted to untreated diabetic rats.
Bayramoglu et al. (2014) reported that increase in the activities of these enzymes in plasma might be principally due to the release of these enzymes from the hepatic cytosol into the blood circulation because of membrane permeability representing severe hepatocellular damage caused by DM. Our results are in line with this statement. Interestingly, the treatment of MEBS is able to defend against increase in the activity of these enzymes in diabetic rats, signifying that protective effect against liver damage and it may be used as a remedy to bring about hepatoprotective effect (Jiang et al. 2020).
Figure 5: Effect of MEBS on liver marker enzymes (A) AST, (B) ALT and (C) LDH in control and experimental animals. All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, **P<0.01.
DM is associated with severe alterations in the serum lipid and lipoprotein profiles and with an increased risk of heart diseases (Hedayatnia et al. 2020). DM is frequently associated with a variety of changes in metabolic and regulatory mechanisms, due to insulin shortage or due to IR is liable for the noticed increase of lipids (Naidu et al. 2016; Swapna et al. 2019). Table 3 displays that the serum lipid profile in control and experimental animals. The data exposed that the significant (P<0.01) increase in the TC, TG, LDL-C, VLDL-C and concomitant reduction in the HDL-C in STZ-induced diabetic rats.
However, treatment with MEBS (200 and 400mg/kg) predominantly (P<0.01) increased the levels of HDL-C and simultaneously reduced the levels of TC, TG, LDL-C and VLDL-C when compared to untreated diabetic rats. Swapna et al. (2019) reported that STZ-induced DM also can produce hyperlipidemia and similarly we noticed that hyperlipidemia in rats and this might be due to an enhance in the activity of hormone-sensitive lipase which catalyzes the mobilization of fatty acids from TG stored in adipocytes (Uddandrao et al. 2020c). This finding was evidenced by the results of the histopathological analysis. The organs of diabetic rats showed STZ-induced cellular damage on pancreatic islets, liver histology, and renal glomeruli and tubules (Swapna et al. 2019).
Table 3. Effect of MEBS on serum lipid profile in control and experimental animals All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, **P<0.01.
| Groups | Total Cholesterol (mg/dL) | Triglycerides
(mg/dL) |
LDL- C
(mg/dL) |
VLDL- C
(mg/dL) |
HDL- C
(mg/dL) |
| Normal control | 110.67±1.022 | 116.33±1.174 | 34.23±1.442 | 23.27±0.2348 | 53.17±0.4773 |
| Diabetic control | 237±1.438a** | 182.33±1.054a** | 169.87±1.520a** | 36.47±0.2108a** | 30.67±0.8819a** |
| Diabetic + MEBS
(200mg/kg) |
141.50±0.9220b** | 152.00±1.713b** | 71.77±1.290b** | 30.40±0.3425b** | 39.33±0.4216b** |
| Diabetic + MEBS
(400 mg/kg) |
126.33±0.4944b** | 130.33±0.760b** | 58.10±0.5905b** | 26.07±0.1520b** | 42.17±0.6540b** |
| Diabetic + Glibenclamide | 121.50±1.057b** | 122.33±0.8819b** | 46.87±1.97b** | 24.47±0.1764b** | 48.50±0.8466b** |
Table 4. Effect of MEBS on liver carbohydrate metabolizing enzymes activities in control and experimental diabetic rats, All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, **P<0.01.
| Groups | Glucokinase
(mU/mg protein) |
Pyruvatekinase
(mU/mg protein) |
Glucose-6- phosphatase
(U/mg protein) |
Glucose-6- phosphate dehydrogenase
(U/min) |
Fructose-1,6- biphosphatase
(U/mg protein) |
| Normal control | 27.33±0.6146 | 0.207±0.0016 | 0.412±0.0007 | 5.28±0.0600 | 0.322±0.0015 |
| Diabetic control | 8.25±0.0885a** | 0.076±0.0014a** | 0.751±0.0010a** | 1.98±0.0508a** | 0.549±0.0052a** |
| Diabetic + MEBS
(200mg/kg) |
12.46±0.1358b** | 0.119±0.0008b** | 0.540±0.0018b** | 3.40±0.0221b** | 0.424±0.0032b** |
| Diabetic + MEBS
(400mg/kg) |
17.07±0.1282b** | 0.151±0.0007b** | 0.339±0.00016b** | 4.32±0.0274b** | 0.381±0.0028b** |
| Diabetic + Glibenclamide | 21.32±0.3535b** | 0.184±0.0008b** | 0.378±0.0011b** | 4.79±0.0554b** | 0.361±0.0022b** |
DM has been shown to slow down the activities of pentose phosphate and glycolytic pathway enzymes while promoting lipolytic and gluconeogenic activities (Jiang et al. 2020). According to previous reports, DM was to be had with alterations in glucose homeostasis that put in to persistent hyperglycemia and liver plays a major role in the regulation of glucose metabolism (Kalidhindi et al. 2020). Table 4 represents the effect of MEBS on liver carbohydrate metabolizing enzymes activities in control and experimental diabetic rats.
There was a significant (P<0.01) reduction in the activities of glucokinase, pyruvatekinase and glucose-6-phosphate dehydrogenase and simultaneous increase in the activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase in STZ-induced diabetic rats when compared to normal control rats. On the other hand, treatment with high dose of MEBS (400mg/kg) predominantly (P<0.01) restored the alterations happen in these enzyme activities and brought back to near normal level. At the same time, low dose of MEBS (200mg/kg) demonstrated moderate activity in the restoration of these altered enzymes activities when compared to high dose. These results are in line with previous study reported by Kalidhindi et al. (2020).
The activity of enzymes like glucokinase, pyruvate kinase, glucose-6-phosphatase, and fructose-1,6-bisphosphatase was noticeably distorted, consequential in hyperglycemia, which leads to the pathogenesis of diabetic complications (Sharabi et al. 2015; Kalidhindi et al. 2020). The altered the activity of glucokinase and pyruvate kinase, diminishing the metabolism of glucose and ATP production in diabetic conditions. The diminution in the activities of these enzymes in the liver of DM rats is a sign of increased gluconeogenesis and decreased glycolysis suggesting that these two pathways are altered in DM (Saravanan et al. 2014). The activities of regulatory enzymes in gluconeogenesis, like glucose-6-phosphatase and fructose-1,6-bisphosphatase, are prominent in DM and improved activities of these enzymes in diabetic rats may be due to lack of insulin (Lekshmi et al. 2015).
Glucose-6-phosphatase and fructose-1,6-bisphosphatase are dephosphorylating enzymes which make worse hepatic glucose consumption. Our results demonstrated that the activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase were considerably diminished by the administration of MEBS. On the other hand, glycogen is a branched polymer and most significant intracellular storable form of glucose residues synthesized by the enzyme glycogen synthase. Its amount in different tissues is a straight manifestation of insulin activity, as insulin supports intracellular glycogen deposition by activating glycogen synthase and restraining glycogen phosphorylase (Nawaz et al. 2020).
In the current study, STZ-induced diabetic animals are predisposed to show diminished glycogen content in liver and reduced activity of glycogen synthase and concurrent raise in the glycogen phosphorylase activity. On the other hand, supplementation of MEBS to diabetic rats increased the activity of glycogen synthase there by increased (P<0.05, 0.01) the glycogen content in the liver via reserve of glycogen phosphorylase activity (Table 5). This might be due to the antidiabetic potential of MEBS and rise in the levels of insulin with MEBS supplementation (Devi and Ramesh 2018). On the other hand, the figure 6 showed the levels of lipidperoxidation in serum (Fig. 6A), liver (Fig. 6B) and kidney (Fig. 6C) in control and experimental diabetic rats. The STZ-induced diabetic rats had a significant (P<0.05, 0.01) elevation in the levels of lipidperoxidation when compared with the normal control rats (Devi and Ramesh 2018).
Stable hyperglycemia in DM prompts intensified formation of oxygen free radicals from glycosylation of protein and autoxidation of glucose which lead to oxidative stress which is associated with diabetic complications. Phytocompounds are the bioactive molecules that have been extensively concerned in treating several clinical disorders in which their pathogenesis is directly or indirectly associated with oxidative stress (Parim et al. 2019; Ghasemi et al. 2020).
Table 5. Effect of MEBS on glycogen and glycogen metabolizing enzymes in control and experimental diabetic rats, All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, *P<0.05, **P<0.01.
| Groups | Glycogen | Glycogen synthase | Glycogen phosphorylase |
| Normal control | 65.82±3.46 | 624.93±43.66 | 398.79±92.64 |
| Diabetic control | 25.19±0.68a* | 404.72±93.48a* | 702.97±63.82a* |
| Diabetic + MEBS (200mg/kg) | 58.25±0.86b* | 523.16±44.6 b* | 565.63±24.83b* |
| Diabetic + MEBS (400 mg/kg) | 64.16±0.58b** | 683.65±24.12b** | 399.63±25.40b* |
| Diabetic + Glibenclamide | 63.61±0.48b** | 622.39±34.22b* | 402.97±29.46b* |
Glycogen: mg of glucose/gm of liver tissue, Glycogen synthase: u moles of UDP formed/hr/mg protein, Glycogen phosphorylase: u moles of pi liberated /hr/mg protein.
Figure 6: Effect of MEBS on lipid peroxidation in (A) serum, (B) liver and (C) kidney in control and experimental animals. All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, *P<0.05, **P<0.01.
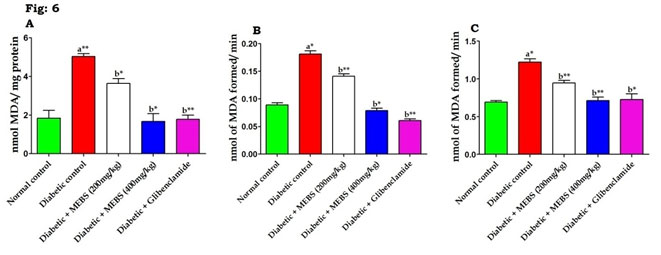
Table 6. Effect of MEBS on Nonenzymic antioxidants in liver and plasma of control and experimental diabetic rats, All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, *P<0.05, **P<0.01. Vitamin A, C, E Units: Plasma: mg/dL, Liver: µg/mg protein. GSH Units: Serum: mg of glutathione/dL, Liver: units/mg/protein.
| Groups | Vitamin A | Vitamin C | Vitamin E | GSH | ||||
| Plasma | Liver | Plasma | Liver | Plasma | Liver | Serum | Liver | |
| Normal control | 1.95±0.03 | 1.87±0.08 | 1.48±0.02 | 1.34±0.07 | 1.92±0.21 | 0.92±0.05 | 36.95±1.42 | 55.12±1.98 |
| Diabetic control | 0.43±0.06a* | 0.65±0.06a* | 0.49±0.08a* | 0.71±0.05a* | 0.76±0.05a* | 0.51±0.08a* | 17.62±2.08a* | 15.65±2.13a* |
| Diabetic + MEBS
(200mg/kg) |
0.98±0.08b* | 1.02±0.08b* | 1.18±0.065b** | 1.23±0.056b** | 1.3±0.08b* | 0.70±0.07b** | 29.85±1.96b* | 36.23±1.85b* |
| Diabetic + MEBS
(400 mg/kg) |
1.75±0.07b** | 1.58±0.06b** | 1.59±0.07b* | 1.63±0.08b* | 1.97±0.49b** | 0.99±0.08b** | 37.83±1.86b** | 56.41±1.83b** |
| Diabetic + Glibenclamide | 1.89±0.08b* | 1.65±0.08b* | 1.38±0.08b* | 1.49±0.06b** | 1.89±0.12b** | 0.87±0.05b** | 38.98±1.73b** | 57.14±2.15b* |
The rats treated with Glibenclamide and the high dose of MEBS (400mg/kg) showed a significant (P<0.05, 0.01) decrease and the low dose of MEBS demonstrated a moderate decrease in the levels of LPO in the serum and tissues (liver and kidney) of the rats. Brahmanaidu et al. (2017) found that lofty levels of blood glucose in DM are linked with elevated lipid peroxidation, which may put in to long-term damage of organs and interestingly, in our study revealed that STZ-administration widely increased lipid peroxidation in serum, liver and kidney and this was attenuated by the MEBS. On the other hand, this study also revealed that STZ administration developed severe oxidative stress in rats which was confirmed by the reduction of non-enzymatic antioxidants (GSH, vitamin A, C and E) and enzymatic antioxidants (SOD, CAT and GPx).
Table 6 demonstrated about the effect of MEBS on non-enzymatic antioxidant markers in control and experimental diabetic rats. There was a significant (P<0.05, 0.01) reduction in the levels of GSH, vitamin A, C and E in STZ-induced diabetic rats when compared with normal control. On the other hand, MEBS (400mg/kg) treatment to diabetic rats notably (P<0.05, 0.01) elevated the levels of non-enzymatic antioxidant markers (GSH, vitamin A, C and E) and at the same time low dose of MEBS (200mg/kg) shown reasonable elevation of these markers (Brahmanaidu et al. 2017; Devi and Ramesh 2018).
Figure 7: Effect of MEBS on enzymatic antioxidants (7.1) SOD, (7.2) CAT and (7.3) GPX in (a) serum, (b) liver and kidney of control and experimental animals. All values expressed in mean ± SEM, n=6, aSignificantly different from the normal control, bSignificantly different from the diabetic control, *P<0.05, **P<0.01.
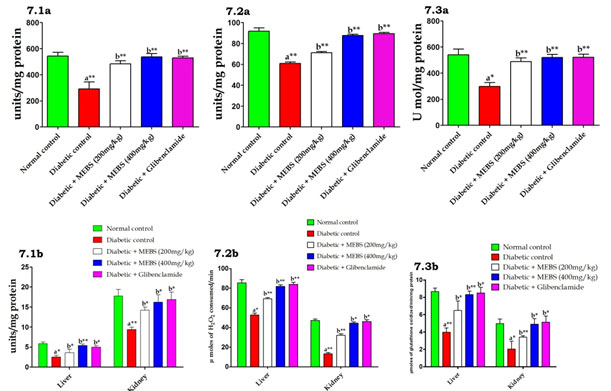
In addition, the increased lipid peroxidation under DM state could be due to enhanced oxidative stress in the cell as a result of the diminution of antioxidant protection mechanisms (Sathibabu et al. 2019). Similarly we also found likewise which was demonstrated by figure 7 which exposed that the effect of MEBS on serum, liver and kidney enzymatic antioxidants in control and experimental diabetic rats. The data exposed that a noteworthy (P<0.05) reduction in the activities of SOD (Fig. 7.1a&b), CAT (Fig. 7.2a&b) and GPX (Fig. 7.3a&b) in STZ-induced diabetic rats.
At the same time, diabetic rats treated with MEBS remarkably (P<0.05, 0.01) restored above enzymatic antioxidant levels to near normal. Antioxidant enzymes form the primary line of protection against reactive oxygen species in the individual include the enzymes SOD, CAT and GPx, which play a vital role in hunting the toxic intermediate of incomplete oxidation (Jansy et al. 2021). On the other hand, in our study, the treatment with MEBS restored enzymatic and non-enzymatic antioxidants in liver and kidney of diabetic rats’ close proximity to normal levels, which in turn reveals the antioxidant potential of MEBS. Concomitantly with the decline in the activity of the antioxidant’s enzymes, cleaned out antioxidant capability of non-enzymatic antioxidants namely GSH, vitamin A, C, and E have been documented in DM (Cammisotto et al. 2021). We also studied histopathology of vital organs such as pancreas, liver and kidney. STZ administration caused severe pathological conditions in these organs.
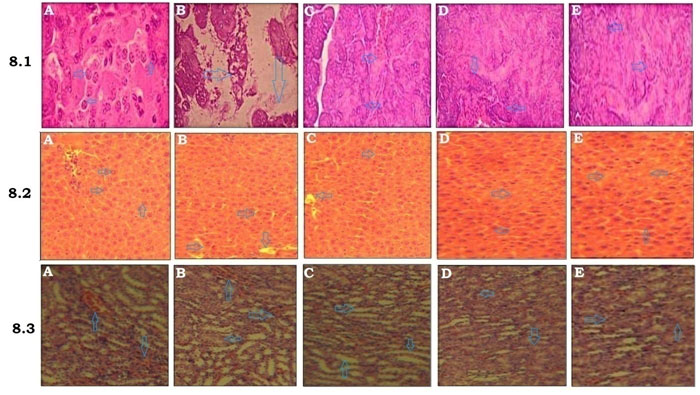
Figure 8.1 summarizes the histoarchestucture of pancreas in control and experimental diabetic rats. The results revealed that the pancreatic islet cells are normal in normal control group with typically arranged islet cells, uniform sinusoid spaces with no vacuolization (Fig.8.1A). Diabetic control rats demonstrated that vascular degranulated islets with severely reduces the volume and number of islets (Fig.8.1B). Diabetic rats treated with MEBS (200mg/kg) shown mild granulation islets with moderately reduced volume and number (Fig.8.1C) (Cammisotto et al. 2021). On the other hand, diabetic rats treated with MEBS (400mg/kg) and Glibenclamide revealed well granulated and regenerated islets with normal cellular characteristics (Fig.8.1D&E). Figure 8.2 depicts the histopathological changes were noted in the liver of control and experimental diabetic rats. The liver of the normal control rats showed the normal architecture of hepatocytes with central lobules (Fig.8.2A). Liver of the diabetic control displayed abnormalities like congestion and cellular necrosis in the liver (Fig.8.2B).
Liver of the MEBS (200mg/kg) treated rats showed the hepatocytes with few lobules (Fig.8.1C). At the same time rats treated with MEBS (400mg/kg) and Glibenclamide demonstrated that heptocytes with almost a normal structure (Fig.8.2D&E). Figure 8.3 explains the histoarchestucture of kidney in control and experimental diabetic rats. The kidney of normal control rats showed the normal structure of kidney with convoluted tubules, glomerulus and tubules (Fig.8.3A). The kidney of STZ-induced diabetic rats displayed congested tubules and glomerulus (Fig.8.3B) (Cammisotto et al. 2021). The kidney of MEBS (200mg/kg) treated rats illustrated a distinguishable glomerules and tubules (Fig.8.3C). Simultaneously, MEBS (400mg/kg) and Glibenclamide treatment for 28 days to diabetic rats displayed that a similar architecture to that of the normal control rats (Fig.8.3D&E).
Figure 8: Histopathological analysis of (8.1) pancreas, (8.2) liver and (8.3) kidney in control and experimental animals, H&E ×40, (A) Normal control, (B) Diabetic control, (C) Diabetic + MEBS (200mg/kg), (D) Diabetic + MEBS (400mg/kg) and (E) Diabetic + Glibenclamide.
CONCLUSION
In conclusion, MEBS remarkably reestablished the activities of carbohydrate and glycogen metabolic enzymes and in this way kept up glucose homeostasis in diabetic rats. Moreover, MEBS had the capability to enhance enzymatic and non-enzymatic antioxidant defense system and to attenuate the lipid peroxidation and oxidative stress in liver and kidney and furthermore secured the pancreas, liver and kidney from the glucose toxicity. Taken together, these outcomes may add to a predominant comprehension of the hepatoprotective and renoprotective potential of MEBS, emphasizing the impact of this therapeutic plant and presumably preventing complications associated with DM.
ACKNOWLEDGEMENTS
Authors thank Navarasam Arts and Science College for Women, Arachalur, Erode District, Tamil Nadu, India and PSG College of Arts and Science, Coimbatore, Tamil Nadu, India for providing facilities to experiments and their constant support throughout the study.
Conflcit of Interests:Authors declare no conflict of interests while preparing for this research.
REFERENCES
Anisa, G., Kanitta, J., and Wannee, J. (2016) Paper Flower, Bougainvillea spectabilis: Update Properties of Traditional Medicinal Plant. Journal of Natural Remedies, 16, 83-87.
Bayramoglu, G., Senturk, H., and Bayramoglu, A. (2014) Carvacrol partially reverses symptoms of diabetes in STZ-induced diabetic rats. Cytotechnology, 66, 251-257.
Brahmanaidu, P., Uddandrao, V.V.S., Sasikumar, V., Naik, R.R., Pothani, S., Begum, M.S., Rajeshkumar, M.P., Varatharaju, C., Meriga, B., Rameshreddy, P., Kalaivani, A., and Saravanan, G. (2017) Reversal of endothelial dysfunction in aorta of streptozotocin-nicotinamide-induced type-2 diabetic rats by S-Allylcysteine. Molecular and Cellular Biochemistry, 432, 25-32.
Cammisotto, V., Nocella, C., Bartimoccia, S., Sanguigni, V., Francomano, D., Sciarretta, S., Pastori, D., Peruzzi, M., Cavarretta, E., D’Amico, A., Castellani, V., Frati, G., Carnevale, R., and Group, S. (2021) The Role of Antioxidants Supplementation in Clinical Practice: Focus on Cardiovascular Risk Factors. Antioxidants, 10(2), 146.
Chitra Devi, M.R., and Ramesh, B. (2018) Hypoglycemic activity of Leaves of Bougainvillea spectabilis extract in Streptozotocin-Induced Diabetic Rats. Asian Journal of Pharmaceutical Research, 8, 99-103.
Fraga, C.G., Leibouitz, B.E., and Toppel, A.L. (1988) Lipid peroxidation measured as TBARS: characterization and comparison with homogenates and microsomes. Free Radical Biology and Medicine, 4, 155-161.
Gancedo, J.M., and Gancedo, C. (1971) Fructose-1,6-bis-phosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting yeast. Archives of Microbiology, 76, 132-8.
Gella, F.J., Olivella, T., Cruz Pastor, M., Arenas, J., Moreno, R., Durban, R., and Gomez, J.A. (1985) A simple procedure for the routine determination of aspartate aminotransferase and alanine aminotransferase with pyridoxal phosphate. Clinica Chimica Acta, 153(3), 241-7.
Ghasemi-Dehnoo, M., Amini-Khoei, H., Lorigooini, Z., and Rafieian-Kopaei, M. (2020) Oxidative stress and antioxidants in diabetes mellitus. Asian Pacific Journal of Tropical Medicine, 13, 431-8.
Ghogar, A., and Jiraungkoorskul, W. (2017) Antifertility Effect of Bougainvillea spectabilis or Paper Flower. Pharmacognosy Reviews, 11, 19-22.
Girard, J. (2017) Glucagon, a key factor in the pathophysiology of type 2 diabetes. Biochimie, 143, 33-36.
Gokçay Canpolat, A., and Sahin, M. (2021) Glucose Lowering Treatment Modalities of Type 2 Diabetes Mellitus. Advances in Experimental Medicine and Biology, 1307, 7-27.
Hedayatnia, M., Asadi, Z., and Zare, F. (2020) Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids in Health and Disease, 19, 42.
Ingeborg, G., and Erich, B. (1974) Pyruvate Kinase Assay in Serum and Erythrocytes, Editor(s): Hans Ulrich Bergmeyer, Methods of Enzymatic Analysis (Second Edition), Academic Press, 774-778.
Jakimiuk, K., Wink, M. and Tomczyk, M. (2021) Flavonoids of the Caryophyllaceae. Phytochemistry Reviews, https://doi.org/10.1007/s11101-021-09755-3
Jamuna, S., and Paulsamy, S. (2016) HPTLC Fingerprints of Various Secondary Metabolites in the Traditional Medicinal Herb Hypochaeris radicata L., Journal of Botany, 2016, 5429625.
Jansy, A.I.R., Uddandrao, V.V.S., Ganapathy, S., Chandrasekaran, P., Sethumathi, S., Tamilmani, P, and Vadivukkarasi, S. (2021) Biochanin A attenuates obesity cardiomyopathy in rats by inhibiting oxidative stress and inflammation through the Nrf-2 pathway. Archives of Physiology and Biochemistry, 10.1080/13813455.2021.1874017.
Jiang, S., Young, J.L., Wang, K., Qian, Y., and Cai, L. (2020) Diabetic-induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Molecular medicine reports, 22(2), 603-611.
Kalidhindi, S., Uddandrao, V.V.S., Sasikumar, V., Raveendran, N., and Ganapathy, S. (2020) Mitigating Perspectives of Asiatic Acid in the Renal Derangements of Streptozotocin-Nicotinamide Induced Diabetic Rats. Cardiovascular & Hematological Agents in Medicinal Chemistry, 18(1), 37-44.
Kleinaki, Z., Kapnisi, S., Theodorelou-Charitou, S.A., Nikas, I.P., and Paschou, S.A. (2020) Type 2 diabetes mellitus management in patients with chronic kidney disease: an update. Hormones (Athens), 19, 467-476.
Koida, H., and Oda, T. (1959) Pathological occurrence of glucose-6-phosphatase in liver disease. Clinica Chimica Acta, 4, 554-61.
Kotb El-Sayed, M.I., Al-Massarani, S., El Gamal, A., El-Shaibany, A., and Al-Mahbashi, H.M. (2020). Mechanism of antidiabetic effects of Plicosepalus acaciae flower in streptozotocin-induced type 2 diabetic rats, as complementary and alternative therapy. BMC Complementary Medicine and Therapies, 20, 290.
Lekshmi, R.K., Sreekutty, M.S., and Mini, S. (2015) The regulatory effects of Cissus quadrangularis on some enzymes involved in carbohydrate metabolism in streptozotocin-induced diabetic rats. Pharmaceutical Biology, 53, 1194-200.
Naidu, P.B., Sathibabu Uddandrao, V.V., Naik, R.R., Pothani, S., Munipally, P.K., Meriga, B., Begum, M.S., Varatharaju, C., Pandiyan, R., and Saravanan, G. (2016) Effects of S-Allylcysteine on Biomarkers of the Polyol Pathway in Rats with Type 2 Diabetes. Canadian Journal of Diabetes, 40, 442-448.
Nawaz, A., Zhang, P., Li, E., Gilbert, R.G., and Sullivan, M.A. (2020). The importance of glycogen molecular structure for blood glucose control. iScience, 24(1), 101953.
Nielsen, J.N., Derave, W., Kristiansen, S., Ralston, E., Ploug, T., and Richter, E.A. (2001) Glycogen synthase localization and activity in rat skeletal muscle is strongly dependent on glycogen content. The Journal of Physiology, 531, 757-769.
Ong, K.C., and Khoo, H.E. (2000) Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Science, 67, 1695-705.
Pallavi, Y., and Hemalatha, K.P.J. (2018) Phytochemical studies and high-performance thin-layer chromatography analysis of Calamus rotang linn leaf extracts. Asian Journal of Pharmaceutical and Clinical Research, 11(2), 269-275.
Parim, B., Sathibabu Uddandrao, V.V., and Saravanan, G. (2019) Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Failure Reviews, 24, 279-299.
Poucher, S.M., Freeman, S., Loxham, S.J., Convey, G., Bartlett, J.B., De Schoolmeester, J., Teague, J., Walker, M., Turnbull, A.V., Charles, A.D., Carey, F., and Berg, S. (2007) An assessment of the in vivo efficacy of the glycogen phosphorylase inhibitor GPi688 in rat models of hyperglycaemia. British Journal of Pharmacology, 152, 1239-1247.
Rudack, D., Chisholm, E.M., and Holten, D. (1971) Rat liver glucose 6-phosphate dehydrogenase. Journal of Biological Chemistry, 246, 1249.
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., Colagiuri, S., Guariguata, L., Motala, A.A., Ogurtsova, K., Shaw, J.E., Bright, D., Williams, R., and IDF Diabetes Atlas Committee. (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice, 157, 107843.
Saravanan, G., and Ponmurugan, P. (2013) S-allylcysteine Improves Streptozotocin-Induced Alterations of Blood Glucose, Liver Cytochrome P450 2E1, Plasma Antioxidant System, and Adipocytes Hormones in Diabetic Rats. International Journal of Endocrinology and Metabolism, 11, 10927.
Saravanan, G., Ponmurugan, P., Deepa, M.A., and Senthilkumar, B. (2014) Modulatory effects of diosgenin on attenuating the key enzymes activities of carbohydrate metabolism and glycogen content in streptozotocin-induced diabetic rats. Canadian Journal of Diabetes, 38, 409-14.
Saravanan, G., Ponmurugan, P., Senthil Kumar, G.P., and Rajarajan, T. (2009) Antidiabetic properties of S-allyl cysteine, a garlic component on streptozotocin-induced diabetes in rats. Journal of Applied Biomedicine, 7, 151-159.
Sathibabu Uddandrao, V.V., Brahmanaidu, P., Nivedha, P.R., Vadivukkarasi, S., and Saravanan, G. (2018) Beneficial Role of Some Natural Products to Attenuate the Diabetic Cardiomyopathy Through Nrf2 Pathway in Cell Culture and Animal Models. Cardiovascular Toxicology, 18, 199-205.
Sathibabu Uddandrao, V.V., Brahmanaidu, P., Ravindarnaik, R., Suresh, P., Vadivukkarasi, S., and Saravanan, G. (2019) Restorative potentiality of S-allylcysteine against diabetic nephropathy through attenuation of oxidative stress and inflammation in streptozotocin-nicotinamide-induced diabetic rats. European Journal of Nutrition, 58, 2425-2437.
Sharabi, K., Tavares, C.D., Rines, A.K., and Puigserver, P. (2015) Molecular pathophysiology of hepatic glucose production. Molecular Aspects of Medicine, 46, 21-33.
Swapna, K., Sathibabu Uddandrao, V.V., Parim, B., and Saravanan, G. (2019) Effects of asiatic acid, an active constituent in Centella asiatica (L.): restorative perspectives of streptozotocin-nicotinamide induced changes on lipid profile and lipid metabolic enzymes in diabetic rats. Comparative Clinical Pathology, 28, 1321-1329.
Thomford, N.E., Senthebane, D.A., Rowe, A., Munro, D., Seele, P., Maroyi, A., and Dzobo, K. (2018) Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. International Journal of Molecular Sciences, 19(6), 1578.
Uddandrao, V.V.S., Parim, B., Ramavat, R., Pothani, S., Vadivukkarasi, S., and Ganapathy, S. (2020a) Effect of S-allylcysteine against diabetic nephropathy via inhibition of MEK1/2-ERK1/2-RSK2 signalling pathway in streptozotocin-nicotinamide-induced diabetic rats. Archives of Physiology and Biochemistry, doi: 10.1080/13813455.2020.1811731
Uddandrao, V.V.S., Brahmanaidu, P., and Ganapathy, S. (2020b) Evaluation of the Antioxidant and Antidiabetic Potential of the Poly Herbal Formulation: Identification of Bioactive Factors. Cardiovascular & Hematological Agents in Medicinal Chemistry, 18, 111-123.
Uddandrao, V.V.S., Rameshreddy, P., Brahmanaidu, P., Ponnusamy, P., Balakrishnan, S., Ramavat, R.N., Swapna, K., Pothani, S., Nemani, H., Meriga, B., Vadivukkarasi, S., P R, N., and Ganapathy, S. (2020c) Antiobesity efficacy of asiatic acid: down-regulation of adipogenic and inflammatory processes in high fat diet induced obese rats. Archives of Physiology and Biochemistry, 126, 453-462.
Walker, D.G., and Rao, S. (1964) The role of glucokinase in the phosphorylation of glucose by rat liver. Biochemical Journal, 90, 360-368.
Win, M.T.T. (2020) Novel effect of medicinal plants on diabetes mellitus. Journal of Diabetes, Metabolic Disorders & Control, 7(2), 73-74.


