1Department of Zoology, Government Arts College, Chidambaram, Tamil Nadu, India.
2Centre of Advanced Study in Marine Biology, Annamalai University, Parangipettai, Tamil Nadu, India.
Corresponding author email: b.balasu84@gmail.com
Article Publishing History
Received: 15/10/2021
Accepted After Revision: 15/12/2021
Pollution from microplastics has recently become a prevalent threat to the ecosystem. Microplastics with a dimension less than or equal to 5 mm are smaller. There are many ways that microplastics can reach the atmosphere. By various mechanisms, the breakdown of macro plastics will happen. Chemical degradation, tire abrasion, is the most common forms of degradation. Microplastics (MPs) pollution in the coastal and marine ecosystem is currently a global problem. Transferring MPs from land to sea and allowing them to enter the food chain has a direct negative impact on marine life and human health. The combined toxicity effects of MicroPlastics (MPs) and other contaminants in marine environments, as well as their toxicity effects and mechanisms based on a variety of environmentally important test organisms, were also covered in this study.
Micro plastic, Marine Environment, Pollution
Balasubramaniyan B, Rajendran N, Kathiresan K, Anandraj P. A Review of Microplastics Risk Assessment in the Coastal Environment. Biosc.Biotech.Res.Comm. 2021;14(4).
Balasubramaniyan B, Rajendran N, Kathiresan K, Anandraj P. A Review of Microplastics Risk Assessment in the Coastal Environment. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3rohA4W“>https://bit.ly/3rohA4W</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, sources the original author and sources are credited.
INTRODUCTION
Microplastics in the atmosphere of the ocean: Microplastics (MPs) are increasingly infiltrating the climate, according to accumulating evidence. Scientists, politicians, and the general public all over the world are debating the role of MPs. It’s alarming to see how quickly Microplastics (MPs) are assimilated into the global culture. Marine environment ends up MPs methods (Stolte et al. 2014).
MP particles have been found all over the country, from the coast to the interior. The origin and place of sources, as well as the local atmosphere, all contribute to the formation of MPs. MPs may come from a variety of places, such as unmaintained plastic waste on land or at ocean, goods transfer leakage, fishing accessories, waste water treatment plants, and so on. A variety of factors affect the distribution of Microplastics MPs in the atmosphere. Microplastics MPs are transported throughout the atmosphere through a variety of processes (Hidalgo-ruzet 2012; Karrman et al.
2016). One consideration is the buoyancy of plastic polymers; for example, since their density is lower than that of water, PE and PP float on the water surface. PVC and PET, on the other hand, have higher densities than water and are thus found under the water’s surface. Microplastics MPs’ distribution in the atmosphere is determined by their particle size (Karrman et al. 2016).
Occurrence in beach sediments: MPs have been present in sediments since the late 1970s, according to studies. Spain, New Zealand, Canada, Bermuda, and Lebanon were among the first countries to make observations. This aids in comprehending the global distribution of MPs since the late 1970s (Lisbeth et al. 2015). MPs reach the marine environment primarily as a result of plastic litter from various sources. According to Karrman et al. (2016), the total amount of plastic waste produced by coastal countries worldwide in 2010 was 192 tons, with 2–5% of that being mismanaged and ending up in the ocean (Karrman et al. 2016).
MPs’ effect on aquatic species: MPs will interact with their surroundings when they arrive in the aquatic environment through various carriers, and their biological fate and mobility will be determined by their size, shape, and other characteristics. MPs in water have a major impact on aquatic life. Marine animals absorb MPs, and this is the main pathway. MPs are contained in sediments, so animals that eat detritus are likely to be affected. The number of laboratories works recently investigated in MP ingestion by marine biota. An accede can be the primary cause of MPs particle ingestion in certain circumstances (Wright 2013; Karrman et al. 2016).
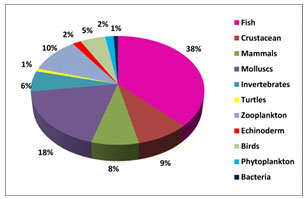
It may also cause inflammation and clog the digestive tract. MPs ingestion has been discovered in a number of marine species as a result of laboratory studies (Auta 2017). Through the use of a variety of applications, the presence of colorants in species indicated an anthropogenic origin. As a result, coastal areas are a hotspot for MP emissions, with filter-feeding bivalves being the most vulnerable. As a result, more research is needed to better understand MP accumulation rates and residence times across food webs. The overall distribution of approximately value (Fig 1) micro plastics studies have been conducted across the world (Silva et al. 2018).
Figure:1
Sample Collecting Methods: This section describes the different sample collection methods used by many researchers during their study. The processing and handling of MP samples is a delicate procedure. As a result, determining the origins of contamination is important. Items such as synthetic fibbers, gears, clothing, and other unwanted items may contaminate Microplastics samples. The equipment to be fully carefully cleaned and to be sealed with polymer free clothing can be worn though for contamination prevention. The following methods of Microplastics sample collection are in the table (Silva et al. 2018).
Table 1: Sampling equipment used to collect MPs Matrix.
| Matrix | Equipment |
| Water
Surface: Mid water level Sediments Bottom sediments Surface samples Seabed samples Biological tissue |
The trawl with rectangular opening and a net bag
The bongo net using for collection
Collection through box corer The iron spoons using for collection core or bottom trawl Dissection & ejection |
Water sample: A variety of nets are used to collect water samples. Neuston nets, manta trawls, and bongo nets are the most commonly used nets. The tail end of these nets has a sample collection bin. The microplastics sample collection nets ranges from 53m to 3mm were used (Silva et al. 2018).
Sampling of sediments: This is a screening technique in which microplastics are obtained from beaches or the bottom of lakes. It is simpler and more convenient to obtain samples on beaches. During the sampling process, there is no one-size-fits-all approach. It is entirely dependent on the person and the sampling location. To collect samples from beaches, most researchers tend to use tidelines and sampling depth (Mai et al. 2018).
Biota sampling: The marine animals like fish, sea gulls, sea cow and other planktons are consumed microplastics, the biota sampling assessment use full for concentration of micro plastic level. Animals’ digestive tracts are used to collect the samples. Laboratories dissect the digestive tracts of marine creatures to collect samples (Mai et al. 2018).
Sample separation and purification: Floatation is a term used to describe the process of Separation and purification of samples can be accomplished in a variety of ways. Density separation is the most common method for sampling microplastics. It is used to distinguish low-density particles such as sand, dirt, and sediments from higher-density particles. Microplastics like PP and PE have a lower density than seawater (1.10 g/cm3).
To differentiate microplastics from higher density microplastics, such as PVC (1.40 g/cm3) or higher density microplastics, various density solutions are used. Saturated NaCl is widely used in this system due to its nonhazardous properties. It’s also reasonably priced and easily available. The use of NaCl solution as a density separator has several drawbacks. During the extraction process, samples with a higher density (PVC) cannot be completely collected (Mai et al. 2018).
During the flotation method, another commonly used solution is ZnCl2, which is effective in extracting almost all microplastics of various densities. The density of the most common products, such as PP and PE, is lower than that of ZnCl2 and CaCl2 solutions. The use of ZnCl2 has the drawback of being harmful. As density separators, other flotation solutions are used, but the overall process remains the same (Stolte et al. 2014; Mai et al. 2018).
Sample purification: The flotation process is ZnCl2 solution widely used, which is efficient in removing almost all microplastics of various densities. Most popular products, such as PE and PP, contain a lesser density than CaCl2 and ZnCl2 solutions (Stolte et al. 2014). The use of ZnCl2 has the drawback of being harmful. As density separators, other flotation solutions are used, but the overall process remains the same. According to a recent analysis, vegetal material such as algae, seagrasses, and various small residues are abundant in microplastics samples collected from beaches (Herrera et al. 2018).
These materials can be sorted by sight or by sieving, but the remaining tiny residue cannot be sorted by sight. There has been a breakthrough in the removal of residues from microplastics samples. which takes less time and is more likely to extract all of the small vegetal residue from the microplastics sample. The method is based on a five-step digestion process that includes the use of chemicals such as HCL, NaOH, KOH, and H2O2, as well as a density separation process using 96 percent ethanol. When it comes to extracting vegetal pollutants from microplastic samples, the digestion method is extremely efficient (Herrera et al. 2018).
Digestion of samples: The most of samples are chemical or enzymatic digestion to break down the organic matter. Hence a chance of damaging plastics due to deterioration and mechanical friction and loss due to heating. The polymer detection system can be used later depending on the detection. The clean water samples only can be filtered directly, for example glass fiber or aluminum oxide filters (Herrera et al. 2018).
Acidic digestion: HNO3 is one option for removing organic compounds. When compared to digestion in HCl, H2O2, and 324 NaOH, the degradation of biogenic compounds was between 94 and 98 percent. However, Avio et al. (2015) demonstrated that polymers and PE dissolving and agglutination occurred. HCl is not recommended because it does not fully kill organic matter and therefore is ineffective (Cole et al. 2011; Avio et al. 2015; Herrera et al. 2018).
Alkaline digestion: The NaOH or KOH solution can be used for sample digestion process (Cole et al. 2011). The 1M NaOH is high efficiency of 90% digestion. While increasing the temperature and morality to achieve the effective digestion process, but the use of 10 M NaOH degraded many polymers, including PC, cellulose acetate (CA), PET, and PVC (Dehaut et al. 2016; Herrera et al. 2018).
Oxidizing digestion: The efficient oxidizer H2O2 for removing organic material. The polymers are slightly getting more transparent, smaller or thinner while using 30% H2O2. However, after a seven-day digestion with 30% H2O2, only 70% of the 343 microplastics were removed. This was most likely due to the development of foam and, as a result, a material loss. PE and PS 345 were unaffected by a 15% H2O2 solution.
After seven days of use of a 35 percent H2O2 346 solution and a time span of seven days, only 25% of the organics were killed, according to another report. Frias et al. (2010) suggest using 10% H2O2 with an exposure period 348 of 18 hours due to the heavy reaction of plastics with 347 high concentrated H2O2 solutions (Nuelle et al. 2014; Herrera et al. 2018; Renzil 2019).
Enzymatic degradation: In certain trials, enzymes were used to remove organic matter and reduce a portion of the 352-biological tissue (Loderet al. 2015). Cellulase 353 (> 30 U/ml), lipase (> 15,000 U/ml), chitinase (> 40 U/ml), and protease (1,100 U/ml) in technical grade were used to incubate microplastics and inorganic content (Cole et al. 2011; Loder et al. 2015; Renzil 2019).
Density separation: Many research (see compilation in review papers) use density separation. It’s often used to separate microplastics from soil or other inorganic matter that didn’t get digested by enzymes or chemicals. A high concentrated or even saturated salt 369 solution is mixed with the sample and shaken (Loder et al. 2015; Renzil 2019).
Water litter has an impact on oceans and marine life, according to Renzil (2019), posing a danger to natural populations of pelagic fish species at the base of the marine food chain. In the years (2013–2014), marine litter and microplastics were measured in the stomach contents of Sardinia pilchardus and Engraulis encrasicolus on a seasonal basis. In the Adriatic Sea, some planktivorous species are of great ecological and commercial value. Data was compared to potential factors that could influence ingested amounts, such as organisms, sampling season, biometry, and animal sex (Renzil 2019).
Almost all of the tested samples (80 organisms for each species) contained marine litter and microplastics (over 90% of samples from both species), but no meso- or macroplastics were found. The average number of registered items per person was 4.63 (S. plichardus) and 1.25 (E. encrasicolus). Sardines had a higher percentage of microplastics of smaller sizes than anchovies. In sardines, sex, the Gastro Somatic Index, and the sampling season had no effect on the amount of ingested litter; however, anchovies showed differences related to both animal sex and the dominant color of ingested materials, with black and blue colors predominating (Mai et al. 2018).
Identification by chemical composition: The molecular structure of plastic polymers is used in this method to establish the polymer’s origin (Bergmann et al. 2015). Furthermore, this process provides a simple way to identify samples using polymer recognition methods such as FTIR, Raman analyses, pyrolysis, GC, and MS (Mai et al. 2018).
Pyrolysis- GC/MS: This is a method for detecting microplastics in the atmosphere that is based on experience. The chemical composition of microplastic particles can be determined using thermal oxidation (Bergmann et al. 2015). The main advantage of this approach is that it can simultaneously analyze both the polymer shape and the organic additives present. Environmental samples are analyzed using gas chromatography and mass spectrometry (Mai et al. 2018).
FTIR spectroscopy: The polymer composition and origin of microplastic particles in samples can be determined using this method (Bergmann et al. 2015). Furthermore, it enables accurate particle detection using their unique IR spectra. This approach has the advantage of causing molecular vibrations when interacting with the sample. In the case of a plastic sample, this technique makes obtaining extremely complex IR spectra with distinct band patterns much simpler. Based on the oxidation level observed, FTIR spectroscopy also provides information on the weathering of sampled plastic particles (Bergmann et al. 2015).
Raman spectroscopy: One of the most dependable analytical techniques for determining the chemical composition of unknown plastic fragments is chromatography (Hidalgo-Ruz et al. 2012). Raman spectroscopy has the advantage of being able to analyze small samples (1 m) and providing a better response to non-polar functional groups than other analytical approaches (Mai et al. 2018).
Routes of exposure: Microplastics are a significant pollutant in the environment. Ingestion of microplastic-containing foods, inhalation of microplastics in the environment, and dermal contact with these particles contained in cosmetics, textiles, and dust are all ways for microplastics to reach the human body (Cox et al. 2019).
Ingestion: Ingestion is thought to be the most common way for humans to come into contact with microplastics (Galloway et al. 2015). Microplastics are expected to be consumed in amounts ranging from 39,000 to 52,000 particles per person per year based on food consumption (Cox et al. 2019). Particles may enter the gastrointestinal system through infected foods or mucociliary clearance after inhalation, causing inflammation, increased permeability, and changes in gut microbe composition and metabolism. Microplastics have been found in mussels, commercial fish, and other foods (Salim et al.2013; Neves et al. 2015; Li et al. 2016; Cox et al. 2019).
Inhalation: Synthetic textiles, abrasion of materials (e.g., car tires, buildings), and resuspension of microplastics in surfaces are all sources of microplastics in the air. Outdoor concentrations of 0.3–1.5 particles m-3 and indoor concentrations of 0.4–56.5 particles m-3 (33 percent of polymers), including inhalable sizes, were one of the first measurements of microplastics in the air (Dris et al. 2017). Individual inhalation of 26 to 130 airborne microplastics per day have been estimated (Prata 2018). A male individual with light activity inhales 272 microplastics every day, according to air sampling using a mannequin (Vianello et al. 2019).
Dermal contact: While nanoplastics have been proposed, dermal contact with microplastics is thought to be a less significant route of contamination.
Microplastic toxicity pathways: Microplastics, which were once thought to be harmless particles with no toxicity, are now thought to be potentially harmful to species, depending on exposure and sensitivity. Microplastics’ large surface area may trigger oxidative stress, cytotoxicity, and translocation to other tissues (Galloway 2015; Anbymani and Kakkar 2018).
Oxidative stress and Cytotoxicity: Oxidative stress may be caused by an overabundance of antioxidant responses. Because of their high surface area, release of oxidizing species adsorbed to their surface (e.g., metals), or reactive oxygen species released during the inflammatory response, microplastics can be at the root of this oxidative stress (Kelly and Fussel 2012; Valavanidis et al. 2013). For example, oxidative stress has been observed in zebrafish and mice after exposure to microplastics (Lu et al. 2016; Deng et al. 2017).
After injection of a polypropylene (PP) prosthesis, an acute inflammatory reaction culminates in the release of oxidants (e.g., hydrogen peroxide, hypochlorous acid), causing the polymer to degrade, hydrolyze, crack, and additively leach, resulting in a positive feedback loop of free radical development and exposing possible pathways of plastic removal from the organism (Anbymani and Kakkar 2018).
Neurotoxicity: Contaminant use has been linked to neurotoxicity and neurodegenerative diseases. In vivo neurotoxicity has been observed in reaction to particulate matter exposure, most likely as a consequence of oxidative stress and activation of the brain’s microglia (immune cells) caused by direct contact with translocated particles or the involvement of circulating pro-inflammatory cytokines (from other sources) (Vianello et al. 2019).
Microplastics as vectors of microorganisms and potentially toxic chemicals: Microplastics can pose a chemical and biological risk in addition to particle toxicity. Within the body, monomer and additives from the microplastics matrix can leach, exposing tissues to chemicals such as phthalates and bisphenol A, which are known as endocrine disruptors – substances that interfere with endogenous hormones even at very low concentrations (Cole et al. 2011).
Microplastics, in addition to their constituents, have a large surface area, making them susceptible to acting as vectors for microorganisms or chemicals they come into contact with. For instance, persistent organic pollutants (POPs) have been identified in microplastics recovered from the environment, including polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) (Vianello et al. 2019).
CONCLUSION
The findings of the present review are risk of microplastic ingestion was evaluated for the various species living in these habitats. Potential trophic transport routes for bioaccumulation of microplastics have been proposed to fill existing knowledge gaps, with a specific emphasis on routes involving some at-risk species. More research in the Indian scenario is required to fully assess the impact of bioaccumulation in specific species and on the coastal ecosystem as a whole. Given the prevalence of microplastics in aquatic habitats and their inability to be removed, we believe it is not too late to combine research on plastic alternatives. Furthermore, immediate steps should be taken to avoid the entry of plastics into the world.
ACKNOWLEDGEMENTS
This study was financially supported by the Government Arts College and the Centre for Advanced Study in Marine Biology, Annamalai University, Tamil Nadu, India.
Conflict of Interests: Authors declare no conflict of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Ajith, N., Arumugam, S., Parthasarathy, S., et al. (2020). Global distribution of microplastics and its impact on marine environment—a review. Environ Sci Pollut Res, 27, 25970–25986.
Anbymani, S., Kakkar, P., (2018). EcoToxicological Effects of Microplastics in biota: A review. Environmental Science and Pollution Research, 25(15), 14373-14396.
Auta, H.E.A., (2017). Distribution and importance of Microplastics in the marine environment: A review of sources, fate, effects and potential solutions. Environment international, Volume, 102, pp.165-176.
Avio, C.G.S., Gorbi, F., and Regoli, (2015). Experimental development of a new protocol for extraction and characterization of Microplastics in Fish Tissues: First observations in commercial species from Adriatic Sea, Mar. Environ. Res. 111, 18-26.
Bergmann, et al. (2015). Methodology used for the Detection and Identification of Microplastics A Critical Appraisal, Helgoland, Germany: Polar and Marine Research, Marine Station.
Boucher, J., Friot, D., (2017). Primary Microplastics in the Oceans: a global evaluation of sources. Gland, Switzerland: Iucn, 178, 483-492, 227-229.
Claessens, M., Meester, S.D., Van Landuyt, L., et al. (2011). Occurrence and distribution of Microplastics in marine sediments along the Belgian coast, Mar. Pollut. Bull, 62 (2011) 2199-2204.
Cole, M., Lindeque, P., Halsband, C., Galloway, T.S., (2011). Microplastics as contaminants in the marine environment: A review, Mar. Pollut. Bull, 62, 2588-2597.
Cox, K.D., Covernton, G.A., Davies, H.L., et al. (2019). Human Consumption of Microplastics. Environmental Science and Technology, 53(12), 7068-7074. https://doi.org/10.1021/acs.est.9b01517
Crawford, C.B. and Quinn, B., (2017). The interactions of microplastics and chemical pollutants. Microplastic pollutants, 1, pp.131-157.
Dehaut, A.L., Cassone, L., Frère, L., et al. (2016). Microplastics in seafood: Benchmark protocol for their extraction and characterization, Environ. Pollut. 215, 223-233.
Deng, Y., Zhang, Y., Lemos, B., et al. (2017). Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Scientific Reports, 7, 46687.
Dris, R., Gasperi, J., Mirande, C., et al. (2010). Organic pollutants in microplastics from two beaches of the Portuguese coast. Marine Pollution Bulletin, 60(11), 1988-1992.
Frias, J., Pagter, E., Nash, R., et al. (2018). Standardised protocol for monitoring Microplastics in sediments. Deliverable 4.2. JPI Oceans BASEMAN project.
Galloway, T.S., (2015). Micro-and nano-plastics and human health. In Marine anthropogenic litter, Springer, Cham, 343-366.
Herrera, A., Garrido-Amador, P., Martínez, I., et al. (2018). Novel methodology to isolate microplastics from vegetal-rich samples. Marine pollution bulletin, 129(1), pp.61-69.
Hidalgo-Ruz, et al. (2012). Microplastics in the marine environment: A review of the methods used for identification and quantification. Environmental Science and Technology, S.1 S.N.
Hidalgo-Ruz, V., Gutow, L., Thompson, R.C. et al. (2012). Microplastics in the marine environment: a review of the methods used for identification and quantification. Environmental science & technology, 46(6), pp.3060-3075.
Kärrman, A., Schönlau, C. and Engwall, M., (2016). Exposure and effects of microplastics on wildlife: A review of existing data.
Kelly, FJ., and Fussel, J.C., (2012). Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmospheric Environment 60, 504- 526.
Lambert, S., and Wagner, M., (2018). Freshwater Microplastics: emerging environmental contaminants? Springer Nature, 303.
Mai, L., Bao, L.J., Shi, L., et al. (2018). A review of methods for measuring microplastics in aquatic environments. Environmental Science and Pollution Research, 25(12), 11319-11332.
Mohan Kumar, S. K. J., Campbell, A., Block, M., et al. (2008). Particulate matter, oxidative stress and neurotoxicity. NeuroToxicology; 29(3), 479-488.
Renzi, M., Specchiulli, A., Blašković, A., et al. (2019). Marine litter in stomach content of small pelagic fishes from the Adriatic Sea: sardines (Sardina pilchardus) and anchovies (Engraulis encrasicolus). Environmental Science and Pollution Research, 26(3), 2771-2781.
Naves, D., Sobral, P., Ferreira, J. L., et al. (2015). Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Marine pollution bulletin; 101(1), 119-126.
Prata, J.C., Costa, J.P.D., Lopes, I., et al. (2020). Environmental exposure to microplastics: An overview on possible human health effects. Science of the Total Environment, 702, 134455.
Salim, S.Y., Kaplan, G.G. and Madsen, K.L., (2014). Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut microbes, 5(2), pp.215-219.
Silva, A.B., (2018). Microplastics in the environment: Challenges in analytical chemistry – A review, Analytical Chimica Acta, s.l: Science direct, Environmental Pollution, 234: 115-12. https://doi.org/10.1016/j.envpol.2017.11.043, 2018. 3060-3075.
Stock, F., Kochleus, C., Bänsch-Baltruschat, B., et al. (2019). Sampling techniques and preparation methods for microplastic analyses in the aquatic environment–A review. TrAC Trends in Analytical Chemistry, 113: 84-92.
Stolte, A., (2014). The detection of microplastics in beahc sediments, Rostock: Universität Rostock Tassin, B., A first overview of textile fibers, including Microplastics, in indoor and outdoor environments. Environmental Pollution 221: 453-458.
Valavanidis, A., Vlachogianni, T., Fiotakis, K., et al. (2013). Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of Lung Carcinogenesis through reactive oxygen species mechanisms. International Journals of Environmental Research and Public Health; 10(9), 3886-3907.
Vianello A, Jensen RL, Liu L, et al. (2019). Simulating human exposure to indoor airborne Microplastics using a Breathing Thermal Manikin. Scientific Reports, 9, 8670.
Wright, S.L., Thompson, R.C. and Galloway, T.S., (2013). The physical impacts of microplastics on marine organisms: a review. Environmental pollution, 178, pp.483-492.


