1Department of Gynecology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, 100026, China.
2Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Center of Excellence in Tissue Engineering Chinese Academy of Medical Sciences, Beijing Key Laboratory (No. BZO381), Beijing 100005, China
Corresponding author email: ludan6268@163.com
Article Publishing History
Received: 17/01/2020
Accepted After Revision: 20/03/2020
Long noncoding RNAs (lncRNAs) have been implicated in a range of developmental processes and diseases, but the roles and mechanisms by which they act in adipogenic differentiation and adipose tissue biology are still unknown. By comparing the different expression patterns of lncRNAs before and after the adipocyte differentiation of human adipose-derived mesenchymal stem cells (hADSCs), we characterized a novel lncRNA, AC092834.1, which is significantly increased in preadipocytes. By gain- and loss-of-function experiments, we demonstrated that lncRNA AC092834.1 potentiated adipogenic differentiation through directly increasing the level of expression of DKK1, which competitively binds with LRP5 to inhibit the Wnt-β-catenin pathway and reduce the inhibition of adipogenesis by Wnt signaling. This finding provides novel mechanistic insights into a critical role for lncRNA AC092834.1 as a regulator of adipogenic differentiation, which expands our knowledge about the molecular mechanisms of obesity and other adipogenic differentiation-related disorders.
long noncoding RNA; adipogenic differentiation; DKK1; hADSCs
Fan L, Xu H, Li D, Li H, Lu D. A Novel Long Noncoding RNA, AC092834.1, Regulates the Adipogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells Via the DKK1/Wnt/Β-Catenin Signaling Pathway. Biosc.Biotech.Res.Comm. 2020;13(1).
Fan L, Xu H, Li D, Li H, Lu D. A Novel Long Noncoding RNA, AC092834.1, Regulates the Adipogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells Via the DKK1/Wnt/Β-Catenin Signaling Pathway. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2PcEHMe
Copyright © Fan et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Obesity has attracted increasing attention worldwide, particularly because obesity is a major risk factor for diabetes, hyperlipidemia, atherosclerosis, fatty liver and chronic inflammation, (Martenstyn, et al., 2020; Sun and Karin, 2012; Shulman, et al., 2014). The rising incidence of obesity has become a serious health challenge with the current prevalence predicted to triple by 2030 according to the World Health Organization (WHO),( Corrales, et al.,2018).
Adipocytes, the main component of adipose tissue, play crucial roles in systemic metabolism and energy homeostasis. In response to abnormal adipogenesis and as adipocytes accumulate excessively, a host of metabolic problems emerge, (Cohen, et al., 2016). Human adipose-derived mesenchymal stem cells (hADSCs) are major sources of adipocyte generation and have the capacity of self-renewal and multi-lineage differentiation potential such as commitment into preadipocytes. The underlying pathophysiology of obesity remains ill-defined and further understanding of the adipogenesis mechanism is of great significance for the prevention and therapy of obesity-related diseases.The mechanisms governing hADSCs adipogenesis are quite complex, and a great deal of investigation has focused on regulating adipocyte lineage commitment by modulating cell signaling pathways or numerous transcription factors. A range of transcription factors such as peroxisome proliferator-activated receptor-gamma (PPARγ) and several members of the CCAAT/enhancer-binding proteins (C/EBPs, specifically C/EBPα/β) have been demonstrated to be critical for the differentiation of hADSCs into adipocytes, (Hadrich and Sayadi, 2018). Importantly, PPARγ can promote adipogenesis in the absence of C/EBPα, while C/EBPα does not function similarly in PPARγ-deficient cells,(Lee, et al., 2019).
In terms of signaling pathways in adipogenesis, multiple extracellular and intracellular signaling pathways such as Wnt/β-catenin, TGF-β, MAPK, and PI3K signaling are vitally important for adipogenesis and are well-studied, (Chen, at al., 2016). On the other hand, epigenetic regulation, including DNA methylation, histone modification, and noncoding RNA modulation also exert important roles in the regulation of adipogenic differentiation of hADSCs. Recently, large-scale sequencing initiatives demonstrated that noncoding RNAs play significant roles in the control of transcription and epigenetic regulators, and, crucially, help establish lineage specification through dependent or independent mechanisms.Long noncoding RNAs (lncRNAs) are a subset of noncoding RNAs that are longer than 200 nt and are characterized by only rarely encoding proteins; instead, lncRNAs are involved in the regulation of gene expression at the epigenetic, transcriptional, and posttranscriptional levels(Kopp and Mendell, 2018).
A growing number of researchers have indicated that lncRNAs are extensively involved in the regulation of numerous cellular processes, including stem cell pluripotency,cell differentiation and human disease pathogenesis(Fico, et al., 2019).Recent studies have prompted us to hypothesize that lncRNAs (such as ADINR, slincRAD and H19) are implicated in adipocytes’ fate decisions of hADSCs through the control of master adipogenic transcriptional factors, including PPARγ and C/EBPα,(Xiao, et al., 2015;; Schmidt, et al., 2018 Yi, et al., 2019). Despite progress in identifying and functionally dissecting lncRNAs involved in adipogenic differentiation and adipose tissue biology, our understanding of how lncRNAs control hADSCs commitment into preadipocytes differentiation and function in particular remains elusive.
To gain molecular insights into these processes, we here performed RNA-sequencing (RNA-Seq) of hADSCs and preadipocytes and characterized a novel adipocyte-specific lncRNA potentiating adipogenic differentiation. This novel lncRNA, named AC092834.1, was significantly increased in preadipocytes and played a positive role in adipogenesis of hADSCs. By gain- and loss-of-function experiments, for the first time, we demonstrated that overexpression of AC092834.1 directly promoted an increase in the level of DKK1, which competitively binds to LRP5 to inhibit the Wnt-β-catenin pathway and reduced inhibition of adipogenesis by Wnt signaling. Our findings provide novel mechanistic insights into a critical role for lncRNA AC092834.1 as a regulator of adipogenic differentiation, which expands our knowledge about molecular mechanisms of obesity and other adipogenic differentiation related disorders.
MATERIALS AND METHODS
Induced adipocyte differentiation and Oil Red O staining: When the hADSCs were 90% confluent, the culture medium was replaced with adipogenesis induction medium (AM), which consists of high-glucose Dulbecco’s modified Eagle medium (DMEM) containing 10% FBS, 0.1 mM ascorbic acid, 1 μΜ dexamethasone and 0.5 mM 3-isobutyl-1-methylxanthine. The AM was changed every 3 days until the intracellular lipid droplets were big and round.
Oil Red O staining was used to monitor the progress of the lipid droplets. Briefly, cells were washed twice with PBS and fixed with 4% paraformaldehyde for 5 min. Then, the paraformaldehyde was washed away with distilled water and the cells were stained with filtered 0.6 mg/mL Oil Red O solution for 30 min at room temperature. After staining, excess stain was removed by rinsing with distilled water and the results were visualized by light microscopy.
RNA extraction and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cultured cells with TRIzol Reagent and treated with DNase I (Ambion, USA) at 37°C for 30 min. cDNAs were synthesized using a high-capacity cDNA reverse transcription kit (Applied Biosystems, USA) and 100 ng of cDNA was used in each sample. Primers used are listed in the Supplementary Table S1. The relative expression level of mRNA was evaluated using the 2-ΔΔCt method and was normalized to the GAPDH expression level.
Cytoplasmic and Nuclear RNA Extraction: The extraction was performed as according to the manufacturer’s protocol of the NE-PER™ Nuclear and Cytoplasmic Extraction Reagent kit (Thermo Scientific, USA). First, harvested cells were washed with ice-cold PBS and centrifuged to remove the supernatant. Then, 100 μL ice-cold CER I was added to each 10 μL cell precipitation and vortexed for 15 sec at high speed and then incubated on ice for 10 min. We repeated this step 2 times to fully lyse the cells. Last, 5.5 μL of ice-cold CER II was added to each 10 μL cell precipitation and vortexed for 5 sec on the highest setting, and then incubated on ice for 2 min. We repeated this step 2 times and the supernatant was collected as the cytoplasmic fraction while the precipitate was the nuclear fraction. For RNA extraction, both fractions were first incubated with Proteinase K (10 mg/mL) at 37℃ for 20 min and then RNA was extracted by TRIzol.
ShRNA transfection and lentiviral vector infection: lncRNA shRNA or the negative control (RiboBio, China) at a concentration of 50 nM was transfected into cells using Lipofectamine 3000 (Invitrogen, USA) and Opti-MEM (Gibco, USA) culture medium, according to the manufacturer’s instructions. Then, 48 h after transfection, cells were harvested and the interference efficiency of the treatments were detected. The lncRNA AC092834.1 sequence was cloned into the LV5-EF1-a-EGFP-Puro vector plasmid, and a vector that expressed a scrambled RNA was used as a negative control. The lentiviruses were produced through the transient transfection of 293T cells using Lipofectamine Plus. Lentivirus vectors and plasmid vectors were prepared according to the protocol from GenePharma (GenePharma, China). The lentiviruses were named lenti-AC092834.1 and lenti-NC. After infection with lentivirus (MOI=10) and selection with puromycin, almost all of the cells were GFP-expressing, indicating stable expression of the lentiviruses.
Western blot analysis: Total protein was extracted using RIPA lysis buffer with 1 mM PMSF, centrifuged and we quantified the supernatant using a BCA Protein Assay Kit (Beyotime, China), and then the proteins were denatured for 10 min in boiling water. A sample of 20 μg of protein from each lysate was separated by SDS-PAGE and transferred onto a PVDF membrane (0.22 μm, Millipore). The PVDF membrane was blocked in 5% nonfat milk in TBST for 1 h and then incubated with primary antibodies at 4℃ overnight, followed by incubation with horseradish peroxidase (HRP)-coupled second antibodies. The specific primary antibodies used were as follows: CEBPA polyclonal antibody (Abcam, #ab2295), PPARG polyclonal antibody (Cell Signaling Technology, #2443), PLIN polyclonal antibody (Abcam, #ab172907), FABP4 polyclonal antibody (Abcam, #ab92501), DKK1 polyclonal antibody (Abcam, #ab61275), β-catenin polyclonal antibody (Abcam, # ab32572) and GAPDH polyclonal antibody (Proteintech, # 60008-1-Ig).
Statistical analysis: All of the staining and western blot images were conducted in triplicate. GAPDH was used as an internal control in the qRT-PCR and western blot analyses. All of the numerical results are presented as the means± S.D from more than three experiments. The significant differences between groups were analyzed using Student’s t-tests (two-tailed) and among multiple groups were assessed by one-way ANOVA. P < 0.05 was considered statistically significant, as indicated by asterisks (*P < 0.05, **P< 0.01, ***P< 0.001)
RESULTS AND DISCUSSION
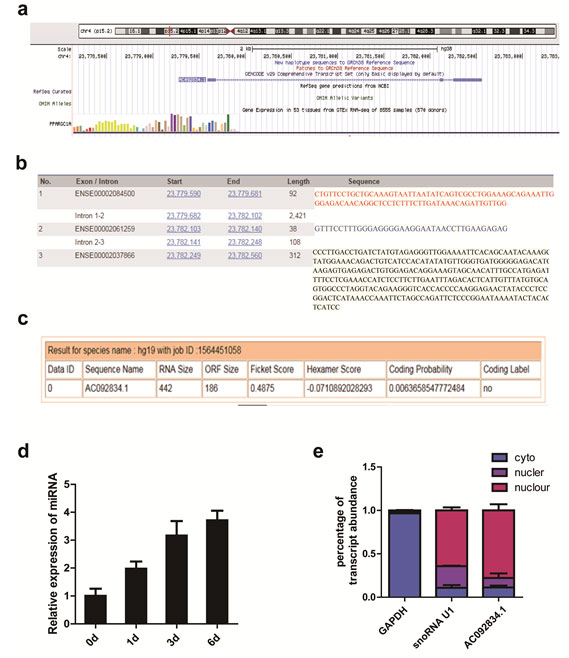
lncRNA AC092834.1 was significantly upregulated during the adipogenic differentiation of hADSCs: HADSCs were cultured and characterized(Fig.S1). RNA-sequencing analyses and qRT-PCR analyses found the lncRNA AC092834.1 (UCSC ID: uc062vpi.1, chr4:23,779,590-23,782,560) was significantly upregulated during adipogenic differentiation of hADSCs.To identify the basic genome information of AC092834.1, the AC092834.1 genomic locus was checked by UCSC (http://genome.ucsc.edu) (Fig. 1a) and Ensemble database (http://www.ensembl.org/index.html) was utilized to obtain its transcriptional variants sequences (Fig. 1b). To further exclude its coding potential, the sequences of lncRNA AC092834.1 were input into the Coding Potential Assessment Tool (CPAT) (http://lilab.research.bcm.edu/cpat/) program to predict its potential to code proteins and the results showed that AC092834.1 has no protein coding probability (Fig. 1c). qRT-PCR analyses detected the expression levels of lncRNA AC092834.1 was significantly upregulated during the adipogenic differentiation of hADSCs (Fig. 1d). Since subcellular localization and cell fractionation are essential for understanding the function and mechanism of lncRNAs, we separated the cytoplasm and nuclear RNA by extraction and detected the AC092834.1 expression level by qRT-PCR analysis. The results showed that AC092834.1 largely displayed a nuclear distribution (>90%), which indicated that AC092834.1 might function at the transcription level (Fig. 1e).
Figure 1: Characterization and expression pattern analysis of lncRNA AC092834.1 a. The genome location locus of AC092834.1 checked in the UCSC database system. b. AC092834.1 exon sequence information shown in the Ensemble database. c. Prediction of protein coding probability of lncRNA AC092834.1 by Coding Potential Assessment Tool (CPAT). d. qRT-PCR analyses detected the expression levels of lncRNA AC092834.1 e. The expression level of AC092834.1 in cytoplasmic and nuclear extracts of hADSCs followed by qRT-PCR analysis. GAPDH served as a cytoplasmic mRNA control and snoRNA U1 served as a nuclear RNA control.
Downregulation of lncRNA AC092834.1 inhibited the adipogenic differentiation of hADSCs
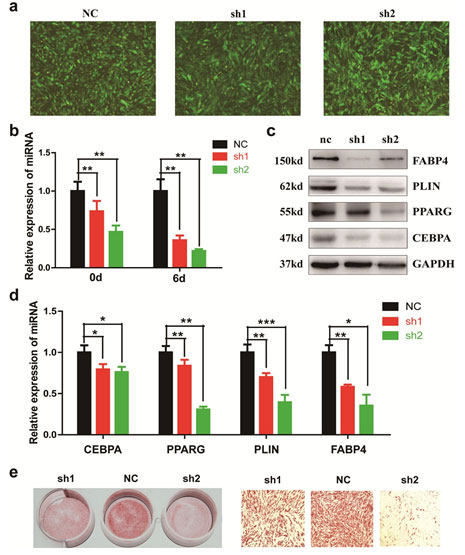
To study the function of lncRNA AC092834.1 during adipogenesis, we inhibited the endogenous expression of lncRNA AC092834.1 in hADSCs by two shRNA lentiviral vectors (sh1, sh2) and a negative control (NC) (Fig. 2a). Then, the qRT-PCR analysis confirmed that the intracellular lncRNA AC092834.1 mRNA levels were significantly downregulated on day 0 and day 6 of adipogenesis (Fig. 2b). qRT-PCR and western blot analyses revealed that when lncRNA AC092834.1 was downregulated, adipogenic transcription factors and marker genes CEBPA, PPARG, PLIN, and FABP4 were markedly decreased at both the mRNA and protein levels (Fig. 2c, 2d). Consistently, we also found knockdown of lncRNA AC092834.1 resulted in an adipogenesis delay, as shown by the decreased number of Oil Red O staining positive cells (Fig. 2e). These results suggested that lncRNA AC092834.1 plays an important role in adipogenesis of hADSCs.
Figure 2: AC092834.1 knockdown inhibited the adipogenic differentiation of hADSCs lncRNA AC092834.1 was silenced using two independent shRNAs (sh1 and sh2). The knockdown efficiency was verified by qRT-PCR compared with the negative control (NC) on day 0 and day 6 of adipogenesis after lncRNA AC092834.1 knockdown. b., c. qRT-PCR and western blot analyses detected the mRNA and protein levels of adipogenesis transcription factors and marker genes CEBPA, PPARG, PLIN, and FABP4 after lncRNA AC092834.1 knockdown. d. Oil Red O staining was performed on day 12 of adipogenic differentiation to evaluate the efficiency of adipogenesis after lncRNA AC092834.1 knockdown.
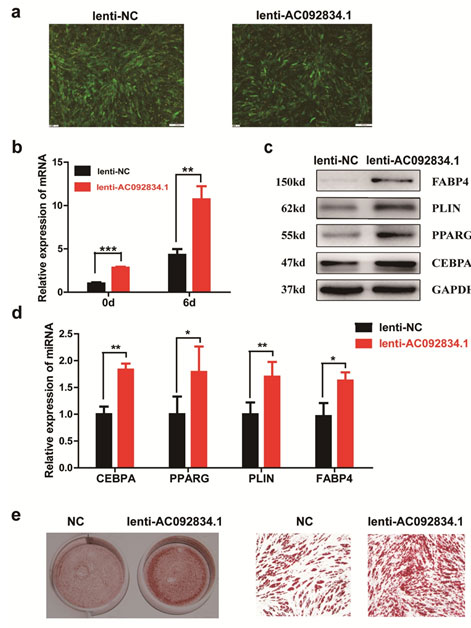
Overexpression of lncRNA AC092834.1 facilitates adipogenesis of hADSCs: To further confirm the role of AC092834.1 in the differentiation of hADSCs to adipocytes, we overexpressed full length AC092834.1 in hADSCs using a lentivirus vector (Fig. 3a), and AC092834.1 expression was thus significantly upregulated on day 0 and day 6 of adipogenesis (Fig. 3b). Compared with the control, ectopic expression of AC092834.1 significantly impaired the expression of the adipogenic transcription factors and marker genes CEBPA, PPARG, PLIN, and FABP4, as verified by qRT-PCR and western blot analyses (Fig. 3c, 3d). Moreover, Oil Red O staining indicated that there were more lipid droplets after the treatments with the lentivirus vector, indicating more mature adipocytes and that the adipogenesis of hADSCs was accelerated (Fig. 3e). Altogether, these data demonstrate that AC092834.1 is a positive regulator in the differentiation of hADSCs towards adipocytes.
Figure 3: AC092834.1 promoted the adipogenic differentiation of hADSCs LncRNA AC092834.1 overexpression lentiviral vectors (lenti-AC092834.1) were used to upregulate AC092834.1 expression. Images show GFP-positive cells under a fluorescence microscope, indicating that the cells were stably transfected. b. qRT-PCR analysis detected the mRNA level of lncRNA AC092834.1. c., d. qRT-PCR and western blot analysis detected the mRNA and protein levels of CEBPA, PPARG, PLIN, and FABP4. e. Oil Red O staining was performed to view the efficiency of adipogenesis.
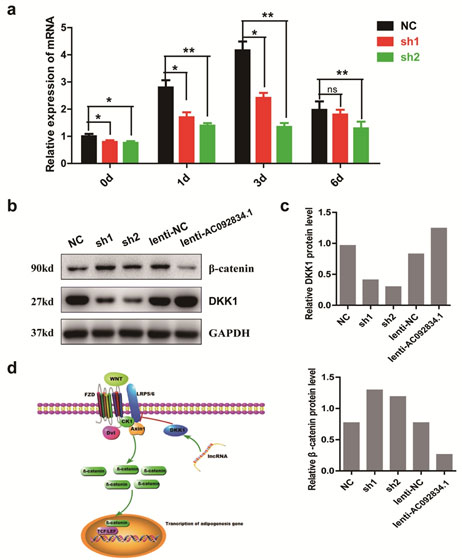
lncRNA AC092834.1 might induce DKK1 expression and antagonize the Wnt/β-catenin pathway: The observations from the AC092834.1 loss-of-function and gain-of-function studies suggested that AC092834.1 plays an important role in adipogenesis gene regulation. Wnt/β-catenin signaling is an important pathway that regulates adipogenesis, and by analyzing changes in important molecules, we found that DKK1 mRNA expression was significantly decreased during adipocytes differentiated from the AC092834.1 knockdown (Fig. 4a). DKK1 antagonizes WNT signaling by binding as high-affinity antagonists to LRP5/6 co-receptors that results in the degradation of cytosolic β-catenin. The results showed that the protein level of DKK1 was reduced while β-catenin was increased in AC092834.1 knockdown cells, and the contrary results were seen in AC092834.1 over expression cells (Fig. 4b, 4c). These results demonstrated that AC092834.1 might promote DKK1 expression and thus more DKK1 was secreted extra cellularly to competitively combine with LRP5/6 and block WNTs binding, subsequently inducing decreases in the β-catenin protein level. Suppression of the Wnt/β-catenin pathway promoted the transcription of adipogenic factors and thus facilitated the differentiation of hADSCs into adipocytes (Fig. 4d).
Figure 4: LncRNA AC092834.1 regulated the expression of DKK1 and β-catenin of the Wnt pathway. qRT-PCR analysis of DKK1 mRNA expression in hADSCs that were transfected with siRNA or infected by lentiviral vectors. b. Western blot analysis of the protein level of DKK1 and β-catenin in hADSCs that were transfected with siRNA or infected by lentiviral vectors. c. Protein quantitative analysis of DKK1 and β-catenin by ImageJ software. d. Schematic diagram of the regulatory effects of AC092834.1 on hADSCs adipogenic differentiation.
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into ectodermal, mesodermal and endodermal lineage cells, and have immune modulatory properties that could benefit patients with autoimmune diseases, (Ullah,et al., 2015). The multipotential capacities and therapeutic potential of hADSCs have prompted numerous clinical studies based on MSC with encouraging results reported to date[Le, et al., 2008]. MSCs have received increasing attention as a new target for obesity therapy, and the results suggested that obesity could be prevented by controlling MSC adipogenesis, (Matsushita and Dzau, 2017). However, understanding of the relationship between MSCs and obesity and its potential clinical implications remains ill defined, and further studies are necessary of the mechanisms, regulation and outcomes of adipogenesis crucial for MSC-based treatments for obesity.
Recently, some studies proved that long noncoding RNAs have a significant and crucial role in the maintenance, commitment and differentiation of MSCs to adipocyte lineages by regulating the principal adipogenic transcription factors and signaling pathways, (Cai, et al., 2018). LncRNA ADINR has been proven to transcriptionally activate C/EBPα by specifically binding to PA1 and recruiting MLL3/4 histone methyl-transferase complexes to increase H3K4me3 and decrease H3K27me3 histone modification, (Xiao, et al., 2015). The PPARγ -activator RBM14-associated lncRNA (Paral1) is restricted to adipocytes and is decreased in humans with an increased body mass index and in diet-induced or genetic mouse models of obesity. LncRNA Paral1 favors adipocyte differentiation and coactivates the master adipogenic regulator PPARγ, and thus it is considered an obesity-sensitive regulator of adipocyte differentiation and function, (Firmin, et al., 2017).
To explore more lncRNAs that play important roles in adipogenic differentiation, we compared the lncRNA expression profiles of preadipocytes and hADSCs through high-throughput RNA-sequencing and selected several lncRNAs that were significantly upregulated in preadipocytes. Among these lncRNAs, a novel lncRNA with the gene number AC092834.1 that was mainly located in the nucleus was found to have significantly upregulated expression. Further study suggested that AC092834.1 may be a positive regulator of adipogenesis progression and knockdown of AC092834.1 could significantly inhibit the expression of adipogenesis transcription factors CEBPα and PPARγ and adipocyte marker genes PLIN and FABP4, thus strongly repressing the adipogenic hADSCs process.
The Wnt/β-catenin signaling pathway is not only featured in multiple biological processes, such as embryonic development, inflammation, and stem cell differentiation (Marchetti,et al., 2020), but also recently emerged as an attractive negative regulator of adipocytes differentiation,(Jeon,et al.,2016). Studies demonstrated that activation of Wnt/β-catenin signaling prevents the induction of C/EBPα, C/EBPβ, and PPARγ transcription factors, resulting in the suppression of adipogenesis of hADSCs; however, blocking endogenous Wnt signaling promotes adipogenic differentiation, suggesting that Wnt acts as a brake for adipogenesis, (Chung,et al.,2012). The canonical Wnt ligands bind with specific cell surface Frizzled (FZD) receptors and low-density lipoprotein-receptor-related protein-5 or -6 (LRP5/6) coreceptors, and then combines with intracellular degradation complex Dishevelled (Dvl), Axin 1 and casein kinase I (CK1), which results in hypophosphorylation of β-catenin and its translocation to the nucleus where it binds to the lymphoid-enhancer-binding factor/T-cell-specific transcription factor (LEF/TCF) family of transcription factors to activate the PPARγ and CEBPα genes,(Nusse and Clevers, 2017). Wnt signaling is modulated by several extracellular antagonists such as dickkopf-1 (DKK1), a newly recognized secreted glycoprotein, that has been demonstrated to inhibit WNT signaling by binding as a high-affinity antagonist to LRP5/6 co-receptors, resulting in the degradation of cytosolic β-catenin,(Rachner and Göbel, 2014).
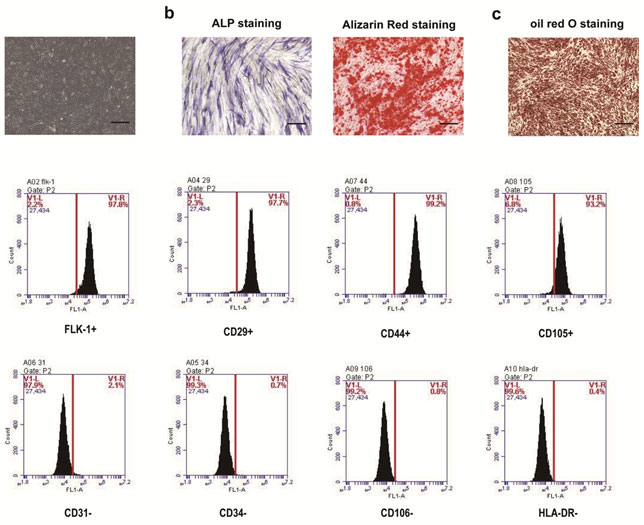
Figure S1. Identification and characterization of hADSCs. a. The morphology of passage 3 of hADSCs under a light microscope. b. The early osteogenic differentiation was identified by ALP staining and Alizarin Red staining. c. The adipogenic differentiation of the hADSCs was confirmed by Oil Red O staining to detect the formation of lipid droplets in the cells. d. Flow cytometry assay detected cell surface marker expression of hADSCs. Scale bar: 100 μm.
Thus, DKK-1 serves as a potential therapeutic target in many diseases such as cancer, diabetic nephropathy, and osteoporosis[Chae,et al.,2019]. Recently, DKK-1 has been found to promote early adipogenesis during human adipogenesis. Studies found that increasing DKK-1 expression during the early stage of adipogenesis and exogenous rhDKK-1 exposure accelerates differentiation by upregulating PPAR-γ and C/EBPα,m (Lu, et al.,2016; Park,et al., 2008). In our study, we found that AC092834.1 could promote DKK1 expression and thus more DKK1 was secreted extracellularly to competitively combine with LRP5/6 and block Wnt binding, subsequently inducing β-catenin degradation leading to a decreased β-catenin protein level. This resulted in suppression of the Wnt/β-catenin pathway and facilitated adipogenic differentiation of hADSCs, which is consistent with the findings of previous studies.
In this study, we identified a novel lncRNA with functions in adipocyte physiology by comparing the expression patterns of lncRNAs before and after the adipocytes differentiation of hADSCs. We found that the expression of lncRNA AC092834.1 was strongly increased during adipogenesis, and for the first time, we demonstrated that lncRNA AC092834.1 acts as a positive regulator of adipogenic differentiation of hADSCs. Importantly, mechanistic analysis revealed that AC092834.1 increased DDK1 expression and subsequently competitively combined with LRP5/6 to accelerate β-catenin degradation, which suppressed the Wnt/β-catenin pathway and, thereby, impacted genes involved in the adipogenesis of hADSCs. This finding adds a new piece to the puzzle of the contribution of lncRNAs to the adipogenesis of hADSCs, and further identification of functional lncRNAs and cell type-specific signaling in adipogenesis and obesity regulation will help to expand the therapeutic repertoire to combat obesity and other adipogenic differentiation-related disorders.
ACKNOWLEDGEMENTS
The authors thank American Journal Experts for providing English language editing of the manuscript.
Conflicts of Interest: None
REFERENCES
Cai R, Sun Y, Qimuge N, Wang G, Wang Y, Chu G, Yu T, Yang G, Pang W. (2018) Adiponectin AS lncRNA inhibits adipogenesis by transferring from nucleus to cytoplasm and attenuating Adiponectin mRNA translation. Biochim Biophys Acta Mol Cell Biol Lipids. Apr;1863(4):420-432.
Chae WJ, Bothwell ALM. Dickkopf (2019) An immunomodulatory ligand and Wnt antagonist in pathological inflammation. Differentiation. Jun 12. pii: S0301-4681(19)30006-4
Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L1, Yang H, Hou J, Wang Y, Shi Y. (2016) Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 23(7):1128-1139.
Chung SS, Lee JS, Kim M, Ahn BY, Jung HS, Lee HM, Kim JW, Park KS. (2012) Regulation of Wnt/β-catenin signaling by CCAAT/enhancer binding protein β during adipogenesis,Obesity (Silver Spring). Mar;20(3):482-487.
Cohen P, Spiegelman BM. (2018) Cell biology of fat storage. Mol Biol Cell. 2016; 27,2523–2527.
Corrales P, Vidal-Puig A, Medina-Gómez G. PPARs and Metabolic Disorders Associated withChallenged Adipose Tissue Plasticity, Int J Mol Sci. 19, 2124-2140.
Fico A, Fiorenzano A, Pascale E, Patriarca EJ, Minchiotti G. (2019) Long non-coding RNA in stem cell pluripotency and lineage commitment: functions and evolutionary conservation. Cell Mol Life Sci. 76(8):1459–1471. doi:10.1007/s00018-018-3000-z
Firmin FF, Oger F, Gheeraert C, Dubois-Chevalier J, Vercoutter-Edouart AS, Alzaid F, Mazuy C1, Dehondt H, Alexandre J, Derudas B, Dhalluin Q, Ploton M, Berthier A, Woitrain E, Lefebvre T, Venteclef N, Pattou F, Staels B, Eeckhoute J, Lefebvre P. (2017) The RBM14/CoAA-interacting, long intergenic non-coding RNA Paral1 regulates adipogenesis and coactivates the nuclear receptor PPARγ. Sci Rep. Oct 26;7(1):14087-14102.
Hadrich F, Sayadi S. (2018) Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 25;17(1):95
Jeon M, Rahman N, Kim YS. (2016) Wnt/β-catenin signaling plays a distinct role in methyl gallate-mediated inhibition of adipogenesis.BiochemBiophys Res Commun. Oct 7;479(1):22-7
Kopp F, Mendell J.T.. (2018) Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. Jan 25;172(3):393-407.
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O. (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. May 10;371(9624):1579-1586.
Lu H, Li X, Mu P, Qian B, Jiang W, Zeng L. (2016) Dickkopf-1 promotes the differentiation and adipocytokines secretion via canonical Wnt signaling pathway in primary cultured human preadipocytes.Obes Res Clin Pract. 10(4):454-464.
Marchetti B, Tirolo C, L’Episcopo F, et al. (2020) Parkinson’s disease, aging and adult neurogenesis: Wnt/β-catenin signalling as the key to unlock the mystery of endogenous brain repair [published online ahead of print, 2020 Feb 12]. Aging Cell. e13101. doi:10.1111/acel.13101.
Martenstyn J, King M, R, (2020) Impact of weight loss interventions on patient-reported outcomes in overweight and obese adults with type 2 diabetes: a systematic review.J Behav Med. Feb 14. doi: 10.1007/s10865-020-00140-7
Nusse R, Clevers H. (2017) Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities,Cell. Jun 1;169(6):985-999.
Park JR, Jung JW, Lee YS, Kang KS. (2008) The roles of Wnt antagonists Dkk1 and sFRP4 during adipogenesis of human adipose tissue-derived mesenchymal stem cells,Cell Prolif. Dec;41(6):859-874.
Rachner TD, Göbel A2, Benad-Mehner P, Hofbauer LC, Rauner M. (2014) Dickkopf-1 as a mediator and novel target in malignant bone disease.Cancer Lett. May 1;346(2):172-177.
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. (2002) C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev.,1;16(1):22-26.
Schmidt E, Dhaouadi I, Gaziano I, Oliverio M, Klemm P, Awazawa M, Mitterer G, Fernandez-Rebollo E, Pradas-Juni M, Wagner W, Hammerschmidt P, Loureiro R, Kiefer C, Hansmeier NR, Khani S, Bergami M, Heine M, Ntini E, Frommolt P, Zentis P, Ørom UA, Heeren J, Blüher M, Bilban M, Kornfeld JW. Linc (2018) RNA H19 protects from dietary obesity by constraining expression of monoallelic genes in brown fat. Nat Commun. Sep 6;9(1):3622-3637
Shulman, G.I. (2014) Ectopic fat in insulin resistance, dyslipidemia,and cardiometabolic disease. N Engl J Med, 371,1131–1141.
Sun, B., and Karin M.. Obesity, inflammation, and liver cancer. J Hepatol, (2012). 56, 704–713.
Ullah I, Subbarao RB, Rho GJ. (2015) Human mesenchymal stem cells – current trends and future prospective.Biosci Rep. Apr 28;35(2). pii: e00191. doi: 10.1042/BSR20150025.
Xiao T, Liu L, Li H, Sun Y, Luo H, Li T, Wang S, Dalton S, Zhao RC, Chen R. Long (2015) Noncoding RNA ADINR Regulates Adipogenesis by Transcriptionally Activating C/EBPα. Stem Cell Reports. Nov 10;5(5):856-865.
Yi F, Zhang P, Wang Y, Xu Y, Zhang Z, Ma W, Xu B, Xia Q, Du Q. (2019) Long non-coding RNA slincRAD functions in methylation regulation during the early stage of mouse adipogenesis. RNA Biol. Jun 19:1-13