Centre for Drug Discovery and Development, Col. Dr. Jeppiaar Research Park, Sathyabama Institute of Science and Technology, Chennai – 600 119. Tamil Nadu. India
Corresponding author email: jerrine.jj@gmail.com
Article Publishing History
Received: 08/01/2020
Accepted After Revision: 07/03/2020
Use of medicinal plants for the treatment of infectious and life style diseases is since time immemorial. This study reports the in vitro antibacterial activity of selected medicinal plant extracts against Mycobacterium tuberculosis H37Rv and other non-mycobacterial pathogens. Fruits of S. torvum, Z. mauritiana and leaves of V.negundo were sequentially extracted using n-hexane, ethyl acetate and methanol. All n-hexane, ethyl acetate and methanol extracts were screened against clinical pathogens viz., S. aureus ATCC 29213, P. aeruginosa ATCC 27853, Carbapenem resistant K. pneumoniae ATCC 700603 and V. parahaemolyticus by agar well diffusion method at 10 μg/μL concentration. The methanol extract of S. torvum and ethyl acetate extract of V.negundo and Z.mauritiana demonstrated 17 and 23 mm inhibition against the non-mycobacterial pathogens, respectively. The three extracts were also screened for anti-tubercular activity against M. tuberculosis H37Rv using Luciferase Reporter Phage (LRP) assay. All the three extracts were exhibited anti TB activity at 500 μg/ml concentration. In particular, the S. torvum extract was showed 98.46% inhibition. GC-MS analysis of the afore mentioned extracts yielded peaks of compounds of ethnomedicinal value/significance. Findings of this study depicted that the medicinal plant S. torvum deserves the potential for isolation of anti TB molecules.
Anti-TB activity, Medicinal plants, Mycobacterium tuberculosis, Tuberculosis
Chandramouli V, Ramasamy A, Manikkam R, Sivaraj A, Aruni W, Joseph J. In Vitro Activity of Selected Medicinal Plant Extracts Against Mycobacterium tuberculosis and other Non Mycobacterial Pathogens. Biosc.Biotech.Res.Comm. 2020;13(1).
Chandramouli V, Ramasamy A, Manikkam R, Sivaraj A, Aruni W, Joseph J. In Vitro Activity of Selected Medicinal Plant Extracts Against Mycobacterium tuberculosis and other Non Mycobacterial Pathogens. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/2Q2LbxB
Copyright © Chandramouli et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Tuberculosis (TB), caused by Mycobacterium tuberculosis, remains among the top ten mortality causing diseases in the world. According to WHO, 10 million new cases of TB and 1.3 million deaths were estimated globally in 2017. This puts the disease burden of TB to be equivalent to 133 deaths per 1,00,000 people. Around 27% of the global TB cases were contributed by India in 2017 which highlights the burden of the disease on the Indian population. The severity of multi-drug resistance TB still persists and the success rates of treatment for MDR/RR-TB (Multi-drug resistant/Rifampicin resistant TB) and XDR-TB (Extensively drug resistant TB) are strikingly low, being 55% and 34% respectively. The problem of drug resistance does not appear to have an easy solution in the near future. Synthetic drugs used in the TB treatment programme pose a heavy burden on the already weakened body of TB patients, (WHO Global TB report 2019).
The drugs are most commonly nephrotoxic (Hussein et al., 2015) and hepatotoxic (Ramappa et al., 2012), thereby leading to harmful side effects of the treatment. This shifts the focus of the treatment regime to natural products and medicinal herbs for treatment of infectious diseases. Use of ethno-medically significant plants for treatment can be initiated by validating the traditional methods that were reported to be used since ancient times. These methods were used to treat multiple ailments including bacterial and viral diseases. With several reports on the activity of herbal medicines against pathogens, there is a need to focus on the potential of herbal medicines in the treatment of infectious diseases,( Ladda and Magdum 2018).
Solanum torvum is a shrub of the Solanaceae family widely available and extensively used in India for the treatment of bacterial diseases and for relief from cough and cold (Yousaf et al., 2013). It is native to other tropical countries and has been traditionally used for treatment of various ailments such as a sedative and diuretic, TB, skin infections, fever and tooth decay (Naimon et al., 2015; Silva et al., 2011). The plant extracts have been reported to be possess cardioprotective, nephroprotective, anti-viral, anti-microbial, anti-oxidant, anti-ulcerogenic and haemostatic properties (Jaiswal et al., 2012). Such properties could be attributed to the presence of phytoconstituents such as flavonoids, steroids, saponins, tannins, alkaloids, vitamin B, vitamin C and phenols, (Jaiswal et al., 2012; Yousaf et al. 2013 Ladda and Magdum (2018) ).
Ziziphus mauritiana is a member of the Rhamnaceae family and is native to the Indian subcontinent, Africa, Iran and parts of Southern Asia. It is known to be traditionally used for the treatment of pain, vomiting and diarrhoea (Mahesh et al., 2008). It has also been reported to possess anti-plasmodial effect (Sameera et al., 2015); its seeds are good sedatives while its leaves are used for treatment of sores, cuts and ulcers. It has also been demonstrated to resolve liver troubles, asthma and experimentally induced liver damage (Abalaka et al., 2010). The juice obtained from the bark of its root is known to alleviate gout and rheumatism according to traditional medicinal practises (Priyanka et al., 2015). It is a pharmacologically important plant since it is known to possess anti-typhoid, anti-cancer, antioxidant and anti-inflammatory properties (Abdallah et al., 2016).
Vitex negundo is a member of the Verbenaceae family and is native to South Asia, East Africa, South America, Indonesia and Japan. It has been demonstrated to possess analgesic, anti-oxidant, anti-inflammatory, hypoglycemic, anti-tumour, anti-rheumatism and insecticidal activities (Zheng et al., 2015, Gupta et al., 2010). The leaves of the plant have been proven to possess anti-convulsant and anti-parasitic activities (Ladda and Magdum 2018) which is an addition to the traditional medicinal properties that the plant has been reported to possess. In this study, we have investigated the antibacterial and anti-mycobacterial properties of different solvent extracts of S. torvum fruits, Z.mauritiana fruits and leaves of V.negundo. GC-MS analysis was performed to identify the potential compounds present in the extracts. With the help of the present in vitro studies, it may be possible to maximize the traditional use of the plants for treatment of various infections.
MATERIALS AND METHODS
The fruits of Solanum torvum, fruits of Ziziphus mauritiana and leaves of Vitex negundo were collected in 2018, from Chennai, India, and duly authenticated by a botanist. The fruits and leaves were processed by washing thrice with distilled water and surface sterilization by rinsing with 70% acetone. Then the samples were shade dried and powdered using mixer grinder. Sequential extraction was performed using the solvents n-hexane, ethyl acetate and methanol to extract the compounds from the powdered plant material. Ten gram of powdered plant material was added to 100 mL n-hexane and incubated at room temperature in an orbital shaker at 80 rpm for 24 hrs. The extract was filtered using Whatman filter paper and the powdered plant material was reused upon drying. To the dried powder, 100 mL of ethyl acetate and subsequently methanol were added and the extracts were filtered and collected in a similar manner. The n-hexane, ethyl acetate and methanol extracts were concentrated using rotary evaporator and dried by incubating the extracts at 50 ºC for 24-36 hrs. The weight of the extracts was determined and the extracts were dissolved in 10% DMSO for evaluating their antibacterial and anti-mycobacterial activities.
Anti-bacterial activity of the extracts was evaluated by agar well diffusion method. Overnight grown cultures of bacterial pathogens viz. Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa ATCC 27853, Carbapenem resistant Klebsiella pneumoniae ATCC 700603 and Vibrio parahemolyticus were swabbed on individual Muller-Hinton Agar plates. Wells of 5 mm diameter were cut on the plate using well cutter and 10 μg/μL of n-hexane (HF), ethyl acetate (EAF) and methanol (MF) extracts were added to each well respectively. The plates were then incubated at 37 ºC for 18 hours and zones of inhibition were measured. The assay was performed in triplicates and mean values were calculated and tabulated.
The antitubercular activity of HF, EAF and MF was screened against M. tuberculosis H37Rv by Luciferase Reporter Phage assay (Radhakrishnan et al., 2016). Briefly, 400 μL of middlebrook 7H9 broth was added to two cryovials (Control) and another 400 μL of rifampicin containing Middlebrook 7H9 broth at concentration of 2 μg/mL was added to a cryovial (drug control). About 350 μL of Middlebrook 7H9 was added to two cryovials (solvent control and test). Fifty μL of extract (HF) was added to test vial to achieve a final concentration of 500 μg/mL and 50 μL of 10% DMSO was added to solvent control vial. All the vials were added with 100 μL of M. tuberculosis H37Rv (McFarland unit 2) and incubated at 37 ºC for 72 hrs. After incubation, 50 μL of phAE202 and 40 μL of 0.1 M CaCl2 were added to all the vials (Cell-phage mixture) and incubated at 37 ºC for 4 hrs. About 100 μL of cell-phage mixture from each vial was added into a luminometer cuvette, to which 100 μL of D- Luciferin was added. The relative light unit (RLU) was measured immediately at 10 sec integration time using the luminometer (Lumat 9508, Berthold, Germany). The above mentioned procedure were followed for the other extracts MF and EAF as well. The percentage reduction was calculated using the formula:% RLU reduction = Control RLU- Test RLU/ Control RLU x 100
On comparison with control, if the sample showed 50% or more reduction of RLU, the extract was deemed to have anti tubercular activity. The potential MF extract was analysed by GC-MS (Shimadzu QP2010 Ultra).
RESULTS AND DISCUSSION
Sequential extraction procedures are commonly used to isolate a number of compounds from plant extracts. The process offers improved phase-specificity due to combined use of multiple solvents of varying polarity. In sequential extraction, the plant residue from the first extraction is used as the material for the second extraction and the process may be continued as required (Kaplan et al., 2009). In this study, sequential extraction was carried out using solvents of increasing polarity. Due to the difference in chemical nature of the solvents, the process is very selective in extraction of compounds from plants. Sequential extraction from 10 g of plant extracts using 100 mL of solvents n-hexane, ethyl acetate and methanol yielded fractions whose stock concentrations were maintained at 10 mg/mL. The crude extracts were diluted and a final concentration of 10 μg/μL was used for evaluating anti-microbial activity against the selected non-mycobacterial pathogens. The inhibitory activity of n-hexane, ethyl acetate and methanol extracts of the fruits of S. torvum, fruits of Z.mauritiana and leaves of V.negundo were screened against clinical pathogens and results were summarized in Table 1.
Table 1. Activity of extracts tested against non-mycobacterial pathogens
| S. No. | Plant | Extract | Zone of inhibition against pathogen
(mm in diameter) |
|||
| S. aureus
ATCC 29213 |
P. aeruginosa ATCC
27853 |
Carbapenem resistant
K. pneumoniae ATCC 700603 |
Vibrio parahem-olyticus | |||
| 1 | Solanum torvum | HF | – | – | – | – |
| EAF | – | 12 | – | 12 | ||
| MF | 17 | 17 | – | 25 | ||
| 2 | Ziziphus mauritiana | HF | – | – | – | – |
| EAF | 23 | 11 | 11 | – | ||
| MF | 12 | – | – | 12 | ||
| 3 | Vitex negundo | HF | – | – | – | 25 |
| EAF | 15 | 15 | – | – | ||
| MF | – | – | – | – | ||
The methanol extract (MF) of S.toruvum fruits demonstrated inhibitory activity against S. aureus ATCC 29213 (17mm), P. aeruginosa ATCC 27853 (17mm) and V. parahaemolyticus (25mm) at a concentration of 10 μg/μL whereas the ethyl acetate extract (EAF) showed inhibition against P. aeruginosa ATCC 27853 (12mm) and V. parahaemolyticus (12mm) at the same concentration. None of the pathogens tested were inhibited by hexane extract (HF). Chah et al. (2000) demonstrated the antimicrobial activity of methanolic and ethanolic extracts of S. torvum against Actinomyces pyogenes, B. subtilis, S.pyogenes, A.niger and C.albicans. In other reports, plant extracts of S. torvum showed inhibition against B.cereus, Staphylococcus epidermidis, E. coli, V. cholerae, Salmonella cibrum and Salmonella typhimurium (Naimon et al., 2015; Sivapriya et al., 2011).
The ethyl acetate extract (EAF) of Z.mauritiana fruits inhibited the growth of S. aureus ATCC 29213 (23mm), P.aeruginosa ATCC 27853 (11mm) and Carbapenem resistant K.pneumoniae ATCC 700603 (11mm) at a concentration of 10 μg/μL. However, 10 μg/μL of MF inhibited the growth of only S.aureus ATCC 29213 (12mm) and V.parahaemolyticus (12mm), while the HF did not inhibit the growth of any of the pathogens tested. The antibacterial activity of the extracts demonstrated against S.aureus is in accordance with previous reports of the same being exhibited by extracts of Z.mauritiana as reported by Mahesh et al., 2008, Abdallah et al., 2016 and Priyanka et al., 2015. Their reports also illustrated the anti-bacterial activity of Z.mauritiana extracts against bacterial pathogens such as B.subtilis, E.coli, Pseudomonas fluorescens and K.pneumoniae.
EAF of V.negundo leaves was inhibited the growth of S.aureus ATCC 29213 (15mm) and P.aeruginosa ATCC 27853 (15mm) at a concentration of 10 μg/μL. The HF only inhibited the growth of V. parahaemolyticus (25 mm), while the MF did not inhibit the growth of any of the pathogens tested. This was similar to the reports of antibacterial activity of V.negundo extracts against S.aureus, V.parahaemolyticus and K.pneumoniae (Zheng et al., 2015). This helps to substantiate the ethnomedicinal use of V.negundo for the treatment of cold, cough and bacterial dysentery (Gupta et al., 2010).
Upon screening of the extracts against M. tuberculosis H37Rv by Luciferase Reporter Phage (LRP) assay, MF of S.torvum showed promising inhibitory activity against M. tuberculosis H37Rv at 500 μg/mL concentration and the RLU reduction in terms of inhibition was found to be 98.46% (Table 2). Our results are in correlation with that of Mohamad et al., (2011), in which hydromethanolic fruit extracts from S.torvum displayed moderate antimycobacterial activity against M. tuberculosis H37Rv. Previously other parts of plants are also evaluated for antimycobacterial activity includes the ethanol extract of leaves from S. torvum, which displayed activity against M. smegmatis and M. tuberculosis H37Rv (Nguta et al., 2016). In this study, Z.mauritiana EAF was showed 84.41% inhibition against M.tuberculosis H37Rv at a concentration of 500 μg/mL. Using the alamar plate assay, Z.mauritiana was reported to have anti-mycobacterial activity against M.tuberculosis H37Ra as reported by Panseeta et al., (2011). V.negundo EAF demonstrated an anti-mycobacterial activity of 59.2% at a concentration of 500 μg/mL. This is along the lines of the published reports of Ladda et al., (2018) and Gupta et al., (2010) where the plant extracts were tested against M. tuberculosis H37Rv.
Table 2. Anti-tubercular activity of extracts against M. tuberculosis H37Rv by LRP
| Extracts | % inhibition against M. tuberculosis H37Rv |
| S.torvum-MF | 98.46 |
| Z.mauritiana-EAF | 84.41 |
| V.negundo-EAF | 59.2 |
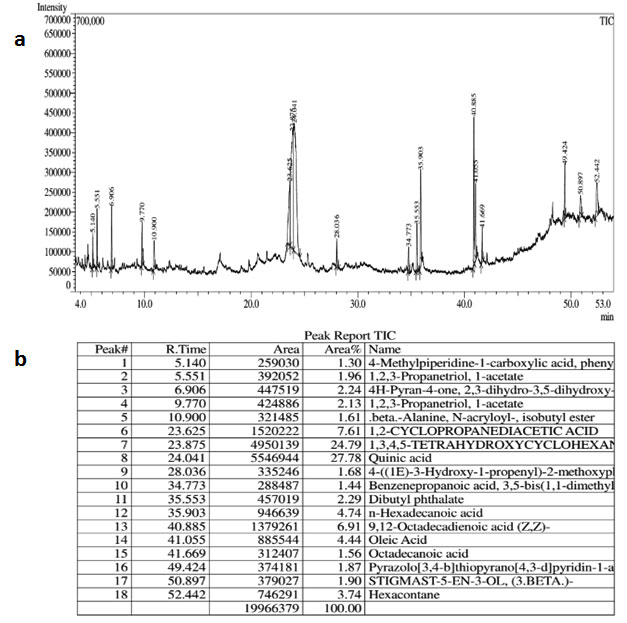
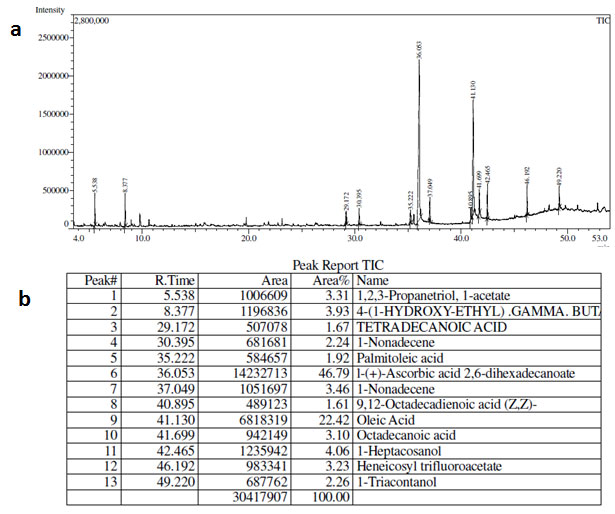
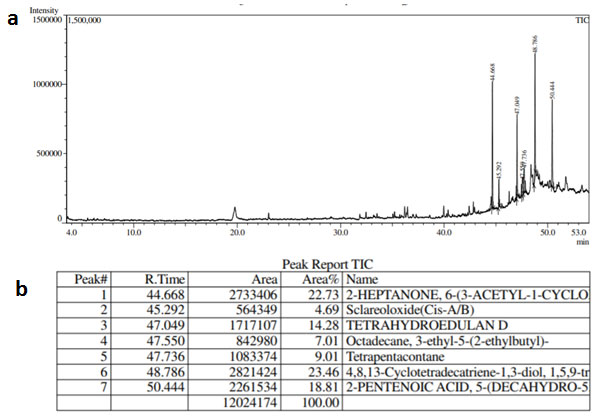
GC-MS analysis of MF of S.torvum (Fig. 1) showed several compounds among which the prominent peaks denotes the presence of Quinic acid, n-hexadecanoic acid, oleic acid and 9,12-octadecadienoic acid. The GC-MS peaks of EAF of Z. mauritiana (Fig. 2) yielded significant peaks corresponding to ascorbic acid-2,6-dihexadecanoate and oleic acid, while those of V.negundo yielded peaks (Fig. 3) which indicated the presence of 2-heptanone, 4,8,13-cyclodecatriene and 2-pentanoic acid. The antimicrobial and anti-mycobacterial activity of the extracts could be attributed to the presence of oleic acid as reported by Ojo et al., (2018; Kalita et al., 2018 and Santhosh et al., 2013). Fatty acids such as n-hexadecanoic acid, otherwise known as palmitic acid has also been reported to possess anti-mycobacterial activity (Ojo et al., 2018). Ascorbic acid, 2,6-dihexadecanoate is an antioxidant which could play a significant role in combatting deficiency or imbalance of essential nutrients which is a common problem that occurs in patients infected by TB. The ascorbic acid from the extracts could compensate for the decreased anti-oxidant levels and further elevate and complement the effects of the treatment (Turchenko et al., 2008).
Figure 1: (a) GC-MS chromatogram (b) GC-MS Peak Report of Methanol fraction of S. torvum
Figure 2:(a) GC-MS chromatogram (b) GC-MS Peak Report of Ethyl acetate fraction of Z.mauritiana.
Figure 3:(a) GC-MS chromatogram (b) GC-MS Peak Report of Ethyl acetate fraction of V.negundo.
CONCLUSION
In conclusion, S. torvum fruits, fruits of Z.mauritiana and leaves of V.negundo have potential anti-mycobacterial properties which can be taken up for further in vitro and in vivo studies. Phyto constituents of the plant extracts can be purified and investigated further to identify lead compounds responsible for anti-bacterial and anti-mycobacterial activity.
ACKNOWLEDGEMENT
Authors thank the authorities of Sathyabama Institute of Science and Technology for the research facilities provided.
Conflict of Interest
There are no conflict of Interest
REFERENCES
Abalaka ME Daniyan SY Mann A (2010) Evaluation of the antimicrobial activities of two Ziziphus species (Ziziphus mauritiana L. and Ziziphus spinachristi L.) on some microbial pathogens African Journal of Pharmacy and Pharmacology Vol 4 No 4 Pages 135-139.
Abdallah EM Elsharkawy ER Ed-Dra A (2016) Biological activities of methanolic leaf extract of Ziziphus mauritiana. Bioscience Biotechnology Research Communications Vol 9 No 4 Pages 605-614
Abhishek RU Thippeswamy S Manjunath K Mohana DC (2015) Antifungal and antimycotoxigenic potency of Solanum torvum Swartz. Leaf extract: Isolation and identification of compound active against mycotoxigenic strains of Aspergillus flavus and Fusarium verticillioides Journal of Applied Microbiology Vol 119 Pages 1624-1636.
Balachandran C Duraipandiyan V Dhabi NAA Balakrishna K et al (2012) Antimicrobial and antimycobacterial activities of methyl caffeate isolated from Solanum torvu, Swartz. fruit Indian Journal of Microbiology Vol 52 No 4 Pages 676-681
Chah KF Muko KN Oboegbulem SI (2000) Antimicrobial activity of methanolic extract of Solanum torvum fruit Fitote Vol 71 Pages 187-189.
Gupta VK Shukla C Bisht GRS Saikia D et al (2010) Detection of anti-tuberculosis activity in some folklore plants by radiometric BACTEC assay Letters in Applied Microbiology Vol 52 Pages 33-40.
Hussein OE Germoush O Mahmoud AM (2016) Ruta graveolens protects against Isoniazid/Rifampicin-induced nephrotoxicity through modulation of oxidative stress and inflammation Global Journal of Biotechnology and Biomaterial Sciences Vol 1 No 1 Pages 017-022
Jaiswal BS (2012) Solanum torvum: A review of its traditional uses, phytochemistry and pharmacology International Journal of Pharmacy and Biological Sciences Vol 3 No 4 Pages 104-111
Kalita RD Hussain I Deka RC Buragohain AK (2018) Antimycobacterial activity of linoleic acid and oleic acid obtained from the hexane extract of the seeds of Mesua ferrea L. and their in silico investigation Indian Journal of Natural Products and Resources Vol 9 No 2 Pages 132-142.
Kaplan O Yaman M (2009) Selective and sequential extraction of lead in soil samples and plant parts taken from a serpentine and copper mining area Atomic Spectrometry Vol 30 No 1
Ladda PL Magdum CS (2018) Antitubercular activity and isolation of chemical constituents from plant Vitex negundo Linn Iranian Journal of Pharmaceutical Research Vol 17 No 4 Pages 1353-1360
Mahesh B Satish S (2008) Antimicrobial activity of some important medicinal plant against plant and human pathogens World Journal of Agricultural Sciences Vol 4 Pages 839-843
Mohamad S Zin NM Wahab HA Ibrahim P Sulaiman SF Zahariluddin ASM (2011) Antituberculosis potential of some ethnobotanically selected Malaysian plants Journal of Ethnopharmacology Vol 133 Pages 1021-1026.
Naimon N Pongchairerk U Suebkhampet A (2015) Phytochemical analysis and antibacterial activity of ethanolic leaf extract of Solanum torvum Sw. against pathogenic bacteria. Kasetsart Journal Natural Science Vol 49 No 4 Pages 516-523
Nguta JM Opong RA Nyarko AK Manu DY Addo PGA Otchere I Twum AK (2016) Antimycobacterial and cytotoxic activity of selected medicinal plant extracts Journal of Ethnopharmacology Vol 182 Pages 10-15.
Ojo PO Babayi H Olayemi IK et al (2018) Anti-tubercular activities and molecular characterization of salivary extract of Leech (Hirudo medicinalis) against Mycobacterium tuberculosis Journal of Tuberculosis Research Vol 6 Pages 1-9.
Panseeta P Lomchoey K Prabpai S Kongsaeree P et al (2011) Anti-plasmodial and antimycobacterial cyclopeptide alkaloids from the root of Ziziphus mauritiana. Phytochemistry Journal Vol 72 Pages 909-915.
Priyanka C Kumar P Bankar SP Karthik L (2015) In vitro antibacterial activity and gas chromatography-mass spectroscopy analysis of Acacia karoo and Ziziphus mauritiana extracts. Journal of Tal Uni Sciences Pages 13-19.
Radhakrishnan M Sekar P Jerrine J Vanaja K (2016) Anti-tubercular activity of pigment from forest soil Streptomyces sp SFA5 Bangladesh Journal of Pharmacology Vol 11 Pages 138-140.
Ramappa V Aithal GP (2013) Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management Journal of Clinical and Experimental Hepatology Vol 3 Pages 37-49
Sameera NS Mandakini BP (2015) Investigations into the antibacterial activity of Ziziphus mauritiana Lam. and Ziziphus xylopyra (Retz.) Willd. International Food Research Journal Vol 22 No 2 Pages 849-853.
Santhosh RS Suriyanarayanan B (2014) A source for new antimycobacterial drugs Planta Medica Vol 80 Pages 9-21.
Silva KN Silva RC Coelho VPM Agra MF (2011) A pharmacobotanical study of vegetative organs of Solanum torvum Brazilian Journal of Pharmacognosy Vol 21 No 4 Pages 568-574
Sivapriya M Dinesha R Harsha R Gowda SST Srinivas L (2011) Antibacterial activity of different extracts of sundakkai (Solanum torvum) fruit coat International Journal of Biological and Chemical Sciences
Turchenko LV Voloshchuk EO Ivanov V et al (2008) Clinical improvement of active tuberculosis patients with complex treatment and nutritional supplementation Open Natural Products Journal Vol 1 Pages 20-26.
WHO, Global tuberculosis report (2018) https://www.who.int/tb/publications/ global_report/en/
Yousaf Z Wang Y Baydoun E (2012) Phytochemistry and pharmacological studies on Solanum torvum Swartz Journal of Applied Pharmaceutical Sciences Vol 3 No 4 Pages 152-160
Zheng CJ Li HQ Ren SC Xu CL et al (2015) Phytochemical and pharmacological profile of Vitex negundo. Phytotherapy Research