1Post Graduate Department of Microbiology, PMB Gujarati Science College, Indore (MP), India.
2V.P. & R.P.T.P. Science College,Vallabh Vidyanagar, Dist. Anand, Gujarat, India.
3Post Graduate Department of Biosciences, Softvision College, Indore (MP), India
4Environment and Microbiology Consultancy, Mumbai (Maharashtra), India.
4Vallabh Vidyanagar, Dist. Anand, Gujarat, India.
Corresponding author email: nandiniphanse2@gmail.com
Article Publishing History
Received: 11/07/2020
Accepted After Revision: 15/09/2020
The corona virus disease (COVID-19) which has been declared a pandemic by the World Health Organization (WHO) in March 2020, is a major concern at present, with 11,635,939 cases and 539,026 deaths worldwide, as on 08 July 2020. The SARS CoV-2 infection had emerged in Wuhan, Hubei Province, China and is likely to have originated from the zoonotic coronaviruses, WHO has initiated an investigation on the zoonotic source of disease, to ascertain how the disease jumped between animals and humans. Scientific community across the globe has been conducting research based on the knowledge from previous outbreaks of Severe Acute Respiratory Syndrome (SARS) in 2002-2003 and Middle East Respiratory Syndrome (MERS) in 2012, caused by other corona viruses, and others similar epidemics to speed up the scientific discoveries and implement solutions. There have been new revelations in this duration of six months in relation to its mode of transmission, efficacy of drugs, development of vaccines, methods of testing and the spread of disease. The research for potential therapeutics as well as combination therapies are under different stages of trials involving about 5500 patients in 39 countries, as on 01 July 2020.
There is no panacea for the treatment of Coronavirus disease, and the current treatment plan is largely symptomatic, which is mostly a combination of classical and compassionate therapeutics. Considering the non-availability of specific therapeutics and vaccines for disease control, and with the increasing knowledge on the varying effects it generates post recovery, it is largely accepted that prevention is paramount. The present review paper aims at providing a comprehensive understanding on origin, transmission, testing, prevention, therapeutics, to provide a guideline for effective planning for controlling COVID-19 spread by suppressing transmission and preventing associated illness and death.
Corona Virus, COVID-19, Epidemiology, Pandemic, Perspectives
Phanse N, Patel B, Rathore P, Matkar K, Patel M. Epidemiological Studies on Outbreaks of Severe Acute Respiratory Syndrome with Special Reference to the COVID-19 Pandemic. Biosc.Biotech.Res.Comm. 2020;13(3).
Phanse N, Patel B, Rathore P, Matkar K, Patel M. Epidemiological Studies on Outbreaks of Severe Acute Respiratory Syndrome with Special Reference to the COVID-19 Pandemic. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2ZM0cZG
Copyright © Phanse et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
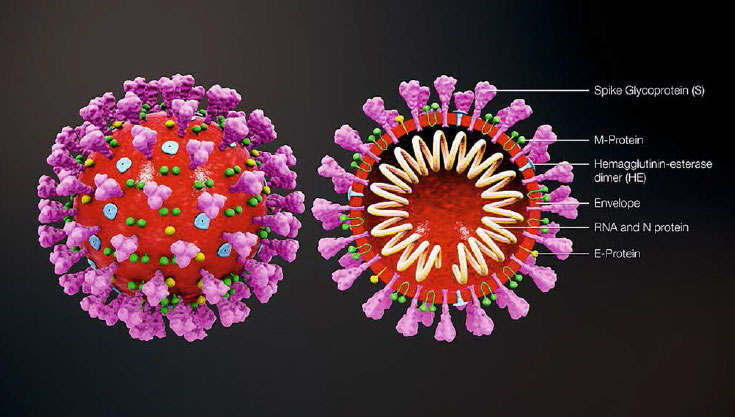
Corona viruses belong to the family Coronaviridae in the order Nidovirales. They are enveloped positive sense RNA viruses ranging from 80 nm to 140 nm in diameter (Cheng et al., 2007). The spike like projections on the outer surface of the virus give it a crown like appearance under the electron microscope, hence the name corona virus. The structure of coronavirus with spike glycoproteins (S), membrane (M) protein, hemagglutinin-esterase (HE) and the envelope (E) protein located in the virus envelope is illustrated in Figure 1 (Jin et al., 2020).
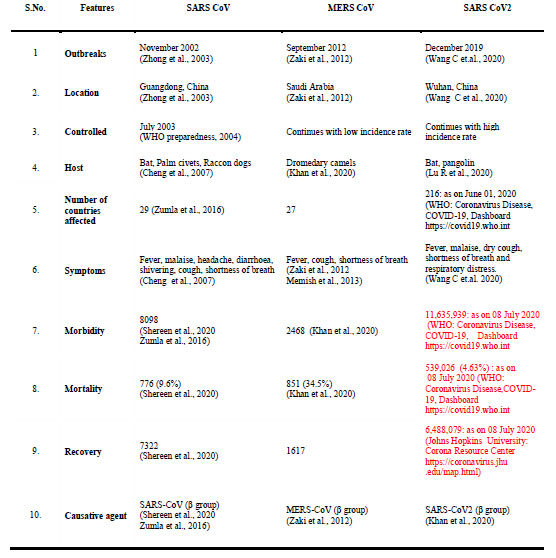
The length of RNA of these viruses ranges from 26-32 kb (Su et al., 2016). There are four subgroups of corona virus namely alpha (⍺), beta (β), gamma (ɣ) and delta (δ) (Woo et al., 2009, Cui et al., 2019). The corona viruses mainly cause Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), Acute Respiratory Distress Syndrome (ARDS) and novel corona virus disease of 2019 (COVID-19). All these lead to serious respiratory illness such as pneumonia and lung failure. A comparison of features of SARS-CoV, MERS-CoV and SARS-CoV-2 outbreaks are summarized in Table 1.
Corona virus family was thought to infect animals before the SARS outbreak in 2002 in China (Zhong et al., 2003). Later on, a similar disease appeared in Middle East countries (MERS) in 2012 (Wang et al., 2013). The present epidemic started during December 2019 and within a short time of three months it converted into a pandemic. The World Health Organization (WHO) on 11 March 2020, declared COVID-19 a pandemic, pointing to over 118,000 cases of the corona virus illness in over 110 countries and territories around the world and the sustained risk of further global spread (WHO media briefing on COVID-19, 11 March 2020). Since then, the number of cases have increased exponentially spreading in 216 countries. Globally, as on 8th July 2020, 11,635,939 confirmed cases of COVID-19, including 539,026 deaths, were reported to WHO (WHO Corona virus Disease Dashboard).
The etiological agent involved in the present outbreak belongs to the beta group of corona virus. This is the same group of virus that was involved in SARS in Guangdong province of China in 2003 with increased infectivity and mortality probably due to high transmission rate because of genetic recombination at S protein in the receptor-binding domain (RBD) (Shereen et al., 2020). The Corona virus Study Group
Figure 1: Coronavirus structure: Coronavirus is an enveloped, non-segmented, positive-sense single-stranded RNA virus with a genome size of approximately 26–32 kb. The genome RNA is complexed with the N protein to form a helical case within the viral membrane. The spike glycoproteins (S), membrane (M) protein, hemagglutinin-esterase (HE) and the envelope (E) protein are located in the virus envelope (Jin et al., 2020). (Image courtesy https://www.scientificanimations.com/ coronavirus-symptoms-and-prevention-explained-through-medical-animation/)
The Corona Virus Study Group (CSG) of the International Committee on Taxonomy of Viruses, which is responsible for developing the official classification of viruses and taxonomy of the Coronaviridae family, assessed the novelty of the human pathogen and tentatively named it 2019-nCoV. Based on phylogeny, taxonomy, and established practice, the CSG designated it as Severe Acute Respiratory Syndrome Corona Virus 2, SARS-CoV-2, (Gorbalenya et al., 2020). The World Health Organization (WHO) officially changed the name of the disease to Corona Virus Disease 2019 (COVID-19) from 2019 novel Corona Virus (2019-nCoV) infection, on 11 February 2020 (WHO, 11 Feb 2020).
ORIGIN OF SARS-CoV, MERS-CoV AND SARS-CoV-2 i)
SARS-CoV:
SARS-CoV (Severe Acute Respiratory Syndrome – Corona Virus) was identified as causative agent in an outbreak in Guangdong, China, during 2003 (Xu et al., 2004). This virus was identified as a member of beta corona virus subgroup and was given the name as SARS- CoV (Shereen et al., 2020, Zhumla et al., 2016). The affected population exhibited pneumonia with diffused alveolar injury that leads to Acute Respiratory Distress Syndrome (ARDS). During this outbreak which had affected 30 countries, a total of 8098 people were infected and there were 774 (9.7%) deaths (WHO emergencies preparedness response, 21.4.2004).
ii) MERS CoV: A coronavirus (CoV) that causes a severe lower respiratory tract infection in humans, emerged in the Middle East region in 2012, which was later named as Middle East Respiratory Syndrome Corona Virus (MERS-CoV).
MERS-CoV was initially isolated from a 60-year-old Saudi patient in September 2012 (Zaki et al., 2012). The MERS-CoV was also identified as a beta corona virus with a different phylogeny. MERS caused infection to upper respiratory tract initially leading to severe respiratory disease, finally leading to pneumonia, ARDS as SARS and causing death (Memish et al., 2013). Until 2020, 2,468 cases and 851 fatalities had been reported globally (Khan et al., 2020).
Table 1: SARS CoV, MERS CoV and SARS CoV2 Outbreaks at a Glance
iii) SARS-CoV-2: In December 2019, the Chinese government informed WHO about severe pneumonia caused by an unknown causative agent. Based on clinical manifestations, blood tests, and chest radiographs, this disease was diagnosed as virus-induced pneumonia by clinicians. The origin was considered to be human seafood market in Wuhan city of China where live animals like bats, frogs, birds, rabbits, marmots, snakes etc are sold. On January 12, 2020, National Health Commission of China declared it as epidemic (Wang et al., 2020). It was presumed that the people who visited seafood market and/or consumed infected birds or animals were infected with pneumonia caused by the novel etiological agent. Later the same was spread from human to human through respiratory activities viz. coughing, sneezing. The respiratory droplets generated, when inhaled through the nose or mouth, can lead to human to human transmission (Li et al., 2020, Parry et al., 2020, Phan et al., 2020, Riou et al., 2020).
EPIDEMIOLOGY
Reservoirs and transmission: In order to understand the spread and control of any disease the first step is to confirm about the origin of the causative agent, its primary reservoir, or intermediate carriers from where the infection may have spread to humans. Based on the history and data collected from infected people at the starting point of outbreak at China it was confirmed that the disease is of zoonotic origin. The reservoirs reported for corona viruses are mammals and birds (Bassetti et al., Ji et al., 2020). The present COVID -19 has 88% genomic sequence similarity with the bat derived SARS (Lu R, et al., Wan et al., 2020).
The cause of transmission, in cases where patients who did not visit seafood market but acquired infection, was as a result of human-human interaction through respiratory activities (Carlos et al., 2020, Wu et al., 2020). The chances of hospital acquired infection at secondary or tertiary stage can’t be neglected. Transfer of virus from pregnant woman to newborn is not yet reported. Migration of infected people from one city to other and one country to other is the possible way of transfer of COVID-19, globally. Importantly, during the initial stage of the outbreak no screening of migrants was done and even if the migrants were screened later, the screening was limited to measuring body temperature which can only detect people with symptoms and not those who are asymptomatic. However, later on many countries adopted the strategy of quarantine of migrants for about 14 days and observed them for the onset of symptoms, but it was too late by then.
Basic Reproductive Number (R0): COVID-19 is a highly infectious disease, with a basic reproductive number (R0) estimates ranging from 1.4 to 3.5 (Chatterjee et al., 2020). It is important to emphasize on the reduction of R0 values for controlling the outbreak size. Between December 10, 2019 and January 4, 2020, analysis of the growth rate of the epidemic gave a R0 of 2.2, meaning each patient was spreading the infection to 2.2 other individuals (Li Q, et al., 2020). The early WHO estimate of R0 was 1.4 to 2.5 (Chatterjee et al., 2020). Preliminary studies, conducted at the beginning of the outbreak, reported higher estimates of R 0, in the range of 2.24-3.58 (Zhao et al., 2020).
Other estimates place it in the range of 2.0-3.1 and at 3.11 (95% confidence interval, CI, 2.39- 4.13) (Majumder et al., 2020, Read et al., 2020). In a data-driven analysis of the probable outbreak size on the Diamond Princess cruise ship, distribution of R0 of COVID-19 was about 2.28 (Zhang et al., 2020). Studies related to transmissibility of COVID-19 indicate that the human-to-human transmission is the most probable explanation for the magnitude of the on-going outbreak (Imai et al., 2020). In general, an epidemic will increase as long as R0 is greater than 1, and control measures aim to reduce the reproductive number to less than 1 (Li Q, et al., 2020).
Incubation Period: In SARS-CoV-2 infection, the period from infection to appearance of symptoms varies. Generally, it is thought to range from 2-14 days with a mean incubation period of 5.2 days (Singhal, 2020, Jin et al., 2020). This period depends on many factors like age, sex, patient’s immune status, environmental conditions, etc. The period from the onset of COVID-19 symptoms to death ranges from 6 to 41 days with a median of 14 days (Wang et al., 2020). SARS-CoV-2 infection can cause five different outcomes: asymptomatically infected persons, mild to medium cases, severe cases, critical cases, and death (Jin et al., 2020).
Receptor Interactions and Cell Entry: Human angiotensin-converting enzyme 2 (ACE2) is a functional receptor that provides a direct binding site for the S proteins of coronavirus. SARS-CoV-2 utilizes ACE2 as a cellular entry receptor. ACE2 is a type I membrane protein expressed in lung, heart, kidney, and intestine mainly associated with cardiovascular diseases (Jin et al., 2020).
Symptoms and pathogenesis: The most common symptoms are fever, cough, fatigue, headache, sputum production, haemoptysis, diarrhea, dyspnoea and lymphopenia (Carlos et al., 2020, Huang et al., 2020, Ren et al., 2020, Wang W et al., 2020, Patel et al., 2020). Other abnormal features include RNAaemia, Acute Respiratory Distress Syndrome (ARDS), cardiac injury, grand glass opacities that leads to death (Huang et al., 2020). The symptoms associated with SARS, MERS and COVID-19 are almost similar. However, people infected with COVID-19 develop gastrointestinal symptoms like diarrhea. A low percentage of SARS or MERS patients exhibited similar GI symptoms. The severity of COVID-19 infection was established with the logarithmic increase in morbidity and mortality.
Started on 29 December 2019 with 05 cases and 01 death the number reached to 51,174 with 1,666 (3.25%) death cases by 16 February 2020, in China alone. The median age in these cases was 75 years (range 48-89 years) (Wang W, et al., 2020). Infected persons show higher leukocyte counts i.e. 2.9 x 109 cells/L of blood of which 70% are neutrophiles. Blood C reactive protein increases up to 16 mg/L of blood. High erythrocyte sedimentation rate and D dimer are also observed along with abnormal respiratory findings, increased level of plasma pro-inflammatory cytokines. The patient’s sample shows a positive real time polymerase chain reaction that confirms COVID-19 infection (Lei et al., 2020).
The clinical manifestations of COVID-19 are heterogeneous. In a study, 20–51% of patients were reported as having at least one comorbidity, with diabetes, hypertension and other cardiovascular and cerebrovascular diseases being most common (Guan et al., 2020). Experts are now also observing that critical patients with COVID-19 show signs of blood clots which can be life threatening. Such blood clots can obstruct a blood vessel and stop blood flow, the condition known as thrombosis and when this clot travels to other organs, phenomena known as embolism occurs which is again severe enough to cause death. In patients with severe clinical features of COVID-19 infection, the proportion of patients with acute pulmonary embolus was 23% (95% CI: 15%, 33%) on pulmonary CT angiography (Grillet et al., 2020).
Prevention and Control: At present there are no potential antiviral drugs or vaccines available against COVID- 19. Hence, prevention is the only way left to control the disease. As mentioned earlier COVID-19 is a lower respiratory virus enters the human through the respiratory path. In order to prevent human-human transmission the first thing is to develop method for effective and early detection of virus. Many companies have developed the PCR based detection kits.
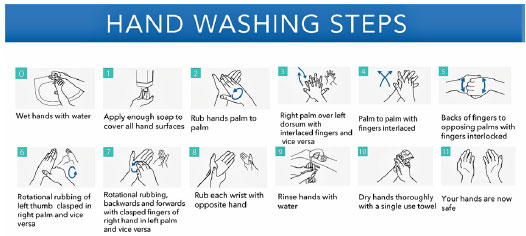
The other important ways to prevent COVID-19 is to keep social distancing (1-3 meters) and observing hand hygiene particularly by the health care workers and family members who are in contact with patients. WHO has already released guidelines for hand hygiene, as shown in Figure 2 (WHO guidelines on Hand Hygiene in Health Care, 2009). PPE including protective mask, clothing, and glasses must be used by healthcare workers. Along with these measures, regular decontamination of surfaces should be done. It is important to disinfect inanimate surfaces in the surgery or hospitals as patients may touch and contaminate surfaces such as door handles, desks, etc. (Kampf et al., 2020).
To reduce the risk of infection with COVID-19, basic preventive measures to be followed at all times, were issued by the Ministry of Health and Family Welfare (MoHFW), Government of India, on 18 May 2020. These include: (i) maintaining physical distancing of at least one meter to be followed at all times, (ii) mandatory use of face covers/masks, (iii) practicing frequent hand washing (for at least 40-60 seconds) and use of alcohol based hand sanitizers (for at least 20 seconds), (iv) following respiratory etiquettes, which involves strict practice of covering one’s mouth and nose while coughing/sneezing with a tissue/handkerchief/flexed elbow and disposing off used tissues properly. (v) self-monitoring of health by all and reporting any illness at the earliest.
Figure 2: Hand washing steps: duration of the entire procedure, 20-30 seconds (Adapted from WHO Guidelines on Hand Hygiene in Health Care, 2009)
POTENTIAL THERAPEUTICS
Potential Antiviral Drugs: Currently, there are no specific antiviral drugs or vaccines for the control of SARS- CoV-2. Present strategy of treatment of COVID-19 cases involves the use of broad spectrum antiviral drugs like nucleoside analogues and also HIV protease inhibitor (Lu H, et al. 2020). ). Use of broad spectrum antiviral drugs like Favipiravir, Remdesivir, Ribavirin, etc., are involved in the present strategy of treatment of COVID-19 cases. Other existing drugs used for the treatment include the corticosteroid, Dexamethasone, and Camostat mesylate, the synthetic serine protease inhibitor, with anti-inflammatory, antifibrotic, and potential antiviral activities (Patel, et al., 2020). Oseltamivir, Lopinavir,
Ritonavir via oral route, and Ganciclovir through intravenous route for 3-14 days are the drugs of choice (Chen et al., 2020). Other compounds like EIDD 2801 that have shown high therapeutic potential against seasonal and pandemic influenza can act as potential drugs to be considered for the treatment of COVID-19 cases (Toots et al.,2019).
It is clear that urgent research is needed to identify new chemotherapeutic drugs and develop vaccines. WHO is running an international therapeutics trial – the Solidarity trial, and as on 01 July 2020, nearly 5500 patients in 39 countries had been recruited into the trial (WHO, 2020 e).
As on 07 July 2020, there are around 139 vaccines in preclinical evaluation phase and around 21 candidate vaccines in clinical evaluation phase (WHO, Draft landscape of COVID-19 candidate vaccines).
ii) Monoclonal antibodies: Tocilizumab, a potential recombinant monoclonal antibody against Interleukin 6 (IL-6) is currently under investigation for the management of ARDS in patients with COVID-19. Treatment of severe COVID-19 cases with Tocilizumab mitigates cytokine storm and averts mechanical ventilation during Acute Respiratory Distress (ARD) (Marovich et al., 2020).
iii) Convalescent plasma therapy: Convalescent plasma therapy has been used for severe respiratory tract infections including SARS and influenza A (H1N1) (Cheng et al., 2005, Hung et al., 2011). Ebola patients had also received the treatment of convalescent plasma (Kraft et al., 2015). A promising approach in combating SARS-CoV-2 during the outbreak would be to use plasma from the convalescent patients. Recently, convalescent plasma has been widely recommended to be used for COVID-19 (Li H, et al., 2020). Neutralizing antibodies (NAbs) against SARS-CoV-2 are important therapeutic agents proposed for the treatment of COVID-19 (Zhou and Zhao,2020).
However, the outcomes of plasma therapy are unpredictable due to variability of sera in different patients.
A patient from South Delhi was the first in India to recover after receiving treatment with plasma therapy, as reported by Jeelani and Mishra in India Today, on 25 April 2020. Indian Council of Medical Research (ICMR) and Drug Controller General of India (DGCI) are two nodal agencies to approve clinical trials for plasma therapy in India.
iv) Antithrombotic therapy: Prophylactic doses of heparin might be associated with improved survival (20%) in patients with evidence of sepsis induced coagulopathy (SIC) (Tang et al., 2020).
Diagnostic Tests: Nucleic acid testing such as real time RT-PCR is the main technique used to detect the novel coronavirus, COVID-19, and to confirm suspected cases (Corman et al., 2020). Other than the molecular-based approaches, serological antibody testing is also valuable in detection of novel corona virus infection (Meyer et al., 2014).
i) Real time RT-PCR: Real time RT-PCR has been a gold standard measure for diagnosis of COVID-19 and is the most accurate way of detecting the presence of SARS-CoV-2 in respiratory specimen (Corman et al., 2020). Various real-time RT-PCR protocols, which differ in the genes they detect, have been proposed for the diagnosis of COVID-19 (Hong et al., 2020). The primers and probes targeting specific genes of SARS-CoV-2 are used in real- time RT-PCR assays as diagnostic tests. The first open reading frames (ORF 1a and 1b), RNA-dependent RNA polymerase gene (RdRp), envelope (E), and nucleocapsid (N) have become key diagnostic targets for SARS-CoV-2 identification (Ahn et al., 2020).
However, RT-PCR has certain limitations. It requires certified laboratories, expensive equipment, and trained technicians to operate. Thus, RT-PR is not scalable due to the lack of testing equipment and consumables (Frost et al., 2020). The human and laboratory resources required in this method makes it difficult to deal with the large volumes of samples. One technique to reduce the number of tests required is the pooling of samples for analysis by RT-PCR prior to testing.
Pooled testing strategy can help accelerate the surveillance for COVID-19 identification in a community or group of people living together. Testing samples from multiple patients with a single PCR test, also known as pooled sampling, has been used previously in the early stages of the HIV epidemic when PCR costs were high (Emmanuel et al., 1988).
Pooled RT-PCR testing can vastly increase testing for COVID-19. In this method multiple swab samples are pooled in a test tube and they are tested using a single RT-PCR test. If the test is negative, all the people tested are negative. If found positive, a pooled sampling exercise, can be done to trace back to the individual(s) (Narayanan et al., 2020). The researchers used mathematical analysis to explore efficient pooling strategies using this technique. They recommend the use of the pooled sample method with a binary hierarchical testing strategy for the detection of SARS-CoV-2 by RT-PCR in community surveillance. This method can enhance the capacity to test in a low-resources setting where testing kits, facilities, and personnel are scarce.
One limitation of pooling multiple RT-PCR samples is that the sensitivity of testing is reduced. To address this, it has been suggested that the number of samples being pooled be kept as low as possible to reduce dilution (Westreich et al., 2008, Muniesa et al., 2014). Based on a study conducted at DHR/ICMR Virus Research & Diagnostic Laboratory (VRDL) at King George’s Medical University (KGMU), Lucknow, India, ICMR has recommended sample pooling for real-time RT-PCR screening for COVID-19 in areas or population with low prevalence of COVID-19. In areas with positivity of 2-5%, sample pooling for PCR screening has been recommended only in community survey or surveillance among asymptomatic individuals. Pooling of sample has not been recommended in areas or population with positivity rates of >5% for COVID-19 (ICMR-Information of testing strategies, 13 April 2020).
ii) Rapid Antibody Tests: Rapid and accurate detection of COVID-19 is very crucial in controlling outbreaks in the population. The limitations of RT-PCR test makes it unsuitable for use in the field for rapid and simple diagnosis and screening of patients. Rapid and simple diagnosis and screening of patients can be achieved by testing of specific antibodies of SARS CoV-2. Rapid antibody tests can detect immunoglobulin M (IgM) and IgG antibodies simultaneously against SARS CoV-2 virus in human blood within 15 minutes. The IgM-IgG combined assay is useful for the rapid screening of SARS CoV-2 carriers, symptomatic or asymptomatic, in the population (Li Z et al., 2020).
THE INDIAN SCENARIO
COVID-19 Cases: In India, the first COVID-19 case was reported in Kerala on 30 January 2020. Only 3 cases were reported till 02 March 2020, however, by 05 March 2020, 29 cases had been reported. One case was reported in an Indian who traveled back from Vienna and exposed a large number of school children in a birthday party at a city hotel (Singhal, 2020). As per the ICMR report of 10 April 2020, total of 1,61,330 samples from 1,47,034 individuals were tested, 6,872 individuals were confirmed positive in India. 764 patients recovered from the disease and there were 246 death cases (ICMR: COVID-2019 data portal).
However, within a span of about 51 days, as on 01 June 2020, India stood seventh in the world in terms of COVID-19 cases with 1,90,535 cases. The cases increased more rapidly thereafter, and India ranked third in the world as on 08 July 2020 with 7,42,417 COVID-19 cases and 20,642 deaths reported (WHO: Coronavirus Disease Dashboard) and there were 4,56,831 recovered cases (Johns Hopkins University: Corona Resource Centre). Up to 08 July 2020, 10740832 samples were tested in India (Official Updates Coronavirus – COVID-19 in India – mygov.in).
COVID-19 Testing Strategies in India: Test, track, treat is the key strategy for early detection and containment of the pandemic. To combat COVID-19, India adopted various testing strategies for tracing infected cases and creating an infrastructure to provide testing facilities and services across the country. To ramp up the testing in the country, ICMR approved of 1049 public and private laboratories for COVID-19 testing as on 01 July 2020. (ICMR portal, Testing Strategy, 01 July 2020).
Along with the existing testing strategies, newer additional strategies for COVID-19 testing have been adapted. To facilitate testing at district level the government has tapped the rich resource of available TrueNat machines, the diagnostic machines used for tuberculosis diagnosis. Along with Real Time RT-PCR, the gold standard test for detecting cases of COVID-19, the TrueNAT and CBNAAT (Cartridge Based Nucleic Acid Amplification Test) systems have also been deployed for diagnosis of COVID-19. These platforms have widespread availability even at district and primary health center level as they are widely used for diagnosis of tuberculosis and other infectious diseases.
The viral lysis buffer that comes with the COVID-19 cartridges inactivates the virus and poses minimum biosafety hazard. The closed nature of these platforms and minimum sample handling further augment their safety. These features have facilitated use of these platforms at grass root level thereby increasing access to testing. Rapid Point-of-Care (PoC) Antigen Detection Test (for diagnosis along with RT-PCR) and IgG Antibody test for COVID-19 (only for surveillance and not diagnosis) are also recommended by ICMR (ICMR Advisory: Newer Additional Strategies for COVID-19 Testing, 23 June 2020).
PERSPECTIVES
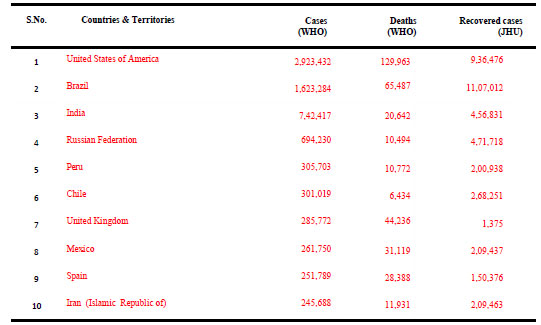
Recommendations for Future: As on 08 July 2020, the novel corona virus, SARS CoV-2, has spread and badly hit about 216 countries across the globe, with 11,635,939 confirmed cases and a mortality of 539,026 cases (4.63%) (WHO: Coronavirus Disease Dashboard). Table 2 summarizes the data of COVID-19 cases in top 10 countries across the globe as on 08 July 2020 (WHO: Coronavirus Disease Dashboard, Johns Hopkins University: Corona Resource Centre).
Looking to the rapid rate of transmission of the disease, measures for disease containment with lower magnitudes of loss to human life and economy need to be taken. Rapid testing methods, screening and isolation of asymptomatic patients and strict implementation of infection prevention and control measures are highly recommended for combating the situation. A few recommendations are mentioned below:
Table 2. Top Ten Countries Affected by COVID-19 as on 08 July 2020
(WHO: Coronavirus Disease (COVID-19) Dashboard), (Johns Hopkins University: Corona Resource Centre)
Globally, as on 08 July 2020, 11.44am CEST, 11,635,939 confirmed cases of COVID-19, including 539,026 deaths, were reported to WHO
i) Adopting strategies of tracing, tracking, testing and treating cases: The approach of focusing on tracing , tracking, testing, and treating cases has been found to be successful to contain the spread of COVID-19, as in the case of Dharavai, the largest slum of Asia, located in Mumbai, India. Dharavi, spread over an area of 2.5 square kilometers and with a population of 650,000, was once declared a COVID-19 hotspot. It recorded its first COVID-19 case on April 1, and till 10 July 2020, 2,359 COVID-19 cases have been recorded in Dharavi of which 1,952 have recovered and there were 166 active cases, till date (Times of India, 11 July 2020).
The Dharavi model of combating COVID-19 has been acknowledged and appreciated by the WHO. On 10 July, 2020, WHO Director-General Tedros Adhanom Ghebreyesus while addressing a virtual media briefing acknowledged the measures adopted to contain the spread of the virus in Italy, Spain, South Korea and in Dharavi, quoting- “a strong focus on community engagement and the basics of testing, tracing, isolating and treating all those that are sick is key to breaking the chains of transmission and suppressing the virus” (WHO Director-General’s media briefing on COVID-19 – 10 July 2020).
ii) Use of pulse oximeter for screening: Early recognition and rapid diagnosis of COVID-19 are essential to prevent transmission and provide supportive care in a timely manner. Widespread pulse oximetry screening for COVID pneumonia could provide an early warning system for the kinds of breathing problems associated with the disease. A pulse oximeter can provide early warning of the kinds of breathing problems associated with COVID-19 pneumonia. This device when placed on a fingertip, displays: oxygen saturation and pulse rate. This was reported in New York Times on 20 April 2020, by Dr Richard Levitan, an emergency physician in Littleton, Town in New Hampshire, United States of America.
According to Dr Levitan, COVID pneumonia initially causes a form of oxygen deprivation, a “silent hypoxia,” which is hard to detect. Normal oxygen saturation for most persons at sea level is 94 to 100 percent; COVID pneumonia patients have lower oxygen saturation. By detecting silent hypoxia early through a common medical device: a pulse oximeter, more asymptomatic patients who have COVID pneumonia could be identified sooner and treated effectively. Although, oximeters are not 100 percent accurate, they may be used as means for screening and early detection of silent hypoxia along with other confirmatory tests.
iii) Convalescent plasma therapy: Convalescent plasma therapy is a promising approach in combating SARS-CoV-2. Passive immunization has been successfully used to treat infectious diseases. High-quality studies and the need for adequate selection of donors with high neutralizing antibody titers should be considered (Cunningham et al., 2020). Convalescent plasma can be given to a sick people to boost their immunity. But a good number of donors must come forward after their recovery and plasma must have good amount of antibodies to help the sick.
iv) Pooled testing strategy: Pooled testing strategy for COVID-19 may benefit India. Pooling of samples can help accelerate the surveillance for COVID-19 identification in a community or group of people living together. This strategy could reduce the time, cost, and resources required and help to identify infected people in a population. Group testing can be beneficial in reducing the number of tests required to assess whether the infection rate in a population is low or high (Narayanan et al. , 2020). Testing on asymptomatic individuals by pooled sample testing can save many test kits in particular.
v) Rapid screening and isolation of asymptomatic patients: Asymptomatic persons are thought to be potential sources of SARS- CoV-2 infection (Rothe et al., 2020) which may have caused the rapid spread of SARS-CoV-2. This asymptomatic spread may be one reason that the control strategy based on the isolation of patients has not been fully successful. Screening of asymptomatic patients and their isolation would help to prevent the random spread of infection by them. Rapid antibody tests are useful for the rapid screening of SARS CoV-2 carriers, symptomatic or asymptomatic, in the population (Li Z et al., 2020).
vi) Following infection prevention and control measures: Infection prevention and control measures as recommended by WHO (WHO interim guidance, March 2020) should be effectively followed. In fact, it should be followed in any such outbreak in future too. Standard precautions include-
- Practice hand and respiratory hygiene
- Offer a medical mask to patients with suspected COVID-19 while they are in waiting/public areas or in cohorting rooms.
- Use of appropriate personal protective equipment (PPE) according to a risk assessment
- Practice safe waste management, environmental cleaning, and sterilization of patient care equipment and linen
- Considering all specimens collected for laboratory investigations as potentially infectious
CONCLUSION
The COVID-19 pandemic has challenged the economic, medical and public health infrastructure of many countries across the globe. The biggest problem is to combat and curb the spread of the outbreak in the absence of vaccines and suitable antiviral agents. Social distancing, isolation and quarantine are useful measures to stay safe from this pandemic infection. It is necessary to develop drugs and vaccines against the COVID-19 infection as soon as possible. Until then, monitoring spread of COVID-19 by screening of symptomatic and asymptomatic patients and their isolation, mass testing of population by rapid testing methods and enforcement of infection prevention and control measures are highly recommended for combating the situation.
REFERENCES
Ahn DG Shin HJ Kim MH Lee S Kim HS Myoung J (2020) Current Status of Epidemiology Diagnosis Therapeutics and Vaccines for Novel Corona Virus Disease 2019 (COVID-19) Journal of Microbiology and Biotechnology Vol 30 No 3 Pages 313-324 doi.org/10.4014/jmb.2003.03011
Bassetti M Vena A Roberto D (2020) The Novel Chinese Corona Virus (2019-nCoV) Infections: Challenges for Fighting the Storm European Journal of Clinical Investigation Vol 50 No 3 doi: 10.1111/eci.13209
Carlos WG Delacruz CS Cao B Pasnick A Jamil S (2020) Novel Wuhan (2019-nCoV) Corona Virus American Journal of Respiratory and Critical Care Medicine Vol 201 No 4 Pages 7-8
Chatterjee P Nagi N Agarwal A Das B Banerjee S Sarkar S Gupta N Gangakhedkar R (2020) The 2019 Novel Coronavirus Disease (COVID-19) Pandemic: A Review of the Current Evidence Indian Journal of Medical Research Vol 151 No 2 Pages 147-159 doi:10.4103/ijmr.IJMR_519_20
Chen N Zhou M Dong X Qu J Gong F Han Y (2020) Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Corona Virus Pneumonia in Wuhan China: A Descriptive Study Lancet Vol 395 No 10223 Pages 507-513
Cheng VC Lau SK Woo PC Yuen KY (2007) Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection Clinical Microbiology Reviews Vol 20 No 4 Pages 660–694 doi: 10.1128/CMR.00023-07
Cheng Y Wong R Soo YOY (2005) Use of Convalescent Plasma Therapy in SARS Patients in Hong Kong European Journal of Clinical Microbiology and Infectious Diseases Vol 24 Pages 44-46 doi.org/10.1007/s10096-004-1271-9
Corman VM Landt O Kaiser M Molenkamp R Meijer A Chu DKW (2020) Detection of 2019 Novel Corona Virus (2019-nCoV) by Real-Time RT-PCR Eurosurveillance Vol 25 No 3 doi: 10.2807/1560-7917
Cui J Li F Shi ZL (2019) Origin and Evolution of Pathogenic Coronaviruses Nature Reviews Microbiology Vol 17 No 3 Pages181–192 doi: 10.1038/s41579-018-0118-9
Cunningham AC Goh HP and Koh D (2020) Treatment of COVID-19: Old Tricks for New Challenges Critical Care Vol 24:91 doi.org/10.1186/s13054-020-2818-6
Emmanuel JC Bassett MT Smith HJ Jacobs JA (1988) Pooling of Sera for Human Immunodeficiency Virus (HIV) Testing: an Economical Method for use in Developing Countries Journal of Clinical Pathology Vol 41 No 8 Pages 582-85
Frost I Tseng K and Laxminarayan R (2020) Pooling RT-PCR or NGS Samples to Cost-Effectively Generate Estimates of COVID-19
Prevalence in Resource-Limited Environments Report by the Center for Disease Dynamics Economics & Policy (CDDEP) Apr. 06 2020.
Gorbalenya AE Baker SC Baric RS Groot RJ de Drosten C Gulyaeva AA (2020) Severe Acute Respiratory Syndrome-Related Coronavirus: The Species and its Viruses – A Statement of the Coronavirus Study Group bioRxiv doi: https://doi. org/10.1101/2020.02.07.937862
Government of India, Ministry of Health and Family Welfare (MoHFW) (2020) Guidelines on preventive measures to contain spread of COVID-19 in workplace-18.5.2020 Available from: https://www.mohfw. gov.in/ pdf/GuidelinesonpreventivemeasurestocontainspreadofCOVID19inworkplacesettings.pdf
Government of India MyGov SARS-CoV-2 (COVID-19) Testing Status Available from: https://www.mygov.in/covid-19
Grillet F Behr J Calame P Aubry S Delabrousse E (2020) Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected by Pulmonary CT Angiography Radiology published online 23 April 2020. https://doi.org/10.1148/radiol.2020201544.
Guan WJ Liang WH Zhao Y Liang H Chen Z et al (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. European Respiratory Journal Vol 55: 2000547 doi: 10.1183/13993003.00547-2020.
Hong KH Lee SW Kim TS Huh HJ Lee J (2020) Guidelines for Laboratory Diagnosis of Corona Virus Disease 2019 (COVID-19) in Korea Annals of Laboratory Medicine Vol 40 Pages 351-360 doi.org/10.3343/alm.2020.40.5.351.
Huang C Wang Y Li X Ren L Zhao J Hu Y Zhang L Fan G Xu J Gu X (2020) Clinical Features of Patients Infected with 2019 Novel Corona Virus in Wuhan China Lancet Vol 395 No 10223 Pages 497-506 doi.org/10.1016/S0140 -6736(20)30183-5.
Hung IF To KK Lee CK Lee KL Chan K Yan WW Liu R Watt CL (2011) Convalescent Plasma Treatment Reduced Mortality in Patients with Severe Pandemic Influenza A (H1N1) 2009 Virus Infection Clinical Infectious Diseases Vol 52 Issue 4 Pages 447-456 doi.org/10.1093/cid/ciq106.
Indian Council of Medical Research, ICMR (2020 a) COVID 2019 data portal https://www.icmr.gov.in Apr.102020. Available from: https://icmr.nic.in/sites/default/fileswhats_newICMR_testing_update_10Apr2020_9AM_IST.pdf
Indian Council of Medical Research, ICMR (2020 b) Information of testing strategies-Advisory on feasibility of using pooled samples for molecular testing of COVID-19, dated 13 April 2020. Available from: https://www.icmr.gov.in/pdf/covid/strategy/Advisory_on_feasibility_of_sample_pooling.pdf
Indian Council of Medical Research, ICMR (2020 c) Testing Strategy: Empowering citizens for testing of SARS-CoV-2 virus to save precious lives and contain the virus, 01 July 2020 Available from: https://www.icmr.gov.in/pdf/covid/strategy/Joint_Letter_Test_Track_Treat.pdf)
Indian Council of Medical Research, ICMR (2020 d) ICMR Advisory: Newer Additional Strategies for COVID-19 Testing, 23 June 2020. Available from: https://www.icmr.gov.in/pdf/covid/strategy/New_additional_Advisory_23062020_3.pdf
Imai N Cori A Dorigatti I Baguelin M Donnelly CA. Riley S Ferguson NM (2020) Report 3: Transmissibility of 2019-nCoVImperial College London COVID-19 Response Team Jan. 25 2020 www.imperial.ac.uk/media/imperial- college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19 transmissibility-25-01-2020.pdf doi.org/10.25561/77148.2020
Jeelani G and Mishra A (2020) Coronavirus: Why Delhi Pins its Hopes on Plasma Therapy India Today Apr. 25 2020.
https://www.indiatoday.in/amp/mail-today/story/coronavirus-why-delhi-pins-its-hopes-on-plasma-therapy-1670848-2020-04-25
Ji W Wang W Zhao X Zai J Li X (2020) Homologous Recombination Within the Spike Glycoprotein of the Newly Identified Corona Virus may Boost Cross Species Transmission From Snake to Human Journal of Medical Virology Vol 92 No 4 Pages 433-440
Jin Y Yang H Ji W Wu W Chen S (2020) Virology Epidemiology Pathogenesis and Control of COVID-19 Viruses Vol 12 No 372 doi:10.3390/v12040372
Johns Hopkins University: Corona Resource Center https://coronavirus.jhu.edu/map.html
Kampf G Todt D Pfaender S (2020) Persistence of Corona Viruses on Inanimate Surfaces and their Inactivation with Biocidal Agents Journal of Hospital Infection Vol 104 No 3 Pages 246-51
Khan S Siddique R Shereen MA Ali A Liu J Bai Q Bashir N Xue M (2020) Emergence of a Novel Coronavirus Severe Acute Respiratory Syndrome Coronavirus 2: Biology and Therapeutic Options The Journal of Clinical Microbiology Vol 58 Issue 5 doi.org/10.1128/JCM .00187-20
Kraft CS Hewlett AL Koepsell S Winkler AM Kratochvil CJ Larson L (2015) The Use of TKM-100802 and Convalescent Plasma in 2 Patients with Ebola Virus Disease in the United States Clinical Infectious Diseases Vol 61 Pages 496-502
Lei J Li J Li X Qi X (2020) CT Imaging of the 2019 Novel Corona Virus (2019-nCoV) Pneumonia Radiology Vol 295 No 1 doi.org/10.1148/radiol.2020200236
Levitan R (2020) The Infection That’s Silently Killing Corona Virus PatientsNew York Times Apr. 20 2020.
Li H Wang YM Xu JY Cao B (2020) Potential Antiviral Therapeutics for 2019 Novel Corona Virus Chinese Journal of Tuberculosis and Respiratory Disease Vol 43 No E002
Li Q Guan X Wu P Wang X Zhou L Tong Y (2020) Early Transmission Dynamics in Wuhan China of Novel Corona Virus Infected Pneumonia New England Journal of Medicine Vol 382 No 13 Pages1199-1207 doi.org/10.1056/NEJMoa2001316
Li Z Yi Y Luo X (2020) Development and Clinical Application of a Rapid IgM IgG Combined Antibody Test for SARS-CoV2 Infection Diagnosis Journal of Medical Virology Pages 1-7 Epub ahead of print https://doi.org/10.1002/jmv.25727
Lu H (2020) Drug Treatment Options for the 2019 New Corona Virus (2019-nCoV) Bioscience Trends Vol 16 No14 (1) Pages 69-71 doi:10.5582/bst.2020.01020 Epub 2020 Jan 28.
Lu R Zhao X Li J Niu P Yang B Wu H (2020) Genomic Characterization and Epidemiology of 2019 Novel Corona Virus: Implications for Virus Origins and Receptor Binding Lancet Vol 395 No 10224 Pages 565–574 doi.org/10.1016/ S0140-6736(20)30251-8
Majumder M Mandl KD (2020) Early Transmissibility Assessment of a Novel Coronavirus in Wuhan China SSRN Electronic Journal Vol 47 doi: http://dx.doi.org/10.2139/ssrn.3524675
Marovich M Mascola JR Cohen MS (2020) Monoclonal Antibodies for Prevention and Treatment of COVID-19 Journal of the American Medical Association Published online 15 June 2020 doi:10.1001/jama.2020.10245
Memish ZA Zumla AI Al-Hakeem RF Al-Rabeeah AA Stephens GM (2013) Family Cluster of Middle East Respiratory Syndrome Corona Virus Infections New England Journal of Medicine Vol 368 No 26 Pages 2487-94
Meyer B Drosten C Muller MA (2014) Serological Assays for Emerging Corona Viruses: Challenges and Pitfalls Virus Research Vol 194 Pages 175-183
Muniesa A Ferreira C Fuertes H Halaihel N de Blas I (2014) Estimation of the Relative Sensitivity of qPCR Analysis Using Pooled Samples PLoS One Vol 09 (4): e93491
Narayanan KR Frost I Heidarzadeh A Tseng KK Banerjee S John J and Laxminarayan R (2020) Pooling RT-PCR or NGS Samples has the Potential to Cost-Effectively Generate Estimates of COVID-19 Prevalence in Resource Limited Environments medRxiv preprint Apr. 06 2020 doi.org/10.1101/2020.04.03.20051995
Parry J (2020) China Corona Virus: Cases Surge as Official Admits Human to Human Transmission British Medical Journal Vol 368 doi:https://doi.org/10.1136/bmj.m236
Phan LT Nguyen TV Luong QC Nguyen HT Le HQ (2020) Importation and Human to Human Transmission of a Novel Corona Virus in Vietnam New England Journal of Medicine Vol 382 Pages 872-874 doi 10.1056/NEJMc2001272.
Patel B Matkar K Phanse N Rathore P Patel M (2020) Epidemiological studies of Corona Virus disease in the state of Gujarat, India International Journal of Scientific Research Vol 09 (7) doi 10.36106/ijsr
Read JM Bridgen JR Cummings DAT Ho A Jewell CP (2020) Novel Coronavirus 2019-nCoV: Early Estimation of Epidemiological Parameters and Epidemic Predictions medRxiv doi.org/10.1101/2020.01.23.20018549
Ren LL Wang YM Wu ZQ Xiang ZC Guo L Xu T (2020) Identification of a Novel Corona Virus Causing Severe Pneumonia in Human: A Descriptive Study Chinese Medical Journal doi: 10.1097/CM9.0000000000000722
Riou J and Althaus CL (2020) Pattern of Early Human to Human Transmission of Wuhan 2019 Novel Corona Virus (2019-nCoV) Eurosurveillance Vol 25 No 4
Rothe C Schunk M Sothmann P Bretzel G Froeschl G (2020) Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany New England Journal of Medicine Vol 382 Pages 970-971 doi.org/10.1056/ NEJMc2001468
Shereen MA Khan S Kazmi A Bashir N Siddique R (2020) COVID-19 Infection: Origin Transmission and Characteristics of Human Coronaviruses Journal of Advanced Research Vol 24 Pages 91–98 doi.org/10.1016/j.jare.2020.03.005
Singhal T (2020) A Review of Corona Virus Disease-2019 (COVID-19) Indian Journal of Pediatrics Vol 87 No 04 Pages 281-286 doi.org/10.1007/s12098-020-03263-6
Su S Wong G Shi W Liu J Lai ACK Zhou Liu JW Bi Y Gao GF (2016) Epidemiology Genetic Recombination and Pathogenesis of Coronaviruses Trends in Microbiology Vol 24 No 6 Pages 490–502 doi: 10.1016/j.tim.2016.03.003
Tang N Bai H Chen X Gong J Li D Sun Z (2020) Anticoagulant Treatment is Associated with Decreased Mortality in Severe Corona Virus Disease 2019 Patients with Coagulopathy Journal of Thrombosis and Haemostasis Vol 18 Pages1094–1099 doi:101111/jth.14817
The Times of India (11 July 2020) Explained: How Mumbai’s Dharavi flattened the Covid-19 curve Available from: https://timesofindia.indiatimes.com/india/expexplai-how-mumbais-dharavi-flattened-the-covid-19-curve/articleshow/76910062.cms
Toots M Yoon JJ Cox RM Hart M Sticher ZM Makhsous N Plesker R Barrena AH Reddy PG Mitchell DG Shean RC (2019) Characterization of Orally Efficacious Influenza Drug with High Resistance Barrier in Ferrets and Human Airway Epithelia Science Translational Medicine Vol 11 Issue 515 doi:10.1126/scitranslmed.aax5866
Wan Y Shang J Graham R Baric RS Li F (2020) Receptor Recognition by Novel Corona Virus from Wuhan: An Analysis Based on Decade Long Structural Studies of SARS Journal of Virology No 94 No 7 doi: 10.1128/JVI.00127-20
Wang N Shi X Jiang L Zhang S Wang D Tong P Guo D Fu L Cui Y Liu X (2013) Structure of MERS-CoV Spike Receptor-Binding Domain Complexed with Human Receptor DPP4 Cell Research Vol 23 Pages 986-993
Wang C Horby PW Hayden FG Gao GF (2020) A Novel Corona Virus Outbreak of Global Health Concern Lancet Vol 395 Issue 10223 doi.org/10.1016/S0140-6736(20)30185-9
Wang W Tang J Wei F (2020) Updated Understanding of the Outbreak of 2019 Novel Corona Virus (2019-nCoV) in Wuhan China Journal of Medical Virology Vol 92 No 4 Pages 441-447 doi.org/10.1002/jmv.25689
Westreich DJ Hudgens MG Fiscus SA Pilcher CD (2008) Optimizing Screening for Acute Human Immunodeficiency Virus Infection with Pooled Nucleic Acid Amplification Tests Journal of Clinical Microbiology Vol 46 Pages 1785-92
Woo PC Lau SK Huang Y Yuen KY (2009) Coronavirus Diversity Phylogeny and Interspecies Jumping Experimental Biology and Medicine Vol 234 Pages 1117–1127 doi.org/10.3181/0903-MR-94
World Health Organization:WHO (2003) Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003 WHO emergencies preparedness response www.who.int/csr/sars/ country/table2004_04_21/en/
World Health Organization:WHO (2009) Guidelines on Hand Hygiene in Health Care: A Summary. © World Health Organization 2009
WHO/IER/PSP/2009.07 www.who.int/gpsc/5may/tools/who_guidelines handhygiene_summary
World Health Organization:WHO (2020 a) WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Available from: https://www.who.int/dg/ speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
World Health Organization, WHO (2020 b) Media Briefing on COVID-19 Mar.11 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
World Health Organization,WHO (2020 c) Interim Guidance: Infection Prevention and Control during Health Care when COVID-19 is Suspected: Interim Guidance 19 Mar. 2020 https://apps.who.int/iris/handle/10665/331495
World Health Organization, WHO (2020 d) Coronavirus Disease (COVID-19) Dashboard https://covid19.who.int
World Health Organization,WHO (2020 e) Global scientific community unites to track progress on COVID-19 R&D, identifies new research priorities and critical gaps Available from
https://www.who.int/news-room/feature-stories/detail/global-scientific-community-unites-to-track-progress-on-covid-19-r-d-identifies-new-research-priorities-and-critical-gaps
World Health Organization,WHO (2020 f) Draft landscape of COVID-19 candidate vaccines. Available from: https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines
World Health Organization, WHO (2020 g) WHO Director-General’s opening remarks at the media briefing on COVID-19 – 10 July 2020 Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on- covid-19—10-july-2020
Wu P. Hao X Lau EHY Wong JY Leung KSM Wu JT (2020) Real Time Tentative Assessment of the Epidemiological Characteristics of Novel Corona Virus Infection in Wuhan China as at 22 January 2020 Eurosurveillance Vol 25 doi:10.2807/1560-7917
Xu RH He JF Evans MR Peng GW Field HE Yu DW Lee CK (2004) Epidemiologic Clues to SARS Origin in China Emerging Infectious Diseases Vol 10 Pages 1030–1037
Zaki AM van Boheemen S Bestebroer TM Osterhaus AD Fouchier RA (2012) Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia New England Journal of Medicine Vol 367 Pages 1814-1820
Zhang S Diao MY Yu W Pei L Lin Z Chen D (2020) Estimation of the Reproductive Number of Novel Coronavirus (COVID-19) and the Probable Outbreak Size on the Diamond Princess Cruise Ship: A Data-Driven AnalysisThe International Journal of Infectious Diseases Vol 93 Pages 201–204 doi.org/10.1016/j.ijid.2020.02.033
Zhao S Lin Q Ran J Musa SS Yang G Wang W (2020) Preliminary Estimation of the Basic Reproduction Number of Novel Coronavirus (2019-nCoV) in China from 2019 to 2020: A Data-Driven Analysis in the early phase of the outbreak International Journal of Infectious Diseases Vol 92 Pages 214-217
Zhong N Zheng Li BY Poon L Xie Z Chan K (2003) Epidemiology and Cause of Severe Acute Respiratory Syndrome (SARS) in Guangdong People’s Republic of China Lancet Vol 362 Issue 9393 Pages 1353-1358
Zhou G and Zhao Q (2020) Perspectives on Therapeutic Neutralizing Antibodies against the Novel Corona Virus SARS-CoV-2 International Journal of Biological Sciences Vol 16 Pages 1718-1723 doi:10.7150/ijbs.45123
Zumla A Chan JFW Azhar EI Hui DSC Yuen KY (2016) Coronaviruses-Drug Discovery and Therapeutic Options Nature Reviews Drug Discovery Vol 15 Pages 327–347 doi.org/10.1038/nrd.2015.37