1Department of Fishery Product Technology, Faculty of Fisheries and Marine Science, Universitas Brawijaya, Malang 65145, Indonesia.
2Bioseafood Research Group, Faculty of Fisheries and Marine Science, Universitas Brawijaya, Malang 65145, Indonesia.
3 Halal Thoyyib Science Center, Universitas Brawijaya, Malang 65145, Indonesia
4Faculty of Food Science and Nutrition, Universiti Malaysia Sabah, Kota Kinabalu, Sabah, 88400, Malaysia
5Adjunct Professor, Universitas Sultas Ageng Tirtayasa, Serang 42124, Banten, Indonesia
Corresponding author email: azizjaziri@ub.ac.id
Article Publishing History
Received: 15/10/2019
Accepted After Revision: 25/12/2019
Mackerel (Scomber japonicus) by-products such as head part is generated by fish processing industries and it may result in serious environmental problem. One of the best approaches to utilize mackerel by-product is to recovery into fish peptone. Fish peptone can be hydrolyzed by adding acid compounds that mostly categorized into positive list compounds in terms of halal or kosher view point. The aim of this research was to characterize peptone extracted from mackerel head by-product as bacterial growth media. Hydrolysate of mackerel head by-product was treated by Hydrochloric acid (HCl) to extract the peptone. The characteristics of extracted peptone was analyzed by determining chemical composition (total nitrogen, fat, ash, and moisture content), color, solubility, bacterial growth, and biomass properties. The results revealed that the chemical composition of peptone extracted from mackerel head by-producs consist of moisture 5.07%, total nitrogen 11.53%, fat 1.33% and ash 3.78%. The color intensity of mackerel by-product peptone was 87%, it showed lower intensity than commercial peptone (90.71%). The values of pH and solubility were 6.9 and 97.07%, respectively. In the bacterial growth test, the peptone extracted from mackerel by-product product showed faster bacterial growth rate (Escherichia coli, Staphylococcus aureus, Salmonella thyphi, and Aeromonas hydrophila) and higher biomass production than the commercial peptone. This findings revealed that mackerel head by-product is a potential raw material for production of peptone as media for bacterial growth.
Bacterial culture media, Mackerel head by-product, Peptone, Protein hydrolysate
Setijawati D, Jaziri A. A, Yufidasari H. S, Wardani D. W, Pratomo M. D, Ersyah D, Huda N. Characteristics of Peptone from the Mackerel, Scomber japonicus Head by-Product as Bacterial Growth Media. Biosc.Biotech.Res.Comm. 2019;12(4).
Setijawati D, Jaziri A. A, Yufidasari H. S, Wardani D. W, Pratomo M. D, Ersyah D, Huda N. Characteristics of Peptone from the Mackerel, Scomber japonicus Head by-Product as Bacterial Growth Media. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/36Oykoq
Copyright © Setijawati et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
FAO (2018) reported the global fisheries production in 2016 reached 170.0 million tonnes, consists of 80 million tonnes (representing 47%) in aquaculture and 91 million tonnes (representing 53%) in capture. Mackerel is one of the economically important species in the world, accounting for 1.6 million tonnes of total global fisheries production in 2016. Generally, mackerel processed in the form of a fillet and canned fish products. Among them, about 30% of the total mackerel processing industry are by-products, including head, viscera, fins, and scales (Villamil et al., 2017).
Mackerel head by-product can be utilized as potential fish peptone that has more acceptable and marketable than other utilized products. On the other hand, utilizing fish by-product could prevent serious environmental problems (Herpandi et al. 2011).Fish peptone can be hydrolized by adding enzyme and acid compounds. Enzymatic hydrolysis could breakdown peptide bonds of proteins or polypeptides into smaller amino acids, but it has drawbacks, such as slow reaction rate and high cost, as a result, less effective in the large scale production (Khalil, 2012). On the contrary, acid hydrolysis has advantages, including low cost, short hydrolysis period, simple operation and applicable to industrial processes (See et al., 2011). Benites et al. (2011) stated that the selection of acidifying agent is based on three factors such as cost, availability, and bactericidal action. In additon, acid solutions are mostly catagorized into positive list compounds in terms of halal or kosher view points.
For microbial growth and biomass production, peptone is an essential source of nitrogen due to the high polypeptides and amino acids. Aspmo et al. (2005) revealed that an important component in microbial growth media is a nitrogen compound. Several studies have been observed that peptone extracted from sole fish by-product was capable of supporting microbial growth rate in the cultures of Pseudomonas aeruginosa, Lactobacillus acidophilus, and Saccharomyces cerevisiae.
Furthemore, recently, Shirahigue et al. (2018) reported peptones hydrolyzed from tilapia and cobia by-product with different acid solutions (citric, formic, and propionate acid) had a significant growth rate and biomass production in the culture of Escherichia coli and Staphylococcus aureus compared to commercial peptone. These findings also in accordance with the peptone isolated from Atlantic cod stomach with formic and phosphoric acid reported by Gildberg et al. (2010). As mentioned above, fish peptones provide better microbial growth, as well as higher biomass production due to the high content of soluble protein compounds, particularly nitrogen compounds. In addition, fish peptones are acceptable for all religions and do not related to dengerous diseases.
However, few investigations on peptone hydrolyzed from mackerel head by-product with acid-adding process. This reseach aimed to characterize peptone from mackerel (Scomber japonicus) head by-product using acid-adding process as bacterial growth media in comparison with commercial peptone.
MATERIALS AND METHODS
Materials
The mackerel heads were obtained from PT. Kelola Mina Laut (Gresik, East Java, Indonesia). The average weight of a mackerel head was 14.5 g. The mackerel heads were put in polyethylene plastic and conditioned at cold temperatures (4oC) during transportation. After the sample arrived at the laboratory, the sample was washed with running water and ground using a milling machine (Maxindo, MHW-80, Indonesia). Then, sample was put into polyethylene plastic and stored in the freezer at a temperature of -20oC for up to 1 week. Escherichia coli, Streptococcus aureus, Salmonella thyphi. and Aeromonas hydrophila strains used in this research was obtained from InaCC LIPI, Indonesia. All chemicals and reagents used were of analytical grade.
Preparation of mackerel head by-product hydrolysate
Hydrolysate of mackerel head by-product according to the method carried out by Khalil (2012) with slight modifications. Samples were weighted 900 g by digital weight machine, and then, added 10% of distilled water with decresing pH around 4.2 using 4N HCl treatment. The treated samples were incubated at room temperature (24-26oC) for 7 days to hydrolyze fish protein by activing endogenous enzymes. After incubation period, the hydrolyzed samples, then treated at 85oC for 20 minutes to inactive enzymes, followed by centrifugation at 5000 rpm for 10 minutes to separate samples into three fractions, namely solid phase, liquid phase and oil phase. The liquid phase compound was then spray-dried at inlet temperature of 160oC and an outlet temperature of 90oC. The obtained peptone was stored at low temperature until used.
Characterization of mackerel peptone
Determination of chemical composition
Total nitrogen, fat, moisture, and ash contents were determined by the method of AOAC (2005). Total nitrogen was determined by the Kjeldahl method. Fat content was determined using Soxhlet method. For moisture content, gravimetric method was used in this study. In addition, ash content of extracted peptone was analyzed by the gravimetric method.
Determination of solubility
Solubility was analyzed using the gravimetric method (Ningsih et al., 2018). About 1 g sample dissolved in 150 mL of distilled water. The solution then filtered using Whatman paper no. 2. Solubility can be determined using the formula:
![]()
Where, a = Whatman paper plus residue
b = initial weight of Whatman paper
c = sample weight
MC = moisture content
Determination of color parameter
The determination of color parameter of peptone extracted from mackerel head by-product with acid-adding treatment was measured using a Konica minolta chroma meter CR-400 (Japan). The color analysis were catagorized into L*, a*, and b* values. The L* value means lightness, the a* value means greeness to redness, and the b* value means blueness and yellowness. The instrument was calibrated with the standard before used in determining color. After calibrating, the peptone sample was put into petri dish, then, the sensor section pointed at the peptone sample and noted the results of color test consist of L*, a*, b*, whiteness and color intensity.
Bacterial Growth Test
Preparation of culture media
Growth media formulations consist of peptone extracted from mackerel head by-product, commercial peptone, sodium chloride and yeast extract, which were equivalent to the composition of Luria Bertani (LB) broth. Media sterilized using autoclaves at 121oC for 15 minutes at a pressure of 1 atm (Andualem and Gessesse, 2013). To obtain the growth performance of each peptone tested in this study, the formulation of culture media was used varies as follows:
Table 1: Formulation of culture media.
| Ingredient | Positive control | Negative control | Mackerel |
| Commercial peptone | 10.00 | – | – |
| Mackerel peptone | – | – | 10.00 |
| Yeast extract | 5.00 | 5.00 | 5.00 |
| Sodium chloride | 10.00 | 10.00 | 10.00 |
Bacterial growth measurement
The Optical Density (OD) was analyzed by testing the growth of Escherichia coli, Staphylococcus aureus, Salmonella thyphi and Aeromonas hydrophila on each media. Incubation was done using a shaker with a speed of 100 rpm at 35oC. Bacterial growth measured at 0-36 hours every 6 hours and measured using a spectrophotometer with a wavelength of 600 nm (Shirahigue et al., 2018).
Biomass production
Biomass production was determined at 24-hour growth. 25 ml sample containing bacteria and media was transferd into a sterile falcon tube then centrifuged at 5000 rpm for 20 minutes. The precipitate was separated, then, added 5 mL of 0.85% NaCl solution (w/v). Afterward, the precipitated sample was centrifuged in the same condition. Finally, the precipitate was dried for 24 hours at 105oC (Poernomo and Buckle, 2002).
Statistical Analysis
Data were examined using analysis of variance (ANOVA), and in the case of significant difference was signed as p<0.05, followed by a Tukey’s post-hoc test using SPSS 25.0 software.
RESULTS AND DISCUSSION
Chemical Composition
The chemical analysis of peptone extracted from mackerel head by-product was observed, and the results are presented in Table 2. The chemical composition of mackerel peptone consisted of total nitrogen, fat, moisture, and ash accounted for 11.53%, 1.33%, 5.07%, and 3.78%, respectively. The peptone extracted from mackerel head by-product was higher in term of nitogen content than that of fish by-product peptone reported by Najim et al. (2015) and it was similar to yellowstripe sead fish peptone (Saputra and Nurhayati, 2013). However, when compared to commercial peptone, the nitrogen content of mackerel peptone was lower than that of commercial peptone. The peptone isolated from mackerel head by-product was higher in fat and moisture content than those of fish by-product peptone and commercial peptone reported by Najim et al. (2015), while the ash content of mackerel peptone was lower when compared to those of peptone mentioned above. It might be indicated that component of peptone (expecially nitrogen content) extracted from mackerel head by-product was still crude compared to the commercial peptone. In addition, the total nitrogen is influenced by the protein content and the hydrolysis process in the raw material (Barokah et al., 2017). Total nitrogen obtained from the peptide bond breaking process becomes simpler during the hydrolysis process and the hydrolysis process is also influenced by various factors such as pH, temperature, and time (Ningsih et al. 2018). However, the mackerel peptone could be used as a essential substrate of bacterial growth media.
Table 2: Chemical characteristics of mackerel head, fish head by-product and commercial peptones.
| Parameter (%) | Mackerel head peptone | Fish by-product peptone* | Commercial peptone** |
| Total Nitrogen | 11.53 | 10.8 | 15.10 |
| Fat | 1.33 | 0.8 | 0.4 |
| Moisture | 5.07 | 3.1 | 1.5 |
| Ash | 3.78 | 8.0 | 5.0 |
* reported by Najim et al. (2015)
** NeoGen Lab peptone
Color Parameter
The color parameter of the peptone isolated from mackerel head by-product was measured adn the results are tabulated in Table 2. and depicted in Fig. 1. The L*, a* and b* value of the mackerel peptone were 85.11%, 0.04%, and 19.04%, respectively. When compared to commercial peptone, the L*, a*, and b* value of mackerel peptone was lower than that of commercial peptone, indicating the color of mackerel peptone (87%) showed slightly lower intensity than that of commercial peptone (90.71%). However, the whiteness of peptone extracted from mackerel head by-product was higher compared to commercial peptone, which was 48.94% in the mackerel peptone, and 40.51% in the commercial peptone. Research has reported that peptone extracted from fish by-products (multi-species of marine fish) has color parameter consists of 52.64 in L*, 2.50% in a*, 7.99% in b* and 51.44% in whiteness (Nurhayati et al. 2015), and Barokah et al. (2017) investigated that microencapsulated peptone from marine fish by-product performed the L*, a*, b*, and whiteness value accounted for 60.01%, 1.70%, 10.33% and 57.44%, respectively.
Table 3. Color parameter of mackerel and commercial peptone.
| Parameter | Mackerel peptone | Commercial peptone* |
| Linghtness (L*) | 85.11±0.06 | 86.45±0.06 |
| Green-red (a*) | 0.04±0.05 | 0.59±0.06 |
| Blue-yellow (b*) | 19.04±0.06 | 27.48±0.04 |
| Whiteness | 48.94±0.07 | 40.51±0.05 |
| Color intensity | 87±0.05 | 90.71±0.06 |
*NeoGen Lab peptone
 |
Figure 1: Color. (a) Commercial peptone (NeoGen Lab) and (b) Mackerel peptone. |
Solubility
Solubility of peptone is an indicator to determine the quality of peptone, as stated by Khalil (2012), peptone is protein hydrolysates that are soluble in water and not coagulable by high temperature. The peptone extracted from mackerel head by-product had solubility around 97.07%, which was lower solubility when compared to that of commercial peptone with solubility around 100%. The solubility of mackerel peptone in accordance with the solubility of yellowstripe sead fish peptone reported by Saputra and Nurhayati (2013). The high solubility value of peptone product is due to the presence of a hydroxy group in the mackerel peptone that interact with water molecules (Ningsih et al. 2018). High solubility value in protein hydrolyzate is caused by the reaction of protein breakdown into smaller peptides (Barokah et al. 2017).
Bacterial Growth Profile
Four different bacterial species (Escherichia coli, Staphylococcus aureus, Salmonella thyphi and Aeromonas hydrophila) were selected to determine the growth rate in the Luria-Bertani (LB) broth with some modifications. The growth profiles of both mackerel and commercial peptones can be shown in Fig. 2. The bacteria grew properly in the LB broth supplemented with the mackerel peptone, as well as commercial peptone. The results depicted in Figs. 2A-2C, almost all the bacterial growth rates supplemented the mackerel peptone showed better performance, compared to commercial peptone (as a positive control), as well as untreated peptone (as a negative control) during the research period. However, only for Aeromonas hydrophila, the growth rate of peptone isolated from mackerel head by-product was in the line with commercial peptone, but both of them grew faster than that of the untreated peptone. This might be suggested that the peptone isolated from mackerel head by-product is more effective for supporting growth of bacteria in the LB-broth media than that of commercial product.
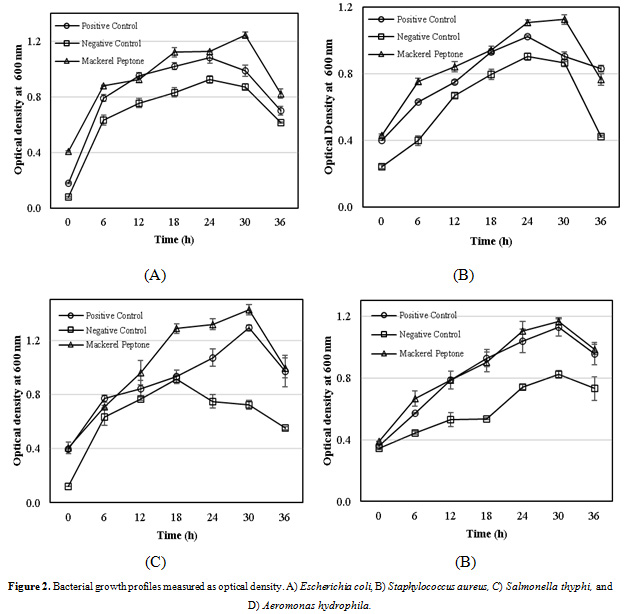 |
Figure 2: Bacterial growth profiles measured as optical density. A) Escherichia coli, B) Staphylococcus aureus, C) Salmonella thyphi, and D) Aeromonas hydrophila. |
Previous studies have investigated that peptones extracted from different fish species, such as cod, salmon, tuna, and unspecified fish showed more higher microbial growth rate than that of a casein peptone (Dufossé et al., 2001). Peptones from cowtail ray (Trygon sephen) have superior performance in supporting different species of microorganims (Aspergillus flavus, Bacillus subtilis, Saccharomyces cerevisiae, Escherichia coli, and Staphylococcus aureus) were compered to commercial peptones (Poernomo and Buckle, 2002). In additon, peptones isolated from different fish species performed better bacterial growth profile than that of commercial peptones (Vieira et al., 2005; Safari et al., 2011). Nevertheless, Najim et al. (2015) reported the fish by-product peptones have lower performance when compared to commercial peptone for Pseudomonas aeruginosa, Lactobacillus acidophilus, and Saccharomyces cerevisiae.
Biomass Production
The bacterial biomass production of peptone produced from mackerel head by-product was observed and the results are illustrated in Fig. 3. The biomass production of all bacterial cultures consisted of Escherichia coli, Staphylococcus aureus, Salmonella thyphi and Aeromonas hydrophila ranged from 30.21-60.32, 30.21-60.32, 30.21-60.32 and 30.21-60.32 (in mg per 100 mL), respectively. For the culture of Escherichia coli, the biomass production in the medium supplemented by the mackerel peptone showed significantly increase (p<0.05) in comparison with either commercial peptone (+ control) and no added peptone (- control). This result was also similar to the cultures of Staphylococcus aureus and Salmonella thyphi, which the mackerel peptone presented higher bacterial biomass yields than those in commercial and no supplemented peptones. However, the culture of Aeromonas hydrophila yielded a significantly increase (p<0.05) of biomass production in the commercial peptone than those in mackerel peptone and negative control. This might be indicated that the peptone produced from mackerel head by-product was effective for supporting bacterial growth in terms of biomass by providing the appropriate sources of nitrogen. Poernomo and Buckle (2002) stated that the high biomass production provides adequate nutrients in supporting the growth rate of microorganisms. In addtion, the higher biomass production obtained, the more effective growth rate of media supplemented with peptones as a microbial substrate.
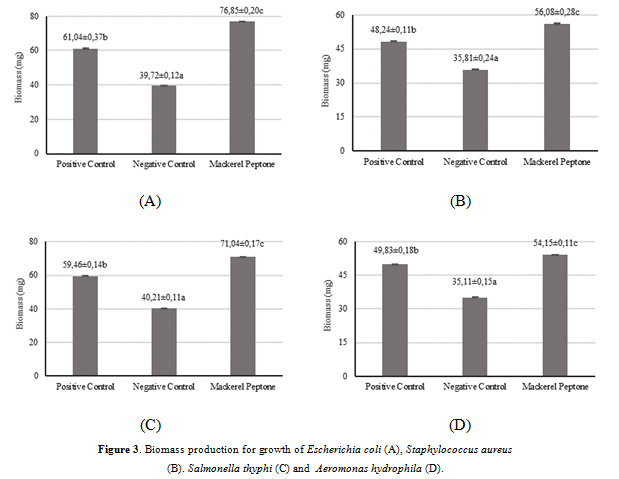 |
Figure 3: Biomass production for growth of Escherichia coli (A), Staphylococcus aureus (B). Salmonella thyphi (C) and Aeromonas hydrophila (D). |
These findings are in the line with some studies revealed that peptones produced from different fish by-products, such as Panulirus argus, Panulirus laevicauda, and Macrobrachium amazonicum showed a significant biomass production of Escherichia coli compared to commercial peptone (OXOID) (Vieira et al., 2005). Moreover, Poernomo and Buckle (2002) and Shirahigue et al. (2018) reported higher result of biomass yield by five microorganisms in Trygon sephen peptones and by two bacteria (Escherichia coli and Staphylococcus aureus) in both Oreochromis niloticus and Rachycentron canadum, respectively.
CONCLUSION
In summary, peptone extracted from mackerel head by-product was characterized by chemical composition, solubility, color parameter, bacterial growth, and biomass production. Though the nitrogen compound, solubility and color intesity of mackerel peptone were lower than that of commercial peptone, almost the bacterial growth rates and biomass productions for Escherichia coli, Staphylococcus aureus, Salmonella thyphi, and Aeromonas hydrophila showed significantly faster and higher in mackerel peptone compared to the commercial peptone. Thus, peptone produced from mackerel head by-product can be an alternative source of as halal substrates for bacterial growth.
ACKNOWLEDGEMENTS
We would like to express our gratitude to the Directorate of Research and Community Service, Ministry of Education and Culture, Republic of Indonesia for financial support through the national grant 2019-2020
Financial Support and Sponsorship
DRPM (No. 330.30/UN10.C10/PN/2019)
Conflict of Interest
There are no conflict of Interest
REFERENCES
Andualem B and A Gessesse (2013) Production of microbial medium from defatted brebra (Milletia ferruginea) seed flour to substitute commercial peptone agar Asian Pacific Journal of Tropical Biomedicine Vol 3 No 10 Pages 790-797
AOAC Association of Official Analytical Chemist (2005) Official Method of Analysis of The Association of Official Analytical Chemist. Arlington Viginia USA
Aspmo SI SJ Horn and VGH Eijsink (2005) Hydrolysates from Atlantic cod (Gadus morhue L.) viscera as components of microbial growth media Process Biochemistry Vol 40 Pages 3714-3722
Barokah GR B Ibrahim and T Nurhayati (2017) Characterization microencapsulated peptone from spoiled by catch fish using spray drying methods Indonesian Fisheries Processing Journal Vol 20 No 2 Pages 401-412 (in Indonesia)
Benites DC Y Verboven D Stroman and L Kodjikian (2011) The role of topical moxifloxacin, a new antibacterial in Europe, in the treatment of bacterial conjunctivitis Vol 31No 8 Pages 543-570
Dufossé L DLB Broise and F Guerard. 2001. Evaluation of nitrogenous substrates such as peptones from fi sh: A new method on gompertz modeling of microbial growth Current Microbiology Vol 42 Pages 32-39
Fallah M S Bahram and SR Javadian (2015) Fish peptone development using enzymatic hydrolysis of silver carp by‐products as a nitrogen source in Staphylococcus aureus media Food Science and Nutrition Vol 3 No 2 Pages 153-157
FAO (2018) Food and Agriculture Organization of United Nation Globe fish highlights of quarterly update on world seafood market [cited 2019 Sept 13] Available from http://www.fao.org/
Gildberg A R Dahl H Milkkelsen and K Nilsen (2010) Peptons from atlantic cod stomach as nitrogen sources in growth media to marine bacteria Journal of Aquatic Food Product Technology Vol 19 Pages 75–83
Herpandi, N Huda, A Rosma WAW Nadiah (2011) The tuna fishing industry: a new outlook on fish protein hydrolysates Comprehensive Reviews in Food Science ad Food Safety Vol 10 No 4 Pages 195-207
Khalil AA (2012) Protein characterization of the aqueous soluble phase of acidified and autolyzed bolti fish (Tilapia nilotica) viscera Asian Journal of Biotechnology Vol 4 No 3 Pages 108-119
Najim SM JM Al-Noor and WA Al-Waely (2015) Extraction of crude peptone fromm fish by-products for use as a nitrogen source in microbiological media Global Journal of Fisheries and Aquaculture Researches Vol 2 Pages 29-37
Ningsih R Sudarno and Agustono (2018) The effect of maltodextrin concentration on characteristic of peptone fish capacity (Lutjanus sp.) Agrointek Vol 12 No 1 Pages 55-60 (in Indonesia)
Nurhayati T B Ibrahim P Suptijah E Salamah RN Fitra and ERW Astuti (2015) Characterization of peptone from spoiled by-catch fish as nutrient source for growth of bacteria and yeast Indonesian Fisheries Processing Journal Vol 25 No 1 Pages 68-77 (in Indonesia)
Poernomo A and KA Buckle (2002) Crude peptone from cowtail ray (Trygon sephen) viscera as microbial growth media World Journal Microbiology Biotechnology Vol 18 Pages 333-340
Safari R HN Saravi R Pourgholam AA Motalebi and A Ghoroghi (2011) Use of hydrolysates from silver carp (Hypophthalmichthys molitrix) head as peptone for Vibrio anguillarum and optimization using response surface method (RSM) Journal of Aquatic Food Production Technology Vol 20 No 2 Pages 247-257
Saputra D and T Nurhayati (2013) Application and production of yellowstrip sead fish peptone for bacteria growth media Indonesian Fisheries Processing Journal Vol 16 No3 Pages 215-223 (in Indonesia)
See SF LL Hoo and AS Babji (2011) Optimization of enzymatic hydrolysis of salmon (Salmo salar) skin by alcalase International Food Research Journal Vol 18 Pages 1359-1365
Shirahigue LD IS Ribeiro LFA Sucasas L Anbe P Vaz-Pires and M Oetterer (2018) Peptones in silage from tilapia (Oreochromis niloticus) and Cobia (Rachycentroncanadum) by-product as a culture medium for bioprocesses Journal of Aquatic Food Product Technology Vol 27 No 6 Pages 712-721
Vieira GH RH Vieira A Macrae and OV Sousa (2005) Peptone preparation from fishing byproducts Journal of the Science of Food and Agriculture Vol 85 No 7 Pages 1235–1237
Villamil O H Vaquiro and JF Solanilla (2017) Fish viscera protein hydrolysates: production, potential applicaions and funcional and biactive properties Food Chemistry Vol 224 Pages 160-171.


