
Histological study on the stages of pollination and
fertilization in the cul tivars of red seedless and
ghezel-ozum grapes
Mahdi Mohammadi
1
*, Mohammad-Reza Dadpoor
1
, Mahboobe Aliasgharpoor
2
,
Elham Mohajel-Kazemi
2
, Hamed Dolati-Baneh
3
and Jaber Panahandeh
4
1
Pomology, Department of Horticultural Science, Faculty of Agricultural Sciences, University of Tabriz,
Tabriz, Iran
2
Plant Sciences, Faculty of Life Sciences, University of Tabriz, Tabriz, Iran
3
Agricultural Research Centre at Seed and Plant Improvement, Kahriz, Urmia, Iran
4
Olericulture, Faculty of Agricultural Sciences, University of Tabriz, Tabriz, Iran
ABSTRACT
The Vitaceae is one of the most important plant families,which it includes the Vitis genus with all its economic
characteristics. In the meantime, the European seedless grapes for their high-quality fruitshave popularity. However,
in the breeding works, the progenies from the seedless cultivars have a low frequency. In Sultana cultivar (known as
Thompson seedless in the United States) which is the most important seedless cultivar, abortion of the embryo after
pollination and fertilization has been mentioned as a reason for being seedless. Investigating the structure of the
seed and determining the time of abortion can be important in terms of tissue culture, embryo rescue and biology.
In this research, the comparative study of the seed development of the red seedless grape with Ghezel-Ozum seeded
cultivars considered using the histological techniques. Samples were harvested from pre-pollination till seed matu-
ration every ve days from the Research Station of Kahriz, Urmia and xed in the FAA xator. The xed samples
were immersed in the paraf n and then were cut with a microtome. After staining with PAS-Hematoxilen, samples
were studied with the light microscope and photography. Observations showed that the growth and development of
zygote and endosperm were delayed and eventually stopped in the red seedless cultivar after the double fertilization.
KEY WORDS: ABORTION; DOUBLE FERTILIZATION; ENDOSPERM; POLLINATION; SEEDLESS GRAPES; SEEDED GRAPES
306
ARTICLE INFORMATION:
*Corresponding Author: MahdiMohamadi1982@yahoo.com
Received 27
th
Dec, 2016
Accepted after revision 2
nd
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
Biosci. Biotech. Res. Comm. Special Issue No 1:306-317 (2017)

Mahdi Mohammadi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION 307
INTRODUCTION
The Vitaceae is one of the most important plant families,
which it includes the Vitis genus with all its economic
characteristics. Furthermore, this family has about 15
genera with 900 species (Zhang et al. 2015). The Vitis
genus has a special importance in horticulture. Itis
divided into two sub-genera of Euvitis and Muscadine.
The commercial cultivars are related to the rst sub-
genus. Their owers have 5 sepals, 5 petals, 5 stamens
linked to the petals and alternating with the disc-shaped
edges and the pistil with di-carpel ovary, in which each
ovary there are the two standing ovules (Ghahraman
1993; Ghanadha et al. 2004).
The different species of the sub-genus Euvitis (2n=38)
have very little differencesin terms of the chromosomal
structure, and their intercourse with each other is pos-
sible. Therefore, only the geographical boundaries and
ecological barriers have separated them from each other
and created the European, American and Asian varieties
(Ghahraman 1993; Ghanadha et al. 2004).
The European grapes due to having high-quality fruit
as the commercial varieties are popular, and it has been
found that polyphenolic compounds existing in those
which include the avonoid compounds are useful in
preventing the heart diseases and cancer in human (Kalt
2001; Lepiniec et al. 2006). The American grapes are also
used as the stock due to resisting pests and diseases. The
varieties that are resistant to cold and frost can also be
found in the Asian grapes (Ghahraman 1993; Ghanadha
et al. 2004).
The origin of the European grapes is the area between
the Caspian Sea to the Black Sea and from there to the
Mediterranean area and Europe has expanded (Wen
2007). Although, grapevines are the dioecious plants,
but following selective breeding done by man that has
continued for many years, the male stocks have been
removed from the European grapes and now the female
stocks and self-pollinated hermaphrodite are cultivated.
Since being seedless is desirable characteristic in many
fruits for table utilizations, in the grape has also paid
special attention to this feature. However, the seed-
less varieties and their progenies have a low frequency.
Perhaps the most main factor is dif culties in making
hybrid and producing seed in these varieties (Bharathy
et al. 2005; Liu et al. 2003; Ramming et al. 1991; Sharma
et al. 1996; Yang et al. 2007).
In Sultana cultivar which is the most important seed-
less cultivar, being seedless is the result of digesting the
embryo after pollination and fertilization. Ebadi et al.
(2010) demonstrated that the high frequency of abnor-
mal ovules and single fertilization can be considered as
two other reasons of seedlessness. The remainders of
undeveloped ovule inside fruitcan be seen until its har-
vest stage. However, due to the small size of these imma-
ture seeds, it does not feel when eating fruit. Although
this feature is very favorable in terms of production,
but in terms of eugenic objectivesand obtaining the
reproductive progenies would be problematic (Bharathy
et al. 2005; Farsi &Bagheri 2004; Farsi &ZolAli 2006; Liu
et al. 2003; Wakana et al. 2002; Yang et al. 2007).
Knowing how to digest the ovule and its morphologi-
cal disorders and the appearance time of this phenom-
enon is very important and necessary. Perhaps only by
this way, the separating of embryo and timely rescue it
can be performed (Bharathy et al. 2005; Liu et al. 2003;
Yang et al. 2007).
Therefore, the comparative study of the development
of the ovule, the formation of embryo sac and seed in the
seedless and seeded cultivars of grapes not only explain
the structural and morphological differences between
them, but it can also use to identify and utilize the
appropriate methods to prevent the hollowness appear-
ance of the seed (in breeding purposes) (Pratt 1971).
More processes of the development of ovule and the
appearance of embryo occur at the microscopic level
thereforethe use of appropriate histological methods for
the structural studying of seedless in the grapes will be
inevitable. However, preparing the microscopic samples
of the perennial trees has speci c problems which the
hardness of tissuesas well as tannins and other phenolic
compounds in them can be noted, and their combina-
tion with chemical materials used in the xator prevents
the optimal xation of the tissues. Therefore, cellular
and histological studies on tree species much less than
herbaceous plants, and vines are not also an exception.
In the annual and biennial plants such problems are less
common (Ruzin 1999).
The purpose of this study was a documentation of his-
tological information in vitis geneus with a comparative
study of the seed development of the red seedless grape
with Ghezel-Ozum seeded cultivar to exact determine
the time of embryo abortion. This study can be useful in
studies and practices of tissue culture and embryo rescue.
Because, to know an appropriate time of exiting seeds can
be more successful in tissue culture and embryo rescue.
MATERIALS AND METHODS
PLANT MATERIAL AND SAMPLING
Samples ( ower buds, owers and fruits during sam-
pling) were collected from Seed and Plant Improvement
Research Institute, Kahriz, Urmia, Iranin Jun to August
2007. Samples were collected from 10 days before and
40 days after the loss of cap (with a 7-day intervals)
(one in orescence per branch, and 10 branches per plant
and 20 plants in the total of the experiment for each

Mahdi Mohammadi et al.
308 HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Table 1. The dates of sampling of the Ghezel-Ozum cultivar
Date Developmentstage Features
2008/05/28 The stage of pollination The peak stage of anthesis
The length of ovule: 1-1.5 mm
2008/06/02 5 days after pollination The length of ovule: 2-3 mm
The length of fruit: 4-6 mm
2008/06/07 10 days after pollination The length of seed: 2-4 mm
The length of fruit: 8-10 mm
2008/06/12 15 days after pollination The length of seed: 5-6 mm
The length of fruit: 10-14 mm
2008/06/17 20 days after pollination The length of seed: 6-7 mm
The length of fruit: 14-16 mm
2008/06/24 27 days after pollination The length of seed: 6-8 mm
The length of fruit: 14-18 mm
2008/06/27 30 days after pollination The length of seed: 6-8 mm
The length of fruit: 18-20 mm
2008/07/03 36 days after pollination The length of seed: 6-8 mm
The length of fruit: 18-20 mm
2008/07/07 40 days after pollination The length of seed: 6-8 mm
The length of fruit: 18-20 mm
2008/07/13 46 days after pollination The length of seed: 6-8 mm
The length of fruit: 18-20 mm
2008/07/18 51 days after pollination The length of seed: 6-8 mm
The length of fruit: 18-20 mm
2008/10/30 5 months after pollination The length of seed: 6-8 mm
The length of fruit: 18-22 mm
The stage offull maturity of
embryo and a ripe fruit
variety), and immediately xed in the FAA xator (for-
maldehyde- acetic acid-alcohol) and transferred to the
laboratory. The local varieties that were used for experi-
ments, including the red seedless cultivar (containing
stenospermocarp seed) and Ghezel-Ozum seeded cultivar
(containing the actual seed) were selected for compari-
son with each other.
Samples were harvested from the clusters that were in
the similar phonological stage, and were marked for next
sampling. Samples were harvested in the early morn-
ing to avoid shrinking and losing the water. The 8-year
plants were used and cultivated by using the method of
Top Wire Cordon (2×4 Meter).
Samples were kept in the refrigerator during the
experiment period. Then, based on the length (in mm) of
pistil (or ovarian), and fruit were dividedin the early and
later stages, respectively. These divisions in the early
stages with the graph paper, and at later stages with the
caliper under the simple (loop) microscope were done In
the maturity stages, seeds were separated from the fruits
and their length were also measured.
Separating under the loop was done using forceps and
sharp-pointed needles, and cap and stamens were pre-
cisely separated, and immediately placed in the xator
materials. Seeds were also separated from the fruit in the
same method. At this stage, it has paid attention to the
time of separating not to damage the samples. Further-
more, in the mature seed, the embryo was removed from
the seed and examined under a microscope. Sampling
of the Ghezel-Ozum and red seedless cultivars were per-
formed in accordance with Tables 1 and 2.
PREPARING THE SAMPLES AND
HISTOLOGICAL ASSAY
The samples were immersed in the FAA xator (formal-
dehyde 37%, 5 ml; ethanol 50%, 90 ml; and 5 ml of
glacial acetic acid) for 12-24 hrs. After suf cient wash-
ing with running water and dehydrating with increasing
levels of ethanol, the samples were clari ed with xylene
and saturated with paraf n. The samples after molding
in paraf n, with the rotary handle microtome (R Jung
Heidelberg) were cut at the thickness of 8 to 10 μm. The
slices after removing paraf n and water were stained
with Hematoxylin and PAS-Hematoxylin (Jenson 1962).
The staining of Light Green, and Sudan Black and Red
were used for studying proteins and lipids, respectively
(Gahan 1984). The microscopic investigatingand photo-
graphing of samples were done with the light micro-
scope (Nickon, E200-LED, USA).
Mahdi Mohammadi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION 309
RESULTS
THE CHANGES OF PRE- AND POST-POLLINATION
STAGES IN THE RED SEEDLESS CULTIVAR
In the owers of Red Seedless cultivar in the anthesis (stage
4; stages 1-3 are not shown here) based on sampling date
and the morphological studies with the light microscopy,
the pollen shad germinated and pollination had occurred.
It should be noted that the owers in grapes are on the
clustered in orescences and at one in orescence, ow-
ers can be in several different phonological stages, and in
examinations should be considered this issue.
Three days after pollination (stage 5), the fertiliza-
tion occurred in all the owers, and micropyle was been
closed, which was due to growing upand becoming mas-
sivethe internal walls.At this stage, the presence of pol-
len tube into the embryo sac was signi cant (Fig.1).
The microscopic sections prepared from owers pol-
linated at the stages of 10 and 15 days after pollination
showed that the double fertilization in this cultivar was
successfully occurred; so that the rst division of the
zygote was observed in 15 days after pollination. The
apical and basal cells were formed in high and low den-
sities, respectively and the basal cells were observed in
its rst division (Fig.2).
In the stage of 8 (20 days after pollination), a number
of the free nuclei within the embryo sac were observed
which have been resulted from the division of synergids
(Fig.2). Whereas at this stage the growth of the zygote
had been stopped and the cell had been degenerating.
The formation of the brown sediments around the cell
that was degenerating was signi cant (Fig.3). The sig-
ni cant point at this stage was distinctively the thick-
ening of the transverse walls of calot cells which had
likely been started from the previous stage (the tangen-
tial walls). Furthermore, sometimes the wall thickening
was observed in the radial walls (Fig.3).
In addition, other notable phenomenon was the sepa-
ration of the inner integumentfrom the outer integument
which was started from this stage and at the following
stages, this space was increased (Fig.4).
In the stage of 9 (30 days after pollination) in the
red seedless cultivar, the nucellus were strongly pressed
and the inner integument completely kept away from
the outer integumentand an empty space was created
between them (Fig. 4b).
In the stage of 10 (40 days after pollination) in the red
seedless cultivar, the shrinkage of nucellus was contin-
ued and nucellus in the form of crumpled on one side of
the seed was observed. The stages of degeneration were
along with the shrinkage and becoming small of the
seed. Thickening in the transverse walls of the calot cells
were also continued at this stage. In the outer integu-
ment, the needle-shaped crystals of calcium oxalate
were observed which large groups of them were formed
within the idioblast (Fig. 5).
THE CHANGES OF PRE- AND POST- POLLINATION
STAGES IN THE GHEZEL-OZUM CULTIVAR
Five days after pollination in the Ghezel-Ozum cultivar,
zygoteand synergids were observed in the embryo sac
(Fig.6).
Table 2. The dates of sampling of the red seedless cultivar
Date Developmentstage Features
2008/06/13 Flower bud
2008/06/14 The beginning of anthesis
2008/06/16 30 to 50 percent anthesis
2008/06/18 The time of pollination The peak stage of anthesis
(80 percent owers in anthesis)
The length of ovule: 1-1.2mm
2008/06/21 5 days after pollination The length of seed: 1.2-1.5 mm
The length of fruit: 2-4 mm
2008/06/27 10 days after pollination The length of seed: 1.5-2 mm
The length of fruit: 4-6 mm
2008/07/03 15 days after pollination The length of seed: 2-2.5 mm
The length of fruit: 6-10 mm
2008/07/08 20 days after pollination The length of seed: 2.5-3 mm
The length of fruit: 10-10 mm
2008/07/15 30 days after pollination The length of seed: 2.5 mm
The length of fruit: 12-14 mm
2008/07/28 40 days after pollination The length of seed: 2-2.3 mm
The length of fruit: 12-14 mm
310 HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mahdi Mohammadi et al.
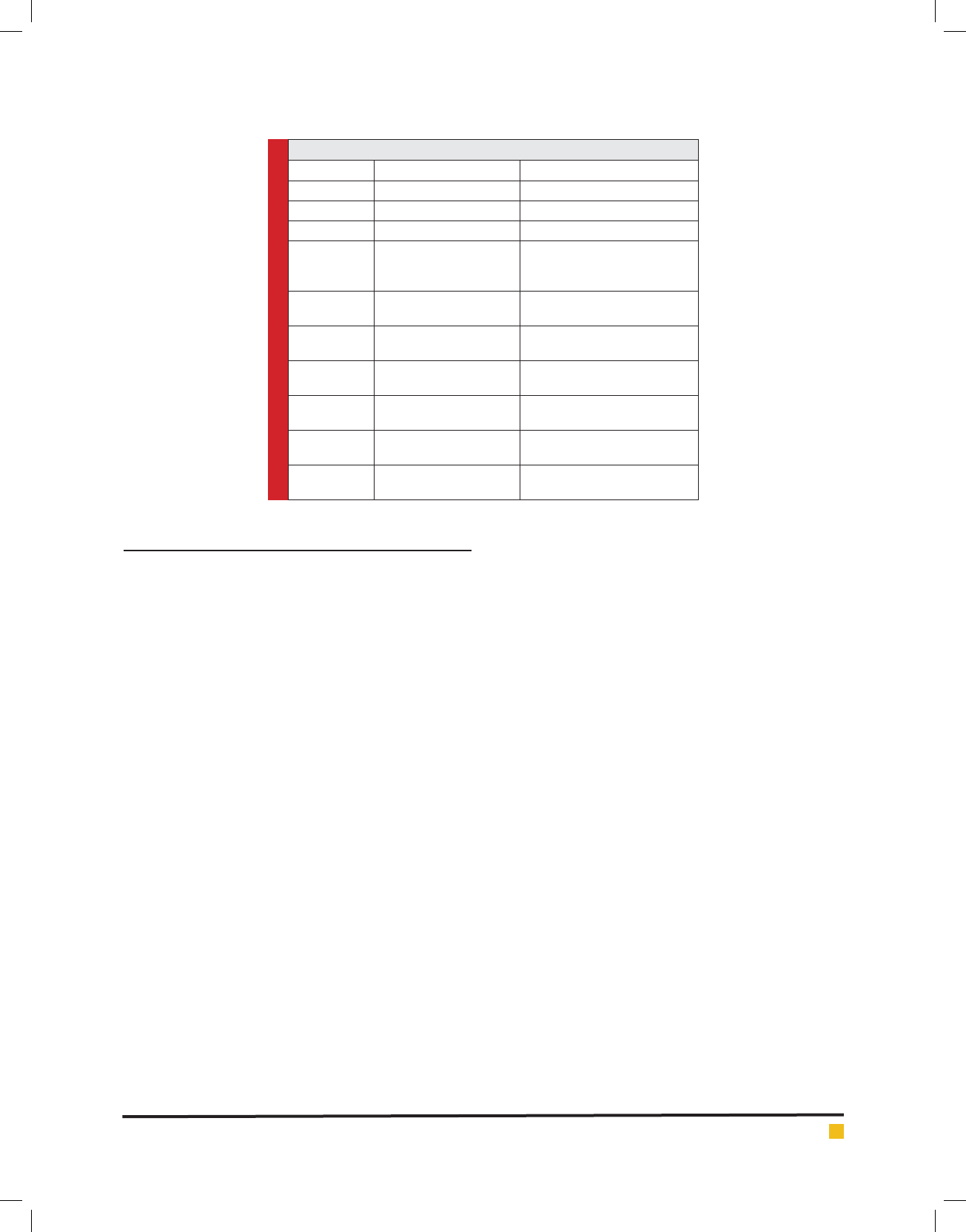
FIGURE 1. The longitudinal section of the seed
of the red seedless cultivar in 3-5 days after
pollination (stage 5), stained with the PAS-
Hematoxilen method, a) Ovule-synergids and
polar nuclei within the embryo sac were distin-
guished. b) Fig.¬a with further magni cation,
the entering of the pollen tube to synergids
was distinguished. c: calot, es: embryo sac, nu:
nucellus, pt: pollen tube.
Ten days after pollination, a number of the free nuclei
were observed within the embryo sac. The free nuclei,
after the double fertilization, were resulted from the divi-
sions of the synergids which would form the endosperm.
The zygote as well as the synergids was observed in the
form of the shrunk mass by the side of that (Fig.7). In the
outer epidermis of the inner integument, the tangential
divisions were seen with the increasing of the lengthin
the radial direction. In the inner epidermis of the outer
integument were also added on the reddish brown com-
pounds.
In Ghezel-Ozum cultivar, the formation of the globu-
lar embryo was observed in simultaneous with the stage
of 8 (20 days after pollination) (Fig.8). In addition, in the
Ghezel-Ozum cultivar, endosperm was formed with cell
wall and also both internal and external integuments grew.
In the nal stages, the entire seed with the mature
embryo and the cell endosperm was observed. The
FIGURE 2. To occur the double fertilization
process in the red seedless cultivar, stained with
the PAS-Hematoxilen method. a) The zygote
has fertilized and its rst division has done. b)
Endosperm nuclei within the embryo sac are
identi ed. en: endosperm nuclei, es: embryo sac
embryo in this cultivar had thoroughly evolved and
consisted of an axial section, which the apical meristems
of the root and stem formed at the two ends of that, and
storages-accumulated cotyledons were on the both sides
of the apical meristem of stem (Fig.9).
In the mature stage of the Ghezel-Ozumseed, the pro-
tein compounds within the endosperm tissue were thor-
oughly observed which were stained with Light Green
(Fig.10). Furthermore, the needle-shaped crystals of cal-
cium oxalate in the form of the groups of idioblast were
also observed in the nal stages in this cultivar.
DISCUSSION
One of the important cultivars of grapes is red Sultana
or red seedless which is one variety of stenospermocarps
and consumed freshly and for producing sultana. So far,
few studies, histologically, was performed on this culti-
var. Stott (1936) was the rst to report stenospermocarpy
and he applied this word for the immature seeds. He

Mahdi Mohammadi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION 311
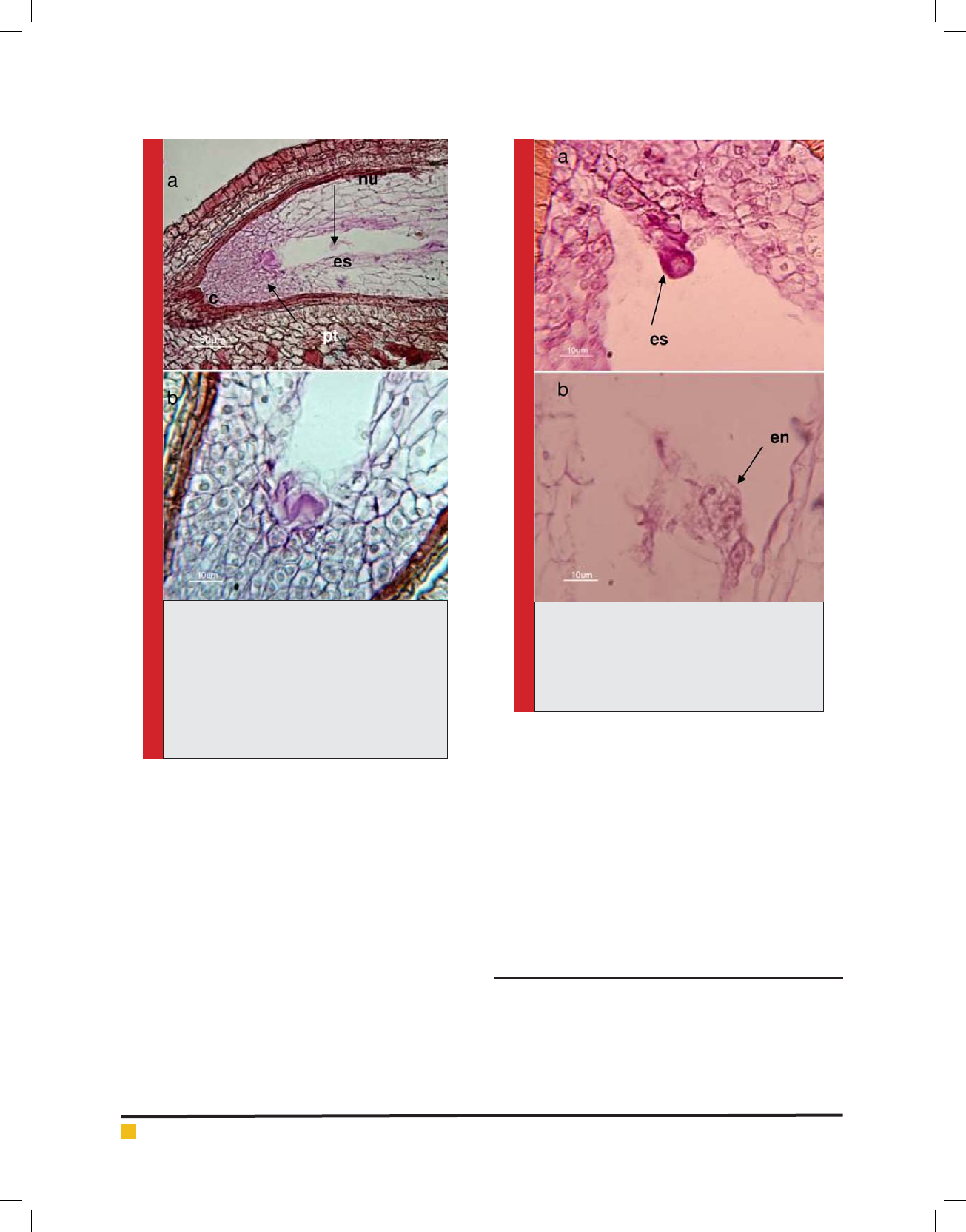
FIGURE 3. Longitudinal section of red seed-
less cultivar in 20 days after pollination,
stained with the method of Hematoxilen .a) The
degenerated pre-embryonic cells were shown
at the micropylar pole. B) Fig.a. With further
magni cation. Brown sediments are observed
around the purple mass. c: calot, de: degener-
ated embryos, mi: micropyle
FIGURE 4. The longitudinal section of red seed-
less cultivar in 20-30 days after pollination
(stages 8 and 9), stained with the method of PAS-
Hematoxilen. a) The initiation of separating the
inner integument from the outer integument is
shown. Photographed with dark eld microscope,
20 days after pollination (stage 8). b) Separating
the inner integument from the outer integument,
30 days after pollination (stage 9). c,e) Fig. a
with further magni cation.d, f) Fig. b with fur-
ther magni cation.The nucellus has degenerated.
es: embryo sac.
stated that the growth of embryo in Thompson Seedless
varieties continues to globular embryo stage (according
to the Pommer et al. 1995).Thereafter, it has been more
considered the breeding aspects of grapevines, which
the most important subjectswere embryo rescue with the
aim of disease resistance, improving fruit quality and
enhancing the performance in the stenospermocarp cul-
tivars. In these studies, genotype, culture medium, sam-
ple age and harvest time have been introduced as impor-
tant factors for successin embryo rescue, however, any
exact research has not been performed on the subject of
ovule and immature seeds of stenospermocarp (Sharma
1996; Yang et al. 2007).
Studies showed that pollination at the stenosper-
mocarp cultivars is just a factor to develop fruit, and fol-
lowing growth and development of endosperm and the
embryo have dif cult (Hanania et al. 2007; Pratt 1971).
Pratt (1971) noted that the outer integument of ovule at
the stenospermocarp cultivars is without sclerenchyma
cells and ovules or aborted seeds remain just as a small
object into cubes. The development of ovule may be
normal, such as seeded cultivars that have nucellus, one
or two integuments and the cord, and or is nearly nor-
mal. Pratt (1971) noted that pollen is usually fertile and
self-pollination occurs in these cultivars. It was found
that the being seedless characteristic is heritable (Bou-
quet and Danglot 1996; Pratt 1971). Liu et al. (2003)
demonstrated that the main reason of stenospermocarp
in Sultana seedless grapeis unknownup to now.
GERMINATION OF POLLEN
It was found that pollen affects the growth and size of
seed, because, the half of the embryo genes and one
third of endosperm genes are sourced from paternal
parent (Ebadi & Dehghani 2002). Pratt (1971) said that
stenospermocarpc ultivars have usually alive pollen. In
our surveys on the red seedless grape, viability and ger-
mination of pollen on the stigma surface were con rmed
by uorescence microscope, however, because of style
tissue thickness, the observation of its penetration from
stigma surface to style was impossible. In sectioning to
view in bright eld light microscope, the pollen tube and
its transition place in the micropylar region and calot
were observed which shows the pollen tube penetrates
into the ovule and then embryo sac.
Mahdi Mohammadi et al.
312 HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
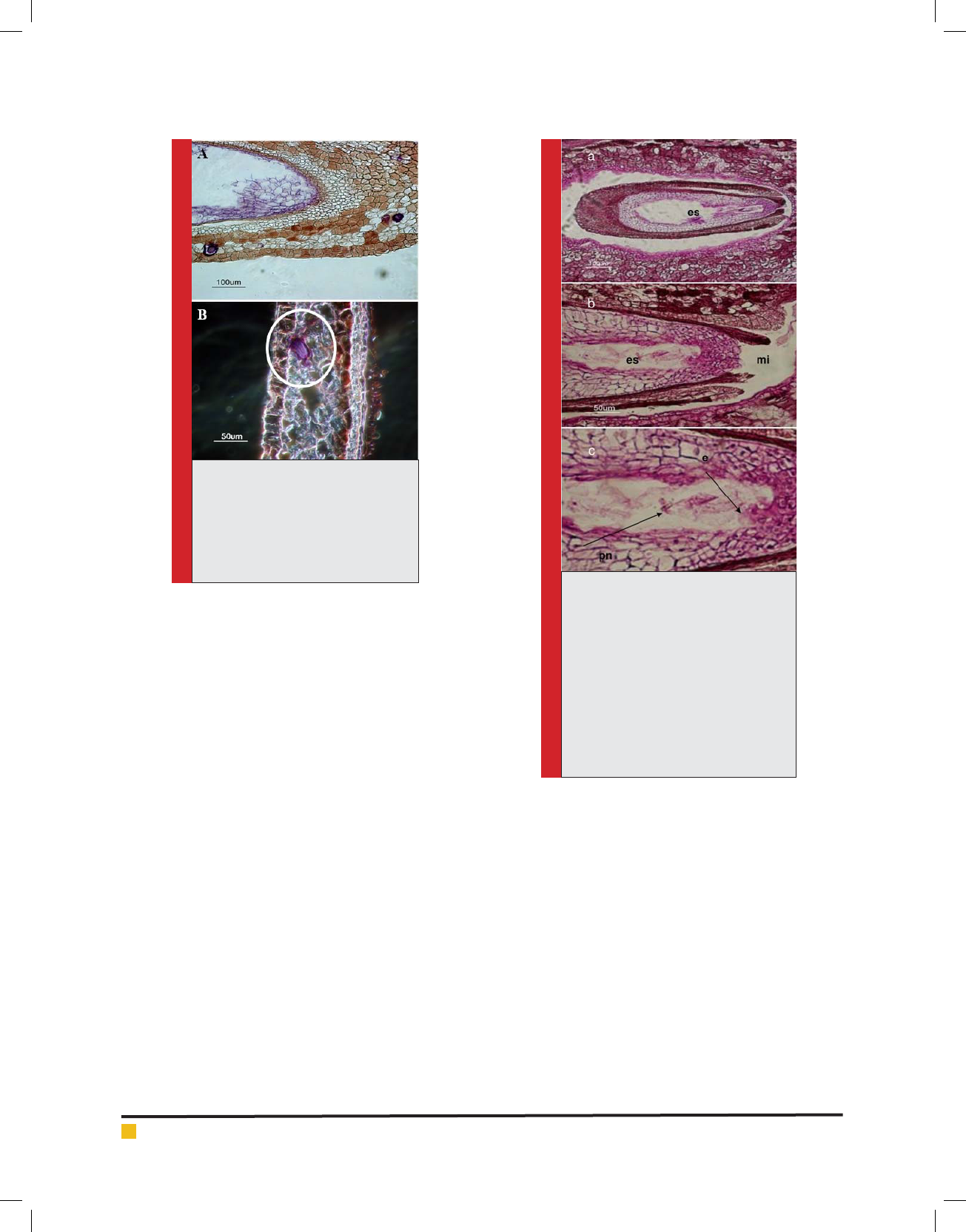
FIGURE 5. The longitudinal section
of red seedless cultivar in 40 days
after pollination (stage 10), Stained
with the method of Hematoxilen.
a,b) Parenchymal cells containing
a mass of calcium oxalate crystals
(Idioblast).
FIGURE 6. The longitudinal section of
Ghezal-Ozum seedless cultivarin pol-
lination and fertilization stage (stage
5), stained with the method of PAS-
Hematoxilen. a) Longitudinal section
of ovule at the Ghezel-Ozum cultivar.
b) Embryo sac in the micropylar pole
with further magni cation has been
determined. c) Zygote cells and ovule
nuclei have been identi ed. e: embryo,
es: embryo sac, pn: polar nuclei, mi:
micropyle
FERTILIZATION AND ZYGOTE FORMATION
In studies, it has been found that the pollen tube reaches
the micropyle within 12 hrs and fertilization take place
24 hrs after pollination (Pratt, 1971). However, some lit-
eratures demonstrated that it occurs 2 to 3 days after
pollination (Batigiana 2006). In an effort to breed seed-
less varieties by Sahijram and Kanamadi (2003), oocytes
were histologically investigated 24 hrs after pollina-
tion, and the formation of zygotes were observed in 4
days after pollination at all studied crosses. Endosperm
mother cell was also formed in all of them, and it means
that double fertilization was successful (Sahijram &
Kanamadi 2003). These ndings correspond with our
observations. The studying sample appeared that ferti-
lization occurred in 3 to 5 days after pollination (due to
a clustering of in orescence and intervals for sampling).
In the studied microscopy sections at this stage, the pres-
ence of pollen tubes inside the synergids con rms this.
Also, fertilization at Ghezel-Ozum cultivar took place in
3 to 5 days after pollination.
POST-FERTILIZATION
Endosperm formation
In our surveys, after fertilization, free nuclei were
formed from divisions of the synergids nucellus in the
embryo sac. However, 30 to 40 days after pollination,
it was found that the endosperm cells were gradually
degenerated at this stage; a type of shrinkage was also
created in nuclei.
In some literatures were noted that the growth of
embryo can be stopped and aborted at the immature
stage which are mentioned different reasons for this. For
example, it can refer to the lack of proper nutrition of
embryo by the endosperm (Bharathy et al., 2005; Liu et
al., 2003; Ramming et al., 1991; Sharma et al.,1996; Yang
et al., 2007). It was also distinguished that the death of
the embryo can be due to toxin production by endosperm
and incompatibility of embryo and endosperm (Bhar-
athy et al. 2005; Yang et al. 2007). In red seedless culti-
var, it seems that one of the reasons for stopping growth
and development of zygote is the stopping the growth
and development of the endosperm; however also the
delay in the growth and development can be another

Mahdi Mohammadi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION 313
FIGURE 7. The longitudinal section
of the seed of Ghezel-Ozum cultivar
in the 10 days after pollination
(stage 6), stained with the method
of PAS-Hematoxilen. a) Zygote and
primary endosperm nuclei are shown
at the micropylar pole. B) Fig.a.with
further magni cation, zygote and
endosperm nuclei which are dividing
have been identi ed. c) Zygote with
further magni cation was shown.
ic: inner integument (coat), mi:
micropyle, ne: nuclear endosperm, z:
zygote, c:calot
cause. Pachno et al. (2014) suggested that, in apomictic
dandelions, the persistent synergids may play a role in
the nutrition of the developing embryo.
In Ghezel-Ozum seeded cultivar, endosperm tissue is
formed and fully developed after double fertilization.
Ten days after pollination, the release endosperm nuclei
were observed in large numbers, which their growth has
continued in the following stages in 15 to 20 days after
pollination and eventually was fully shaped into cell. In
our investigations, proteins, polysaccharides, oil com-
pounds and star-shaped crystals of calcium oxalate were
observed in the mature endosperm.
Endosperm in the seeded cultivars of grape has irreg-
ular shape and is mainly composed of thick-wall cells
(Pratt 1971). It was found that the endosperm can con-
tinue to grow even without fertilization (Chaudhury et
al. 1998; Raghavan 2006). Endosperm effect on embryo
morphogenesis is probably due to physical pressures and
in the production of somatic embryos can be observed
abnormal mode due to imbalances in pressure (Farsi
&Bagheri 2004; Farsi&ZolAli2006).
GROWTH OF INTEGUMENT AND CLOSING
THE MICROPYLE
Batigiana (2006) demonstrated that a series of severe
divisions after fertilization occurs in the funicle, navel,
chalaza and integuments.Also, in the seedless cultivar,
after fertilization, the growth of cells of inner integu-
ment was observed and therefore micropyle was closed.
Then any division was not observed at the integument
cells, while in Ghezel-Ozum seeded cultivar, the growth
of outer integument was impressive. Carraro et al. (1979)
reported when micropyle remains open it is due to the
disruption of pollination, and increases the embryo
abortion and stenospermic in grapes. In our observa-
tions, micropyle opened before fertilization, and after
fertilization due to the growth of inner integument cells
had been closed. Haughnand Chaudhury (2005) report-
edthat there is a relationship between the growth of
endosperm and integument which is related to genetic
control. It means that with growth and development of
the endosperm, integument growth continues. Striem et
al. (1992) showed that seed integument and endosperm
formation and development in the stenospermocarp
grapes are independent of each other, and therefore, seed
integument will be emerging without endosperm and
embryo. In our study, independent of the integument
formation from endosperm and embryo was rejected;
because in this sample, growing integument of embryo,
i.e. the seed, was stopped from 20 days after pollination
and even earlier when also stops growth of endosperm.
In our studies, it was found that the outer integu-
ment at micropylar pole was formed from two to three
cell layers and in chalazal pole was added to the num-
ber of layers and it is 6-7 cell layers.In the red seed-
less cultivar, this integument has color compounds that
are likely polyphenols or anthocyanins. Vessels at the
chalazal pole of outer integument were completely vis-
ible.The Color of outer integument was different with
inner integument and has transparent cells that contain
clearcontents.The inner integument by two to three lay-
ers has the compressed, stretched and attened cells
astangential. The inner integument surface was covered
by the cuticle layer which was determined after staining
with black Sudan. These observations correspond with
the ndings of Pratt (1971).
In the following stages of the red seedless cultivar,
we saw the separation of the inner integument from the
outer integument. The beginning of this phenomenon
was observed 20 days after pollination. Furthermore,
nuclei were gradually suffering from degeneration and
Mahdi Mohammadi et al.
314 HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
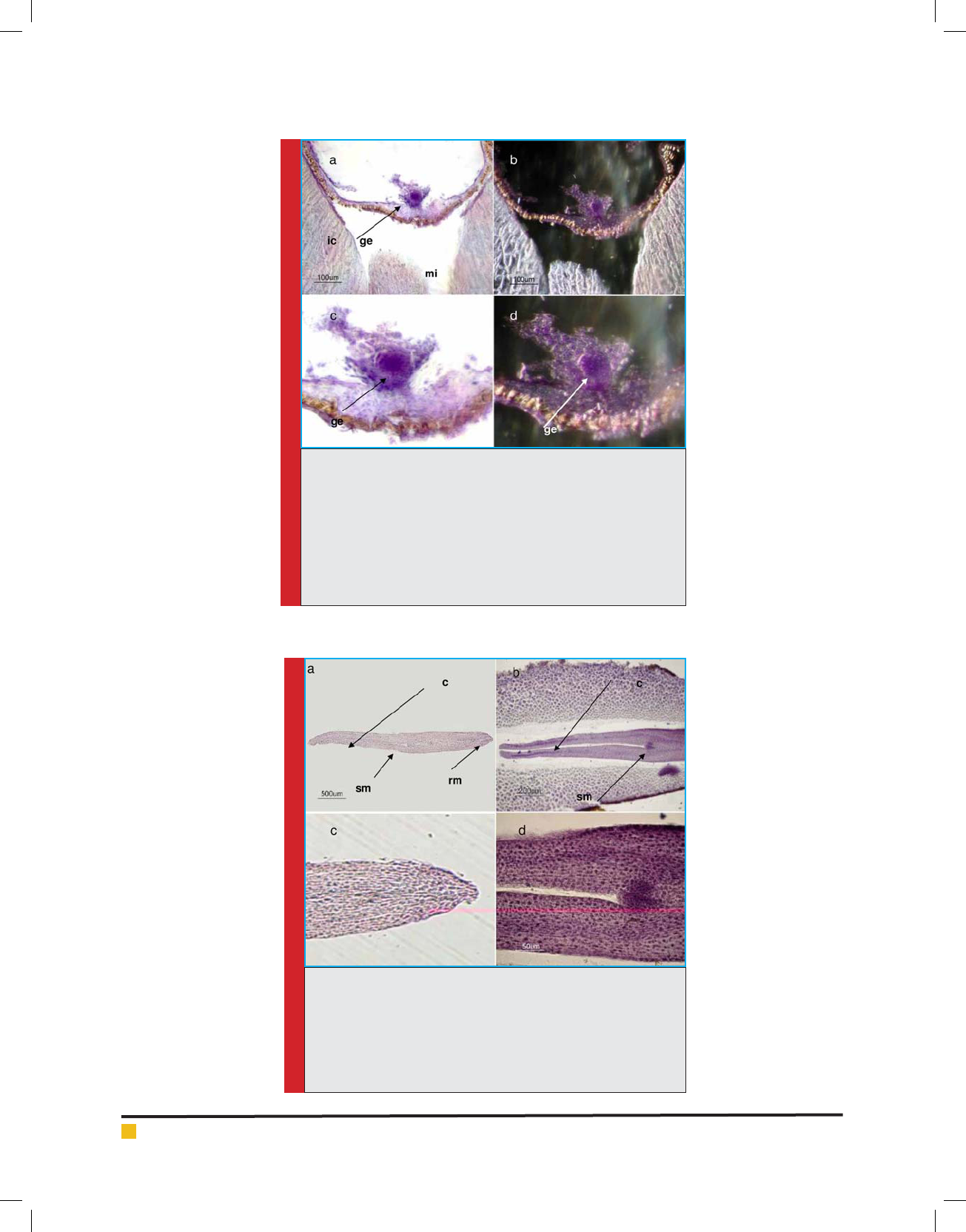
FIGURE 8. Axial sections of the seed of Ghezel-Ozum cultivar in
20 days after pollination (stage 8), stained with method of Hema-
toxilen. Photographed with dark and bright eld microscope.a)
Globular embryo is shown at the micropylar pole. Photographed
with a bright eld microscope.b) Globular embryo is shown at
the micropylar pole. Photographed with a dark eld microscope.
c) Fig. a is shown with further magni cation. d) Fig.b. Is shown
with further magni cation. ge: globular embryo, ic: inner integu-
ment (coat), mi: micropyle.
FIGURE 9. Longitudinal sections of mature embryo of the
Ghezel-Ozum cultivar, stained with the method of Hematoxilen.
a) Longitudinal section from isolated embryo, meristem of roots,
shoots and cotyledons has been speci ed. b) Longitudinal section
from embryo inside the seed, meristem of shoots and cotyledons
has been speci ed. c) Root meristem. d) Shoot meristem. c: coty-
ledons, rm: root meristem, sm: shoot meristem.

Mahdi Mohammadi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION 315
FIGURE 10. Longitudinal sections of mature seed of the Ghezel-Ozum cultivar, the different
parts of the embryo and endosperm components are shown, stained with the method of
PAS_Hematoxilen. a) Embryo is shown inside the endosperm tissue. b) Endosperm tissue
that stained with the method of PAS- Light. In this gure, polysaccharide walls and intra-
cellular storage are shown green.c) Endosperm tissue that stained with the method of Light
green. In this gure, protein components are shown. d,e) Endosperm tissue that stained
with the method of black and red Sudan, plasma membrane of cells with Sudan Black, is
stained black. c: cotyledon, e: endosperm, rm: root meristem, sm: shoot meristem.
shrinkage. These ndings are corresponded with studies
of Vallania et al. (1987) in terms of shrinkage of nucleo-
lus and separating integument.
FINAL STAGES
With further investigations it was found that the zygote
divided 7 or 15 days after pollination. In fact, the zygote
has done st division and then stopped.The maximum
size of seed is created at the end of the rst stage and
then its growth stops and just continuing the growth
and development of the embryo, endosperm and berries
would be observed. In our observations and investiga-
tions, also seed size was completed 20 days after polli-
nation, but then, growth of embryo had stopped.
In the red seedless cultivar, following the investiga-
tions, on 20 to 30 days after pollination, brown sediments
around the zygote which is dividing, were observed that
seems be the compressed polyphenols and tannins and
may be effective in the death of embryo.This compact
tannin role as an agent for dormancy and preventing
earlier germination of the seed was documented which
are in the seed coat (Debeaujon et al. 2000). Further-
more, nucleolus had been suffering from shrinkage in
this stage. At the nal stages, needle-shaped crystals
of calcium oxalate were observed at the outer wall that
large groups of them formed within idioblast, which
their roles are for regulation of calcium, plant protec-
tion, detoxi cation of heavy metals, ion balance, rm-
ness and so on (Vincent et al. 2005).Also, Rosianski et
al. (2016) reported that the pollinated fruit of g had a
larger diameter and weight and improved rmness com-
pared to the parthenocarpic fruit. These groups of cal-
cium oxalate were also observed in the seeded cultivar.

Mahdi Mohammadi et al.
316 HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
The calcium oxalate crystals were also observed within
the seedin the nal stages. Pratt (1971) noted that this
case can be observed in samples with abnormal ovules
that oxalate crystals are replaced by nucellar cells. These
abnormal ovules consist inovules with nucleolus and
embryo sac with defection at growth and development,
abnormal curvature of ovule in the ovary, necrosis of
part of chalaza and so on.
CONCLUSION
The ndings showed that growth and development of
zygote and endosperm were delayed and nally stopped
in red seedless cultivar after double fertilization. It means
that rst zygote was divided in 15-20 days after pollina-
tion when apical and basal cells had been formed, but
after ward their divisions were ceased. Also in 20 days
after pollination the series of brown sediments were
observed around the zygote. Mean while, in Ghezel-
Ozum cultivar, the formation of globular embryo and
endosperm were observed in 20 days after pollination.
In red seedless cultivar, endosperm cell was divided as
nuclear that release nuclei were observed in the embryo
sac and then divisions were stopped. Moreover, in the
transverse walls of calot cells, thickening was seen quite
clear. At the nuclei in the nal stages, shrinkage and
degeneration were observed. Also, internal integument
was separated from outer integument.
ACKNOWLEDGMENT
Authors like to thank Agricultural Research Centre at
Seed and Plant Improvement, Kahriz, Uremia, Iran for
facilities that give in conducting this project.
REFERENCES
BATIGINA, T.B. 2006. Embryology of owering plants: Termi-
nology and concepts. Science Publishers.
Bharathy, P.V. - Karibasappa G.S. - Patil, S.G. - Agrawal, D.C.
2005. In ovule rescue of hybrid embryos in Flame seedless
grapes-In uence of pre-bloom sprays of benzyladenine. In
Scientia Horticulturae, vol. 106, pp. 353-359.
Bouquet, A. - Danglot, Y. 1996. Inheritence of seedlessness
in grapevine (Vitisvinifera). In Vitis,vol. 35, no. 1, pp. 35-42.
Carraro, L. - Lombardo, G. - Cargenello G. - Gerola, F.M. 1979.
Studies on the embryo sac and on the stigmatic receptivity
of Vitis cultivars with different productivity (Picolitgiallo and
Verduzzofriulano). In Vitis,vol. 18, pp. 285-290.
Chaudhury, A.M. - Stuart, C. - Dennis, E.S. - Peacock, W.J.1998.
Ovule and embryo development, apomixis and fertilization. In
Plant Biology,vol. 1, pp. 26-31.
Debeaujon, I.M. - Leon-Kloosterziel, M. - Koornneef, M.2000.
In uence of the testa on seed dormancy, Germination, an
Logevity in Arabidopsis. In Plant Physiology,vol. 122, pp. 403-
413.
Ebadi, A. - Dehghani, Y. 2002. Sexual reproduction in tree
crops.Tehran University Press (In Persian).
Ebadi, A. - Rezaei, M. - Fatahi, R. 2010. Mechanism of seed-
lessness in Iranian seedless barberry (Berberis vulgaris L. var.
asperma). In Scientia Horticulturae, vol. 125, pp. 486–493.
Farsi, M. - Bagheri, A.R. 2004. Principles of Plant Breeding,
Jihad Daneshgahi of Mashhad publications (In Persian).
Farsi M. - Zolali, J. 2006. Principles of Plant Biotechnology.
Mashhad University of Ferdousi (In Persian).
Gahan, P.B. 1984. Plant histochemistry and cytochemistry.
Harcourt brace Jovanovich publishers.
Ghahraman, A. 1993. Cromophytes of Iran (Plant Systematics),
Volume II, Tehran University Press (In Persian).
Ghanadha, M.r. - Zahravi, M. - Vahdati, K. 2004. Horticultural
Plant Breeding. Dibagaran Art Institute of Tehran (In Persian).
Hanania, U. - Velcheva, M. - Or, E. - Flaishman, M. - Sahar,
N. - Perl, A. 2007. Silencing of chaperonin 21, that was dif-
ferentially expressed in in orescence of seedless and seeded
grapes promoted seed abortion in tobacco and tomato fruits.
In Transgenic Researches, vol. 16, pp. 515-525.
Haughn, G. - Chaudhury, A. 2005. Genetic analysis of seed
coat development in Arabidopsis. In Plant Science, vol. 10,
pp. 472-477.
Jenson, W.A. 1962. Botanical histochemistry. Freeman, W.H.
and company
Kalt, W. 2001. Health functional phytochemicals of fruit.
Horticultural reviews, edited by Jules janick, John wiley &
sons.
Lepiniec, L. - Debeaujon, I. - Routaboul, J.m. - Baudry, A. -
Pourcel, L. - Nesi, N. - Caboche, M. 2006. Genetics and Bio-
chemistry of Seed Flavonoids. In Annual Review of Plant
Biology,vol.
57, pp. 405–30.
Liu, S.m. - Sykes, S.r. - Clingeleffer, P.R. 2003. Improved in
ovule embryo culture for stenospermocarpic grapes (Vitisvin-
ifera L.). In Australian Journal of Agricultural Reasearch, vol.
54, no. 9, pp. 869-876.
Pachno, B.j. - Musia, K. - S
´
wi
˛
a
tek, P. - Tuleja, M. - Marciniuk,
J. -Grabowska-Joachimiak, A.
2014.Synergids and liform
apparatus in the sexual and apomicticdandelions from section
Palustria (Taraxacum, Asteraceae)
. In Protoplasma, vol.251,
pp. 211–217
.
Pommer, C.v. - Ramming, D.w. - Emershad, R.L.1995. In uence
of grape genotype, ripening season, seed trace size, and culture
date on in ovule embryo development and plant formation. In
Bragantia Campinas,vol. 54, no. 2, pp. 237-249.
Pratt, C.1971.Reproductive anatomy in cultivated grapes - a
review.In American. Journal of Enology and Viticulture, vol.
22, pp. 92-109.
Raghavan, C. 2006. Double fertilization. Emberyo and
endosperm development in owering plants. Springer-Verlag,
Heidelberg, Berlin.

Mahdi Mohammadi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOLOGICAL STUDY ON THE STAGES OF POLLINATION AND FERTILIZATION 317
Ramming, D.w. - Emershad, R.l. - Tarailo, R. - Chaparro,
J.x. - Mowray, B.D. 1991. The zygotic origin of hybrids from
Thompson Seedless grape, Vitisvinifera L. In Vitis, vol. 30, pp.
11-15.
Rosianski, Y. - Freiman, Z.e. - Cochavi, S.m. - Yablovitz, Z. -
Kerem, Z. - Flaishman, M.A. 2016. Advanced analysis of devel-
opmental and ripening characteristics of pollinated common-
type g (Ficuscarica L.). In Scientia Horticulturae, vol. 198,
pp. 98–106.
Ruzin, S.E. 1999. Plant microtechnique and microscopy. Oxford
University Press, New York.
Sahijram, L. - Kanamadi, V.C. 2003. In ovulo hybrid embryo
culture in controlled grape crosses involving stenosper-
mocarpic parents. ISHS ActaHorticulturae 662: VII Interna-
tional Symposium on Temperate Zone Fruits in the Tropics and
Subtropics.
Sharma, D.r. - Kaur, R. - Kumar, K. 1996. Embryo rescue in
plants- a review. In Euphytica, vol. 89, pp. 325-337.
Striem, M.j. - Spiegel-Roy, P. - Baron, I. - Sahar, N. 1992. The
degrees of development of the seed-coat and the endosperm as
separate subtraits of stenospermocarpicseedlessness in grapes.
In Vitis, vol. 31, pp. 149-155.
Vallania, R. - Botta, R. - ME, G. 1987. Investigations on anom-
alies of ovule development and on pollination in mutated
grapevines, cv. Barbera. In Vitis, vol. 26, pp. 1-8.
Vincent, R.f. - Paul, A.N. 2005. Calcium oxalate in plants: For-
mation and Function. In Annual Review of Plant Biology, vol.
56, pp. 41–71.
Wakana, A. -Hiramatsu, M. -Park, S.m. -Hanada, N. -Fuku-
dome, I. -Ngo, B. -Ngo, B.X. 2002. Degree of abortion and
germination rates in triploid seeds from crosses between dip-
loid and tetraploid grapes (Vitisvinifera L. and V. complex). In
Journal of the Faculty of Agriculture Kyushu University, vol.
46, no. 2, pp. 281-294.
Wen, J. 2007. Vitaceae.In: Kubitzki K, editor. The Families and
Genera of Vascular Plants. 9. Berlin:Springer; pp. 466–78.
Yang, D. - Li, W. - Li, S. - Yang, X. - Wu, J. - Cao, Z. 2007.
In vitro embryo rescue culture of F1 progenies from crosses
between diploid and tetraploid grape varieties. In Plant Growth
Regulation, vol. 51, pp. 63-71.
Zhang, N. - Wen, J. - Zimmer, E.A. 2015. Congruent Deep Rela-
tionships in the Grape Family (Vitaceae) Based on Sequences
of Chloroplast Genomes and Mitochondrial Genes via Genome
Skimming. In Plos One, vol. 10, no. 12: e0144701. doi:10.1371/
journal.pone.0144701.