Effects of high intensity interval training on plasma
levels of growth hormone and insulin like growth
factor-1 in healthy males
Seyyed Mahmoud Hejazi
Department of Physical Education, Mashhad Branch, Islamic Azad University, Mashhad, Iran
ABSTRACT
It is well-recognized that exercise has a signi cant impact on the growth hormone / insulin-like growth factor GH/
IGF system but less is known about the effects of high intensity training (HIT) on this axis. Aim of the present study
was to evaluate the effect of ten weeks of HIT on plasma levels of GH and IGF-I in healthy men. Twenty young men
(age 23.34±2.56 weight 72.47±12.01 height 174.10 ± 5.75) recruited and randomly assigned into Control (n=10) and
HIT (n=12) groups. HIT protocol was started with 4 cycles. Then, every two weeks one cycle was added to the previ-
ous ones. Finally it was to 8 cycles/session in tenth weeks that lasted 16 minutes. Blood samples were collected prior
to and after HIT program for all subjects and IGF-I and GH levels were measured. HIT subjects showed a significant
increase in IGF-I (P=0.002, F=12.38). However no signi cant change was shown in GH levels (P=0.716, F=0.62).Our
findings indicate that the HIT caused increase in circulating levels of IGF-I independently from GH levels. Both hor-
mones may contribute to positive effects of anabolic conditions.
KEY WORDS: IGF-I/GH AXIS, HIT, ADAPTATION
199
ARTICLE INFORMATION:
*Corresponding Author: Sm.hejazi37@gmail.com
Received 27
th
Dec, 2016
Accepted after revision 2
nd
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
Biosci. Biotech. Res. Comm. Special Issue No 1:199-202 (2017)
INTRODUCTION
Growth hormone (GH) is the principal regulator of the
hepatic synthesis of insulin-like growth factor I (IGF-
1). IGF-1 itself is the primary downstream mediator of
GH actions, and circulating IGF-1 plays an important
role in the feedback regulation of GH secretion. However
IGF-1, produced in skeletal muscle during exercise, is
also released into the circulation which might explain
an increase in Circulating IGF-1 levels as well Frystyk
(2010) and Nindl (2010). IGF-1 has widespread anabolic
and insulin-sensitizing effects, and plays a critical role
in formation, maintenance, and regeneration of skeletal
muscles. IGF-1 also plays a direct role in whole body
glucose homeostasis primarily by stimulating skeletal
muscle glucose uptake (Berg and Bang 2004).
200 EFFECTS OF HIGH INTENSITY INTERVAL TRAINING ON PLASMA LEVELS OF GROWTH HORMONE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Seyyed Mahmoud Hejazi
Nindl et al. (2010) stated that IGF-1 is an important
metabolic biomarker associated with a variety of health
and exercise-related outcomes. It is well-recognized that
exercise has a signi cant impact on the GH/IGF sys-
tem, number of factors have led to interest in the effect
of exercise on the growth hormone/insulin-like growth
factor-I (GH/IGF-I) axis, including its possible role in
maintenance of lean mass in a variety of physiological
actions like protein synthesis, cellular proliferation and
glucose metabolism, (Brill et al., 2002 and Weltman et
al 2003).
Although most modes of exercise stimulate an
increased GH secretory response that is linear with
exercise intensity evidence suggests IGF-I responses are
independent of GH. Insulin-like growth factor I (IGF-1)
is a polypeptide of 70 amino acids (7650 daltons), and
is one of a number of related insulin-like growth factors
present in the circulation.The molecule has a number of
biological activities similar to insulin. IGF-1 concentra-
tions change with age, nutritional status, body composi-
tion and physical activity, m(Roy et al 1985, Hornum et
al 1985, Stitt et al.2004).Whether previous studies have
reported exercise-induced alterations of IGF-I seems to
depend on several factors, including exercise model.
Both low- and high-intensity cycling have been shown
to increase IGF-I concentrations. However, neither low-
volume nor high-volume resistance exercise has been
shown to change total IGF-I concentrations, (Cappon et
al., 1994 and Nindl et al., 2001).
Moreover, no change in IGF-I concentrations has
been found following a marathon, a 20 km run, and
treadmill exercise at 60% of Vo max. The ability of IGF-I
to promote muscle hypertrophy is unchallenged; how-
ever, several lines of evidence have demonstrated that
load-induced hypertrophy can occur independently of
IGF-I and/or activation of the IGF-I receptor. Conversely,
evidence in support of IGF-I as a “regulator or amplifier”
of muscle remodeling cascades also exists, (Hagberg et
al., 1988, Ban et al., 1994, Spangenburg et al., 2008
and Flueck and Goldspink 2011).
HIT exercises are high intensity and interval that can
be done in a short time, although has the bene ts of
long-term endurance exercise. Even though the recent
studies have shown endurance training can induce an
increase of GH, IGF-1, levels in the circulation, but less
is known about the effects of different training inten-
sities (e. g. high-intensity training (HIT) on circulating
levels of these growth factors. Only a few studies have
addressed this issue in a strictly experimental way. The
purpose of the present study was to evaluate the effect
of ten weeks of HIT on plasma levels of GH and IGF-I
in healthy men. The present study differs from previ-
ous studies, the protocol was more rigorous and exercise
responses were compared with a non-exercise control
trial, (Schwarz et al 1996 and Laursens and Jenkins
2002).
METHODS
STUDY DESIGN /PARTICIPANTS
Trial design was semi-experimental with control
group. Twenty two young men (age 23.34±2.56 weight
72.47±12.01 height 174.10 ± 5.75) recruited via a recall
in Ferdowsi university of Mashhad campuses and those
approved participation were randomized into either a
training group (HIT) or a control group (CON). Informed
consentwas obtained from each patient included in the
study and the study protocol conforms to the ethical
guidelinesof the1975 Declaration of Helsinki.Exclusion
criteria include professional athletics history as well as
the current regular exercise, smoking, cardiovascular
and metabolic disease or any complication that disrupt
the implementation of exercise.
HIT group after became acquainted with the correct
training performances, carried out the exercises, every
other day, three sessions a week, for 10 weeks. Exer-
cises included warm up, HIT training, cool down. Sub-
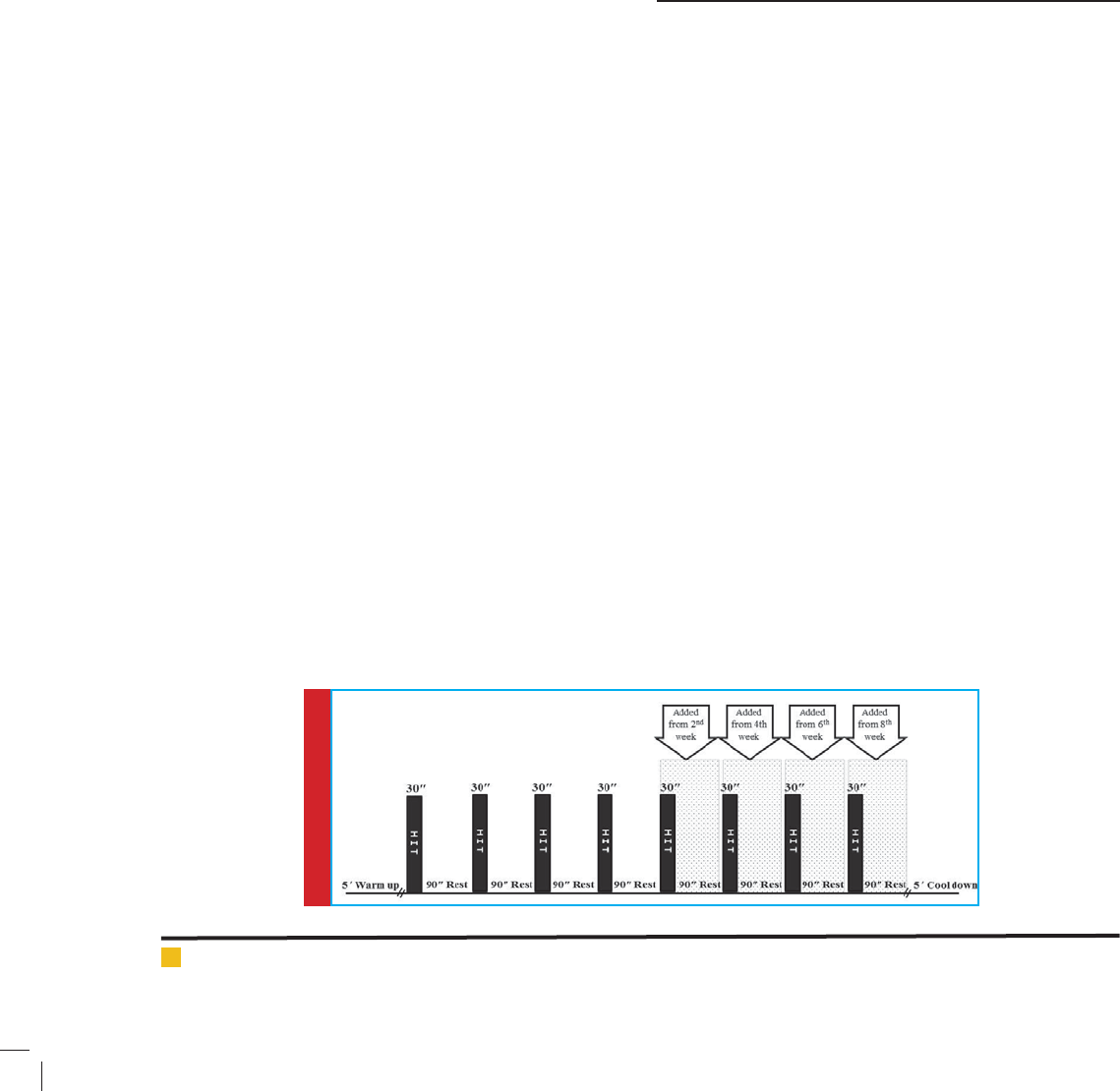
jects warmed up by stretching and easy walking for 5
minutes. The HIT interventions consisted of four 30 s
maximal effort (“all-out”) shuttle run bouts (from cone-1
to cone-2 ,20 meter sweep) separated by 1.5 min passive
rest each (Fig. 1).
HIT protocol began with 4 cycles and every two weeks
added one cycle. Finally it was 8 cycles in tenth week
that lasted 16 minutes. Cooling Down also included 5
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EFFECTS OF HIGH INTENSITY INTERVAL TRAINING ON PLASMA LEVELS OF GROWTH HORMONE 201
Seyyed Mahmoud Hejazi
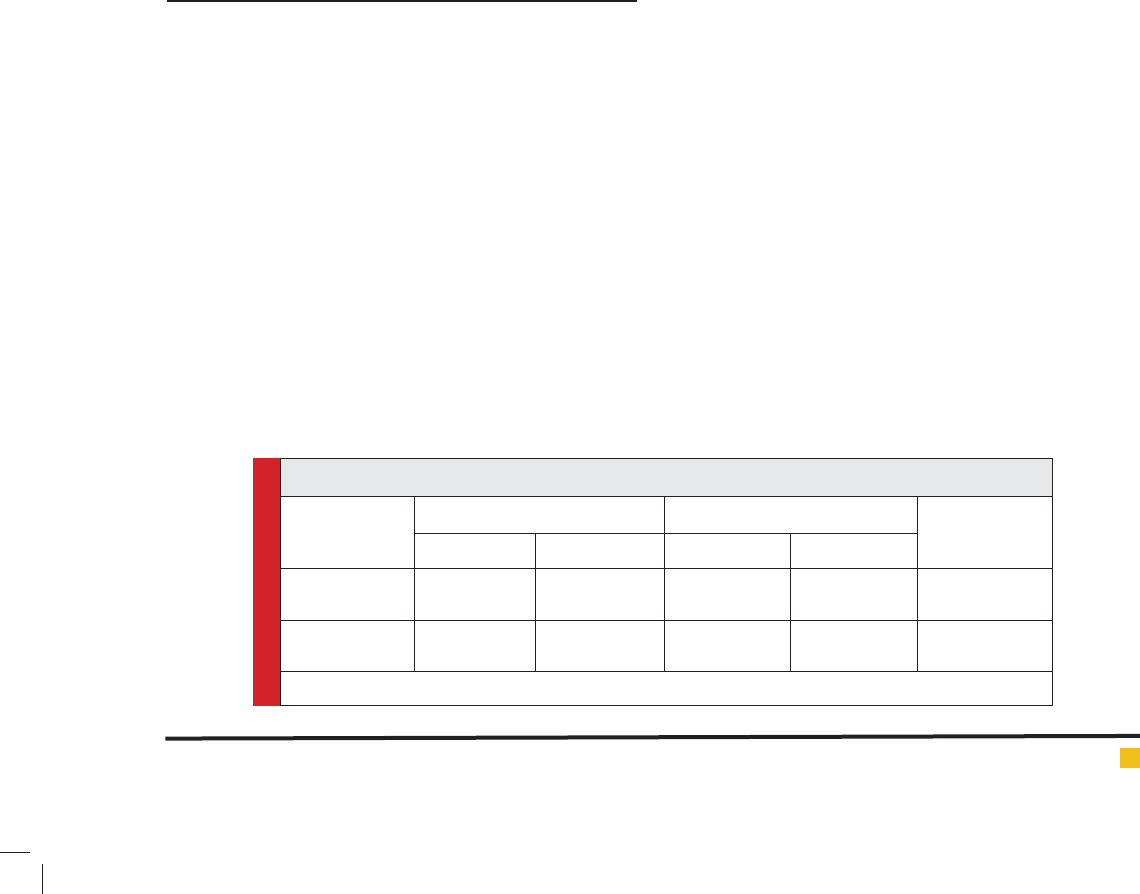
Table 1. Study variables before (pre) and after (post) 10 weeks of study protocol
Variable Control HIT ANOVA
(Repeated
Measurement)
Pre test Post test Pre test Post test
IGF-I (ng /mL) 193.10± 33.54 190.60 ± 32.32 199.27 ± 28.69 222.27 ± 32.90 F=12.38
P=0.002
GH (ng /mL ) 3.053±0.73 3.004±0.53 3.241±0.41 3.158±0.61 F=0.62
P=0.716
Data are mean ± SD
min of walking and stretching. During the HIT interven-
tions all subjects were vocally encouraged to maintain
Maximal effort. Quality of training was controlled by a
physical education expert and subject’s heartbeat was
constantly checked by polar device. Control group asked
to be sedentary in this period.
Twenty four hours before starting the exercise pro-
gram, while all the subjects were fasten; 5 cc of blood
was taken from their brachial vein. Also sampling
repeated after 48 hours of last session in same condition.
Blood samples were frozen in -20 for future analysis.
LDN (Germany) ELISA assay Kit was used to measure
IGF-I concentrations which Sensitivity was 1.292 ng /
mL and monobind (USA) ELISA assay Kit was used to
measure GH concentrations which Sensitivity was 0.072
ng /mL.
Data normality was tested using Shapiro-wilk and the
homogeneity of the variances was tested using levene.
After making sure of the normality and equality of the
groups, variance analysis with repeated measuring was
used to study the differences between groups. Statistical
analysis was done by the SPSS Software.
RESULTS AND DISCUSSION
After ten weeks HIT subjects showed a significant
increase in IGF-I (P=0.002, F=12.38). However no signif-
icant change was shown in GH levels (P=0.716, F=0.62).
(Table 1).
Previous studies have been reported that exercise has
a signi cant impact on the levels of several hormones,
and can increase resistance and performance, as well
as muscle mass. Hormone levels can change according
to several parameters, including the type and length of
exercise, the duration of time following exercise, the
age and gender of the athletes, among others, Kraemer
et al (2006). Based on the ndings from the present
research, plasma levels of IGF-I had been signi cantly
increased due to HIT protocol. To our knowledge, no
previous research has directly investigated the impacts
of HIT on IGF-I/GH axis. However, other types of train-
ing have been published. Most of the training time dur-
ing the HIT intervention was spent in recovery between
short, intense bursts of all-out shuttle running. This is
in accordance with previously published studies, (Wahl
et al 2010).
Several studies have indicated that anabolic hor-
mones, such as insulin, GH, testosterone and IGF-1,
stimulate neural tissue and muscle development dur-
ing resistance exercise, (Crewther et al 2006). The serum
concentration of anabolic hormones is elevated during
and following resistance exercise compared to the level
at rest, which leads to hypertrophy and remodeling of
muscle ( Widdowson et al., 2009). Circadian rhythm
has speci c effects on the release of IGF-1 in the body,
where the hormone levels are higher in the morning and
lower in the afternoon, (Hayes et al., 2010).
In the present study GH concentrations did not sig-
ni cantly changed as a result of relative long term HIT.
That regard should be considered that due to our study
limitation, GH only measured at one point in time. Also
regarding Circadian rhythm and the pulsatile man-
ner of GH it will probably cause different results when
compared with multi-time point, whereas long-term
exercise training approximately doubles integrated GH
concentrations when measured on non-exercising days.
Linnamo et al. reports that GH levels are increased in
response to submaximal and maximal heavy resistance
exercise. However, the prominent increase was detected
just after the exercise session was completed, and the
response returned to normal level two hours post exer-
cise, (Weltman et al., 1992 and Linnamo et al., 2005).
Different training intensities, such as high-intensity
training and high volume, low-intensity training may
have a different impact on hormone levels. Although pH
is generally well regulated, a more increase in the acidity
of the circulating blood and the skeletal muscle occurs
when performing HIT. One can speculate that these sys-
temic and local changes in the extracellular environment
might in uence the release, the af nity, and association/
dissociation of GH, IGF-1. The extracellular pH has been
recognized to regulate the IGF-1 interactions with dif-
ferent cells, components of the extracellular, (Gordon
et al 1994 and Gibala et al 2006).
Seyyed Mahmoud Hejazi
202 EFFECTS OF HIGH INTENSITY INTERVAL TRAINING ON PLASMA LEVELS OF GROWTH HORMONE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
In summary, we undertook a randomized trial of the
impact of 10 weeks of HIT on IGF-I/GH axis in previ-
ously untrained subjects. The major nding was HIT
caused increase in circulating levels of IGF-I indepen-
dently from GH levels. Both hormones may contribute to
the positive effects of anabolic conditions as it has been
shown by previous studies.
REFERENCES
Ban G, Marinelli M, Roi G, Colombini A, Pontillo M, Giaco-
metti M, et al. Growth hormone and insulin-like growth factor
I in athletes performing a marathon at 4000 m of altitude.
Growth regulation. 1994;4(2):82-6.
Berg U, Bang P. Exercise and circulating insulin-like growth
factor I. Hormone Research in Paediatrics. 2004; 62 (Suppl.
1): 50-8.
Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks
JB, et al. Single and combined effects of growth hormone and
testosterone administration on measures of body composition,
physical performance, mood, sexual function, bone turnover,
and muscle gene expression in healthy older men. The Journal
of clinical endocrinology and metabolism. 2002;87(12):5649-57.
Cappo n J, Brasel J, Mohan S, Cooper D. Effect of brief exercise
on circulating insulin-like growth factor I. Journal of Applied
Physiology. 1994;76(6):2490-6.
Crewt her B, Keogh J, Cronin J, Cook C. Possible stimuli for strength
and power adaptation. Sports medicine. 2006; 36(3): 215-38.
Fluec k M, Goldspink G. Counterpoint: IGF is not the major
physiological regulator of muscle mass. Journal of Applied
Physiology. 2010;108(6):1821-3.
Forst en‐Williams K, Cassino TR, Delo LJ, Bellis AD, Robinson
AS, Ryan TE. Enhanced insulin‐like growth factor‐I (IGF‐I) cell
association at reduced pH is dependent on IGF binding pro-
tein‐3 (IGFBP‐3) interaction. Journal of cellular physiology.
2007;210(2):298-308.
Frystyk J. Exercise and the growth hormone-insulin-like
growth factor axis. Medicine and science in sports and exer-
cise. 2010;42(1):58-66.
Gordon SE, Kraemer WJ, Vos NH, Lynch JM, Knuttgen HG.
Effect of acid-base balance on the growth hormone response to
acute high-intensity cycle exercise. Journal of Applied Physi-
ology. 1994;76(2):821-9.
Hagberg JM, Seals DR, Yerg JE, Gavin J, Gingerich R,
Premachandra B, et al. Metabolic responses to exercise in
young and older athletes and sedentary men. Journal of
Applied Physiology. 1988;65(2):900-8.
Hayes LD, B ickerstaff GF, Baker JS. Interactions of cortisol,
testosterone, and resistance training: in uence of circadian
rhythms. Chronobiology international. 2010;27(4):675-705.
Hornum M, C ooper DM, Brasel JA, Bueno A, Sietsema KE.
Exercise-induced changes in circulating growth factors with
cyclic variation in plasma estradiol in women. Journal of
applied physiology (Bethesda, Md : 1985). 1997;82(6):1946-51.
Kraemer RR, Hollander DB, Reeves GV, Francois M, Ramadan
ZG, Meeker B, et al. Similar hormonal responses to concentric
and eccentric muscle actions using relative loading. European
journal of applied physiology. 2006;96(5):551-7.
Laursen PB, Jenkins DG. The scienti c basis for high-intensity
interval training. Sports Medicine. 2002;32(1):53-73.
Linnamo V, Pakarinen A, Komi PV, Kraemer WJ, Häkkinen K.
Acute hormonal responses to submaximal and maximal heavy
resistance and explosive exercises in men and women. The Jour-
nal of Strength & Conditioning Research. 2005;19(3):566-71.
Nindl BC, Kr aemer WJ, Marx JO, Arciero PJ, Dohi K, Kellogg
MD, et al. Overnight responses of the circulating IGF-I sys-
tem after acute, heavy-resistance exercise. Journal of Applied
Physiology. 2001;90(4):1319-26.
Nindl BC. Insulin-like growth factor-I, physical activity, and
control of cellular anabolism. Medicine and science in sports
and exercise. 2010;42(1):35-8.
Roy P., CJ, Widemen L, Weltman JY, Abbott R, Gutgesell M,
Hartman ML, et al. Gender governs the relationship between
exercise intensity and growth hormone release in young
adults. Journal of applied physiology (Bethesda, Md : 1985).
2002;92(5):2053-60.
Schwarz AJ, Brasel J, Hintz RL, Mohan S, Cooper D. Acute
effect of brief low-and high-intensity exercise on circulating
insulin-like growth factor (IGF) I, II, and IGF-binding protein-3
and its proteolysis in young healthy men. The Journal of Clini-
cal Endocrinology & Metabolism. 1996;81(10):3492-7.
Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A func-
tional insulin‐like growth factor receptor is not necessary
for load‐induced skeletal muscle hypertrophy. The Journal of
physiology. 2008;586(1):283-91.
Stitt TN, Druj an D, Clarke BA, Panaro F, Timofeyva Y, Kline
WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of
muscle atrophy-induced ubiquitin ligases by inhibiting FOXO
transcription factors. Molecular cell. 2004;14(3):395-403.
Wahl P, Zinner C, Achtzehn S, Bloch W, Mester J. Effect of
high-and low-intensity exercise and metabolic acidosis on lev-
els of GH, IGF-I, IGFBP-3 and cortisol. Growth Hormone & IGF
Research. 2010;20(5):380-5.
Weltman A, Des pres JP, Clasey JL, Weltman JY, Wideman L, Kan-
aley J, et al. Impact of abdominal visceral fat, growth hormone,
tness, and insulin on lipids and lipoproteins in older adults.
Metabolism: clinical and experimental. 2003;52(1):73-80.
Weltman A, Wel tman JY, Schurrer R, Evans WS, Veldhuis JD,
Rogol AD. Endurance training ampli es the pulsatile release
of growth hormone: effects of training intensity. Journal of
Applied Physiology. 1992;72(6):2188-96.
Widdowson WM, Healy M-L, Sönksen PH, Gibney J. The phys-
iology of growth hormone and sport. Growth Hormone & IGF
Research. 2009;19(4):308-19.