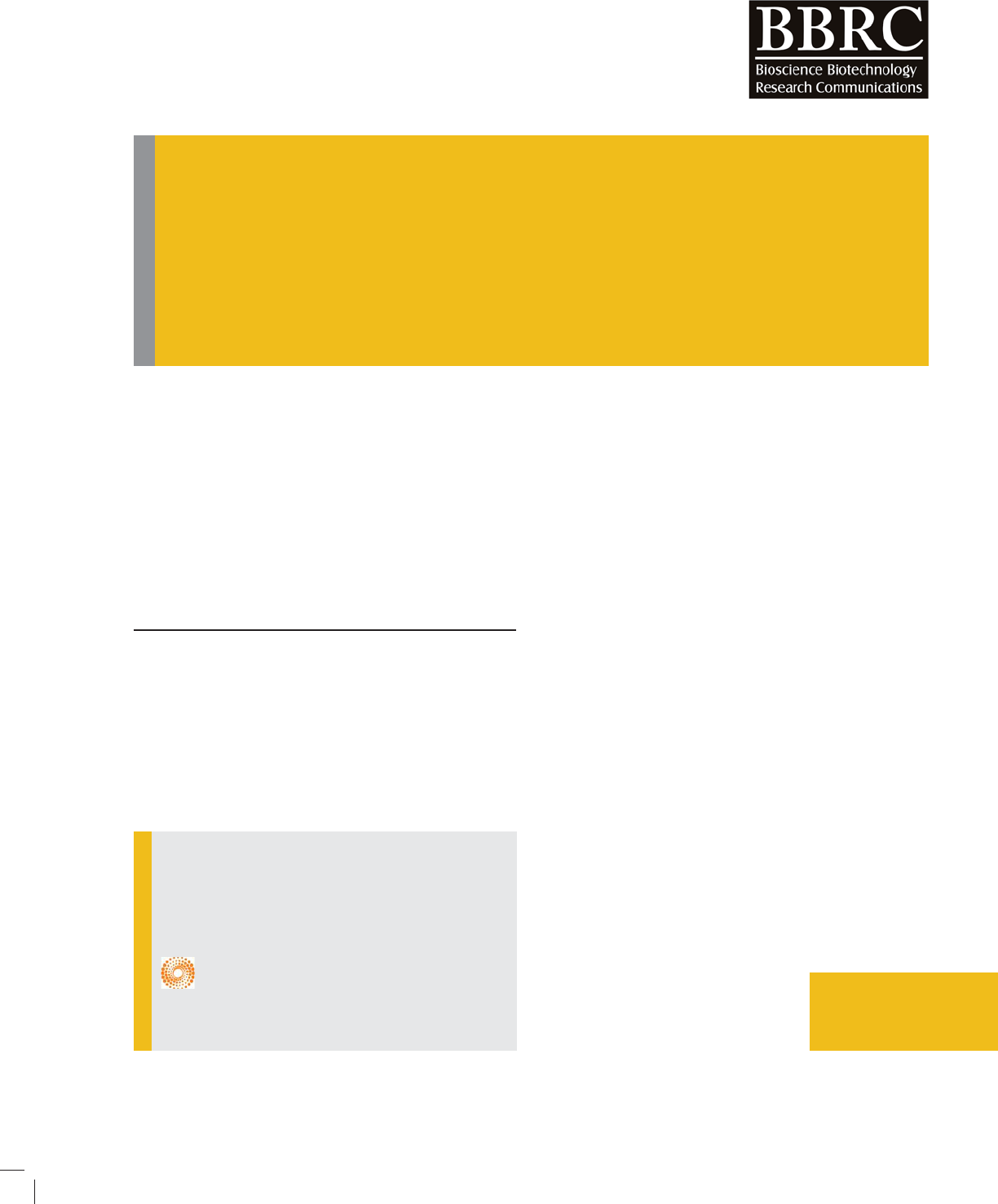
Synthesis and some properties of 3-substituted -5,5-
Dimethyl-5,6-dihydrobenzo[H]quinazolin(1h,3h)2,
4-dione
Najmeh Torshirzad
Department of Food Science and Technology, Torbat-e Heydarieh Branch, Islamic Azad University
Torbat-e Heydarieh, Iran
ABSTRACT
Ethyl 2-cyano-3,3-dimethyl-4-phenylbutanoate was cyclized into 1-amino-3,3-dimethyl-3,4-dihydronaphtha-
lene-2-ethylcarboxyiate condensation of which with primary amines synthesized 3 – substituted-5,5-dimethyl-
5,6-dihydrobenzo[h]quinazolin-(1H,3H)2,4-dions. The method is based on the interaction of carbamate (II) with pri-
mary amines, which resulted in the preparation of 1,3-disubstituted urea without isolation formate reaction medium
subjected to cyclization in the presence of alkali
KEY WORDS: CYCLIZATION, AMINOESTER, BENZO[H]QUINAZOLINE, SUBSTITUTION
130
ARTICLE INFORMATION:
*Corresponding Author: najmetorshirzad@yahoo.com
Received 11
th
Dec, 2016
Accepted after revision 19
th
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
For this, ethyl 2-cyano-3,3-dimethyl-4- phenyl-
butanoate(I)in H
2
SO
4
solution was cyclized into
1-amino-3,3-dimethyl-3,4-dihydronaphthalene-
2-ethylcarboxyiate(II), which was then reacted with
chlorophenylformate (III)a method for the synthesis of
3-substituted 5,5-dimethyl-5,6-dihydrobenzo[h]quina
zoline(1H,3H)2,4-dione. (IV) was developed. The method
is based on the interaction of carbamate (II) with pri-
mary amines, which resulted in the preparation of
1,3-disubstituted urea without isolation formate reaction
medium subjected to cyclization in the presence of alkali
(Graddon & Nickel, 2012; Kwatkowski & Trojanowski,
2010)
Biosci. Biotech. Res. Comm. Special Issue No 1:130-132 (2017)
Najmeh Torshirzad
Table 1. 3-substituted-5,5-dimethyl-5,6-dihydrobenzo[h]quinazolin-(1H,3H)2,4-dions
Compound R Yield, % Mp,°
C Rf*
Theoretical, % experimental, %
CHNCCN
22
NH
2
96
228-230
0.43 (a) 65.48 5.97 16.50 65.33 5.88 16.32
23
CH
3
43
>250
0.67 (a) 70.44 6.39 11.12 70.29 6.29 10.93
24
C
2
H
5
63 230-232
0.75 (B) 71.25 6.86 10.24 71.09 6.71 10.36
25
C
3
H
7
63 228-230
0.78 (a) 71.97 7.23 9.70 71.81 7.09 9.85
26
ISO-C
3
H
7
56 219-220
0.76 () 71.88 7.19 10.01 71.81 7.09 9.85
27
C
4
H
9
83 180-182
0.53 () 72.59 7.56 9.57 72.46 7.43 9.39
28
Cyclopentyl 70 >250
0.56 () 73.68 7.32 9.18 73.52 7.14 9.03
29
Cyclohexane 68 238-240
0.80 (r) 73.95 7.62 8.77 74.04 7.46 8.64
30
2-Furfuryl 68 219-220
0.71 (a) 70.86 5.77 8.50 70.79 5.63 8.69
31
C
6
H
5
94 >250
0.69 (a) 75.64 5.56 8.63 75.45 5.70 8.80
32
CH
2
C
6
H
5
78 218-220
0.58 () 75.95 6.22 8.58 75.88 6.06 8.43
33
CH
2
CH
2
C
6
H
5
81 >250
0.73 (a) 76.43 6.57 7.95 76.28 6.40 8.09
34
3-ClC
6
H
4
55 >250
0.76 () 67.93 5.01 7.82 68.09 4.86 7.94
TLC using Acetate:Benzen (1:2)
METHODOLOGY
EXPERIMENTAL CHEMICAL PART
IR spectra were taken in mineral oil UR-20 and FT-IR
Nexus instrument. PMR spectra, with or HMDS internal
standard on a Varian Mercury-300 spectrometer (USA)
IV: R=NH
2
;VII:R=CH
3
; VIII: R=C
2
H
5
; IX: R=C
3
H
7
; X:
R=izo-C
3
H
7
; XI: R=C
4
H
9
;
XI: R=cyclopentyl; XII: R=cyclohegzil; XIII: R=2-furfu-
ryl; XIV: R=C
6
H
5
;
XV: R=CH
2
C
6
H
5
; XVI:R=CH
2
CH
2
C
6
H
5
.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SYNTHESIS AND SOME PROPERTIES OF 3-SUBSTITUTED 131
Najmeh Torshirzad
.Mass spectra were obtained on an MX-1321A spectrom-
eter (USSR) using direct sample introduction into the
ion source. TLC was carried out on Silufol R plates with
detection by I
2
vapor (Patel & Woods, 2001).
1-Amino-3,3-dimethyl-3,4-dihydronaphthalene-2-
ethylcarboxylate(II)
Compound I (50g,0.193mol) was placed into a 250-mL
ask, stirred at 10-15°C, treated in portions with conc.
H
2
SO
4
(100mL), stirred at the same temperature for 7 h,
neutralized with aqueous NH
4
OH, and extracted with
Et2O(500 mL). The extract was washed twice with H2O
and dried over anhydrous Na
2
SO
4
. The solvent was dis-
tilled off. The solid was crystallized. The crystals were
washed with EtOH(70%) and dried in air to afford II,
23g(46%yield), MP 58-60°c,Rf 0.67 (isooctane-ethyl-
acetate,2:1) IR spectrum, v, cm-1: 1600 (c=c atom) , 1643
(c=o), 3334, 3438(NH
2
). PMR spectrum (DMSO-d
6
), ,
ppm:1.16 (s, 6H, 2× CH
3
), 1.33 (t, J 7.1 Hz, 3H, OCH
2
CH
3
),
2.60 (s, 2H, 4-CH
2
), 4.17 (q, J 7.1 Hz, 2H, OCH
2
), 7.10 (m,
3H, 5-CH, NH
2
), 7.20 – 7.28 (m, 2H, 6-CH, 7-CH), 7.59
(m, 1H, 8-CH) (Popp et al., 2006).
ETHYL–3,3–DIMETHYL–1–( (PHENOXYCARBONYL)
AMINO)–3,4–DIHYDRONAPHTALIN–2–CARBOXYL (III)
A mixture of II (24.5g, 0.1 moL), chlorophenyl for-
mate (15.6 g, 0.1 mol) and Benzen (150 mol) was
re uxed for 20h. The resulting crystals were l-
tered off, washed with H
2
O, and crystallized from
EtOH and wather (3:1) to afford (III) 31.5 gr (86.2%
yield), mp 122-124°c, Rf 0.76 (Ethyl Acetate – Benzen,
1:2) IR spectrum, V, cm-1 :1600 (c=c arom), 1625 (c=c
ethylen), 1710 (c=o), 1750 (NH). PMR spectrum (DMSO-
d6), ppm : 9.10 (bs, 1H, NH) (Sahai & Singh, 1998).
3–Substituted 5, 5–dimethyl– 5, 6–dihydrobenzo [h]
quinazolin–(1h, 3h ) 2,4–dions (v)
A mixture of carbamate (III) (3.65 gr, 0.01 mol), primary
amine (0.01 mol) and ethanol (20 ml) was re uxed for 7
h , treated with KOH solution (1.1 gr 0.02 mol) in H
2
O (10
ml), re uxed for another 3 h, cooled, stirred, and acidi ed
with HCL solution (18%) to PH 3.0 – 3.5. The resulting
crystals were ltered off, washed with H
2
O and recrystal-
lized from EtOH to afford (IV - XVI), MP >250 °C, R
f
0.74
(benzoyl ethyl acetate, 2:1). IR spectrum: , CM
-1
: 1605
(C=Carom.); 1646 (C=C-C=O); 1711 (C=O); 3240 (NH).
1
HPMR spectrum (DMSO -d
6
), , ppm, Hz: 1.28 (6H, c,
2CH
3
); 2.76 (2H, c, 6-CH
2
); 7.14-7.51 (5H, m, arom.); 7.93
(1H,dd, J
1
=7.7, J
2
=1.2, 10-CH); 11.24 (1H, c, NH).
REFERENCES
Graddon, D.P.; Heng, K.B. Nickel.(2010) Complexes of Some
3-Substituted Pentane-2,4-diones. Aust. J. Chem. 2012, 25,
2247-2250.
Kwatkowski, E.; Trojanowski, H. Complexes of Tertiary Amides
with Acetylacetonates of Oxovanadium(IV) and Copper(II) in
Methylene Chloride Solution. J. Inorg. Nucl. Chem. 37, 979-983.
Patel, K.S.; Woods, J.A.O. (2001) Preparation and Physicochemi-
cal Studies of Some 3-substituted 2,4-Pentanedionatocopper(II)
Complexes and their Adducts. Synth. React. Inorg. Met.-Org.
Chem 20, 97-109, and references therein.
Popp, C.J.; Nelson, J.H.; Ragsdale, R.O.(2006) Thermodynamic
and Infrared Studies of Tertiary Amine Oxides with Bis(2,4-
Pentanedionato) oxovanadium(IV). J. Amer. Chem. Soc. 91,
610-614.
Sahai, R.; Singh, P.R.(1998) Spectroscopic Studies in Metal
b-Diketonates I: Preparation and Study of Halogenated Metal
Acetylacetonates. Aust. J. Chem. 20, 639-648.
132 SYNTHESIS AND SOME PROPERTIES OF 3-SUBSTITUTED BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS