
Synthesis of 4, 4’-(1, 3 and 1, 4-phenylene) bis
(6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-
carboxylate) via a one- pot three-component reaction
of Urea with dialdehydes and acetoacetates in the
presence of hydrochloric acid and heteropolyacid
Haniyeh Mahmoudi Esgandani, Mina Roshani, Ehsan Akhondi Ranjbar and
Mohammad Shaker*
Department of Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, PO Box 91735-413, Iran
ABSTRACT
A simple and ef cient synthesis of 4, 4’-(1, 3 and 1, 4-phenylene) bis (6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-
5-carboxylate) via a one- pot three-component reaction of Urea with dialdehydes and acetoacetates in the presence
of hydrochloric acid and heteropolyacid is described. All synthesized compounds were characterized on the basis of
their spectral and microanalytical data. In the rst stage we report a convenient synthesis of new 4, 4’-(phenylene)
bis tetrahydropyrimidines 4a-4f by a one-pot three-component reaction of Urea 1, dialdehydes 2a-4b, and acetoac-
etates 3a-3c in re uxing hydrochloric acid. And then, in the second stage, we used heteropolyacids in this reaction.
We report reaction conditions (solvent, temperature, reaction time, catalyst type, and concentration) were studied to
optimize in this procedure. Melting points were recorded on a Stuart SMP3 melting point apparatus. The IR spectra
were obtained using a Bruker Tensor 27 spectrophotometer using KBr discs. The
1
H NMR (300 MHz) was recorded
with Bruker-300 MHz spectrometers. The
13
C-NMR was recorded with Bruker-300 MHz spectrometers at 75 MHz
frequencies. The mass spectra were scanned on an Agilent Technologies instrument at 70 eV. Elemental analysis was
performed on a Thermo Finnigan Flash EA microanalyzer.
KEY WORDS: BIS (1, 2, 3, 4-TETRAHYDROPYRIMIDINE-5-CARBOXYLATE), HETEROPOLYACID, UREA, ACETOACETATES, DIALDEHYDES,
BIGINELLI REACTION
112
ARTICLE INFORMATION:
*Corresponding Author: mshaker985@gmail.com
Received 31
st
Dec, 2016
Accepted after revision 29
th
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
Biosci. Biotech. Res. Comm. Special Issue No 1:112-117 (2017)
Haniyeh et al.
INTRODUTION
3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) and their
derivatives gained considerable interest from the rst
reported in 1891 until today both in academia and
industry because of their important and promising
therapeutic and pharmacological properties (Biginelli
et al. 1891). For instance, they have emerged as integral
backbones of several channel blockers, antihypertensive
agent -1antagonists, neuropeptide Y (NPY) antagonists
and anticancer activities (Singh et al. 2009; Russowsky
et al. 2006; Kumar et al. 2009; Da Silva et al. 2012).
The classical Biginelli reaction involves the strong
acid-catalyzed cyclocondensation reaction of ethyl ace-
toacetate, benzaldehyde and urea in ethanol at re ux
temperature for long reaction time. Furthermore, this
one-pot three-component procedure often provides with
relatively low yields of the dihydropyrimidine deriva-
tives. In order to improve the ef ency and synthetic pro-
cedure of the classical one-pot Biginelli reaction, using
different types of catalysts and conditions have been
reported by different research groups. The most of these
procedures are all similar, using different acid catalyst
such as BF
3
.OEt
2
(Hu et al. 1998), FeCl
3
.6H
2
O (Xu et al.
2004), MgCl
2
.6H
2
O (Zhang et al. 2004), MgBr
2
(Gulten,
2013; Salehi, 2004), BiCl
3
(Ramalinga et al. 2001), InCl
3
(Ranu et al. 2000), ZnO nano particles (Hassanpour et al.
2015), zeolites (Radha et al. 2001), LaCl
3
.7H
2
O (Lu et al.
2000), LiClO
4
(Yadav et al. 2001), Mn(OAc)
3
.2H
2
O (Kumar
et al. 2001), NiCl
2
.6H
2
O (Lu, 2010), and so on, in solvent
such as CH
3
CN, CH
2
Cl
2
, THF, EtOH or H
2
O. In addition
procedures employing ultrasound, microwave (Kappe,
1999), solid and uorous (Studer et al. 1997) phase syn-
theses have reported.
A number of procedures under solvent free conditions
using different acid catalyst have also been reported
(Zhang et al. 2015). However, despite their potential
utility some these procedures use expensive catalysts,
strong acidic conditions, higher temperatures, stoicho-
metric amounts of catalyst, toxic reagents, large amount
of solvents, unsatisfactory yields, inconvenient pruri -
cation techniques, incompatibility with other functional
groups and require longer reaction times are not all
acceptable in the context of green synthesis.
On the other hand, Polyoxometalates (POMs) are a
large class of metal oxide cluster compounds consist-
ing of transi-tion metal atoms bridged by oxygen atoms.
POMs can exist in a variety of different size and struc-
ture, and compounds belonging to this class have been
studied extensively because they possess interesting
electronic and molecular properties, such as wide-rang-
ing reduction potentials, acidities, and polarities. Based
on their attractive properties, POMs have also been used
in a variety of different application, including catalysis,
biomedicine, magnetism, nanotechnology, and materi-
als science (Müller et al. 1998; Pope and Müller, 2001;
Davoodnia et al. 2013; Coronado et al. 1998; Okuhara
et al. 1996). The development of methods using heter-
opolyacids (HPAs) as Catalyst for the synthesis of ne
chemicals, such as avors, pharmaceuticals, and in
food industries, has gained attention in the last decade
(Chwegler et al. 1991).
Catalysts based on heteropolyacids have many advan-
tages over liquid-acid catalysts. They are not corrosive
and are environmentally benign and present fewer dis-
posal problems.
Solid heteropolyacids have attracted much attention
organic synthesis owing to easy work-up procedures,
easy ltration, and reduction of cost and waste genera-
tion through reuse and recycling of the catalysts.
MATERIAL AND METHODS
In spite of much work on the synthesis of substituted
tetrahydropyrimidines, to the best of our knowledge,
the synthesis of 4, 4’-(1, 3 and 1, 4-phenylene) bis
(6-methyl-2-oxo-1, 2, 3, 4-tetrahydropyrimidine-5-car-
boxylate) has not been reported in the literature. In this
paper, in the rst stage we report a convenient synthe-
sis of new 4, 4’-(phenylene) bis tetrahydropyrimidines
4a-4f by a one-pot three-component reaction of Urea 1,
dialdehydes 2a-4b, and acetoacetates 3a-3c in re ux-
ing hydrochloric acid. And then, in the second stage, we
used heteropolyacids in this reaction. We report reaction
conditions (solvent, temperature, reaction time, catalyst
type, and concentration) were studied to optimize in this
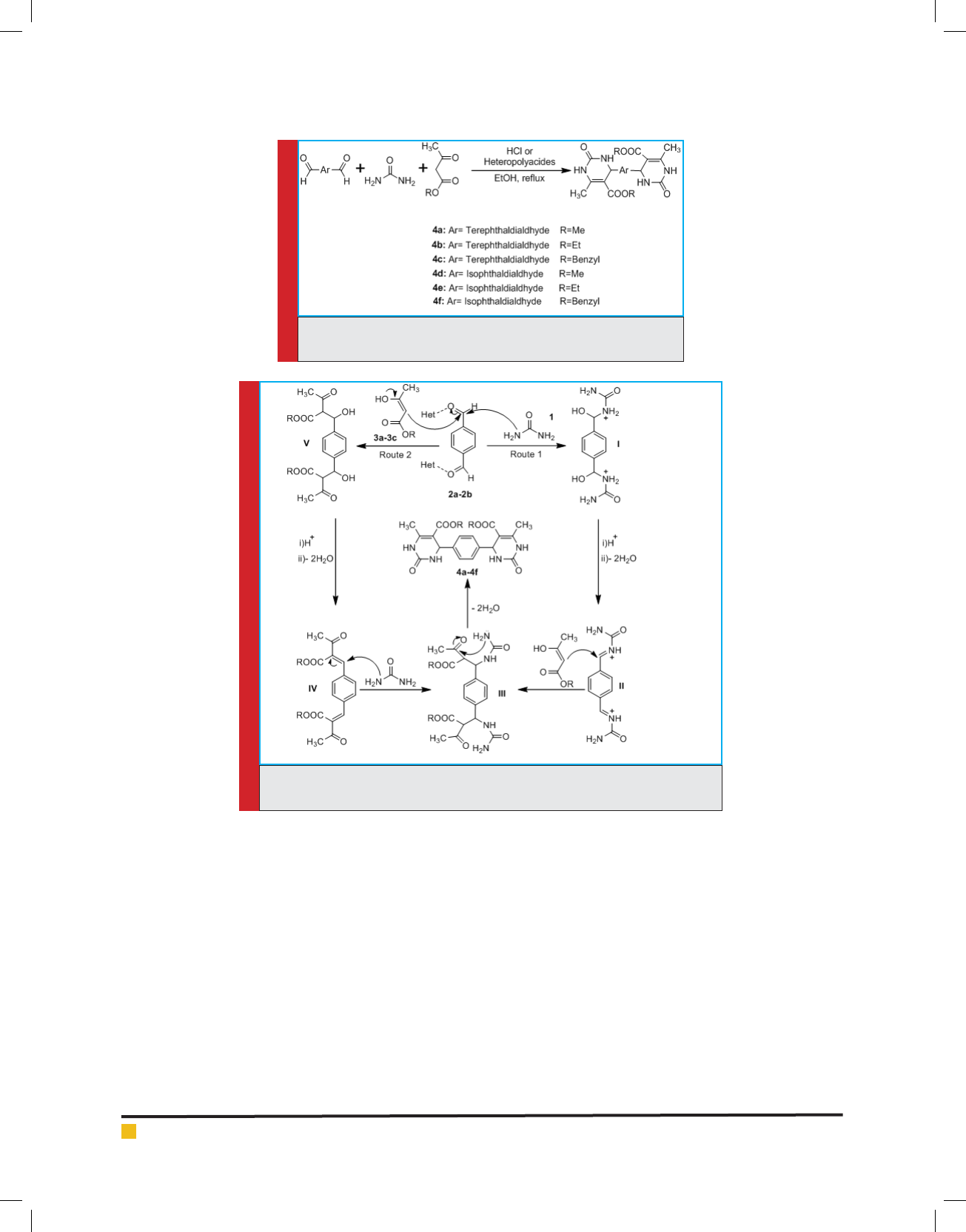
procedure (Scheme 1).
RESULTS AND DISCUSSION
Although we did not investigate the reaction mechanism,
two plausible mechanisms for this three-component
reaction as have been depicted in Scheme 2. For exam-
ple in route 1, it is proposed that the reaction occurs
via initial formation of the intermediate I as a result
of a nucleophilic attack of Urea at the carbonyl group
of dialdehydes. Dehydration of the intermediate I fol-
lows the intermediate II. Reaction of acetoacetates with
this intermediate then gives the intermediate III which
after cyclization followed by dehydration afforded nal
products 4a-4f. As shown in Scheme 2, we propose that
Hydrochloric acid and Heteropolyacid Het activate the
reactants and the intermediates in this reaction.
A one-pot three-component reaction of Urea 1, an
dialdehydes 2a-2b and acetoacetates 3a-3c in the pres-
ence of hydrochloric acid under re ux for 4h leads to
the facile formation of 4, 4’-(1, 3 and 1, 4-phenylene)
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SYNTHESIS OF 4, 4’-(1, 3 AND 1, 4-PHENYLENE) BIS 113
Haniyeh et al.
bis (6-methyl-2-oxo-1, 2, 3, 4-tetrahydropyrimidine-
5-carboxylate) 4a-4f in 80-90% yields (Table 1).
The structures of the products were deduced from
their spectral and microanalytical data. For example, the
1
H NMR spectrum of compound 4a which this is a sym-
metrical produce, exhibited one sharp signal at 2.250
and 3.541ppm for methyl groups, 5.109ppm for CH
groups, 7.736 and 9.231ppm for NH groups, as well
as the signals in the aromatic region, 7.189 ppm, due
to 4 aromatic protons indicating the formation of the
compound 4a. The IR spectrum of 4a showed strong
absorptions at 3430 cm
-1
for NH absorption, 3028 and
2943 cm
-1
due to the aromatic and aliphatic protons, two
strong absorptions in 1697 and 1661 cm
-1
for stretching
C=O in the pyrimidine ring, two strong absorptions as
doublet in 1433 and 1385 cm
-1
for stretching C=C in
rings and a medium absorption in 1238 cm
-1
for C-N
respectively.
The MS (APCI) of 4a showed a peak at m/z 414.1([M]
+
)
corresponding to the molecular formula C
20
H
22
N
4
O
6
. This
product gave also satisfactory proton decoupled
13
C
NMR data in 18.30, 51.32, 53.99, 99.40, 129.78, 144.25,
149.15, 152.62, 166.28 ppm.
In the second stage, we studied the ef ciency using
two heteropolyacids contain Keggin-type H
3
[PMo
12
O
40
]
and preyssler H
14
[NaP
5
W
30
O
110
]. The results are reported
in Table1 with the order of ef ciency as follows:
H
3
[PMo
12
O
40
]> H
14
[NaP
5
W
30
O
110
].
SCHEME 1. Synthesis of some 4,4’-(phenylene)bis tetrahydropyri-
midines in HCI=Het
SCHEME 2. Plausible mechanism for the formation of some 4,4’-(phenylene)bis
tetrahydropyrimidines in HCI=Het
114 SYNTHESIS OF 4, 4’-(1, 3 AND 1, 4-PHENYLENE) BIS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Haniyeh et al.
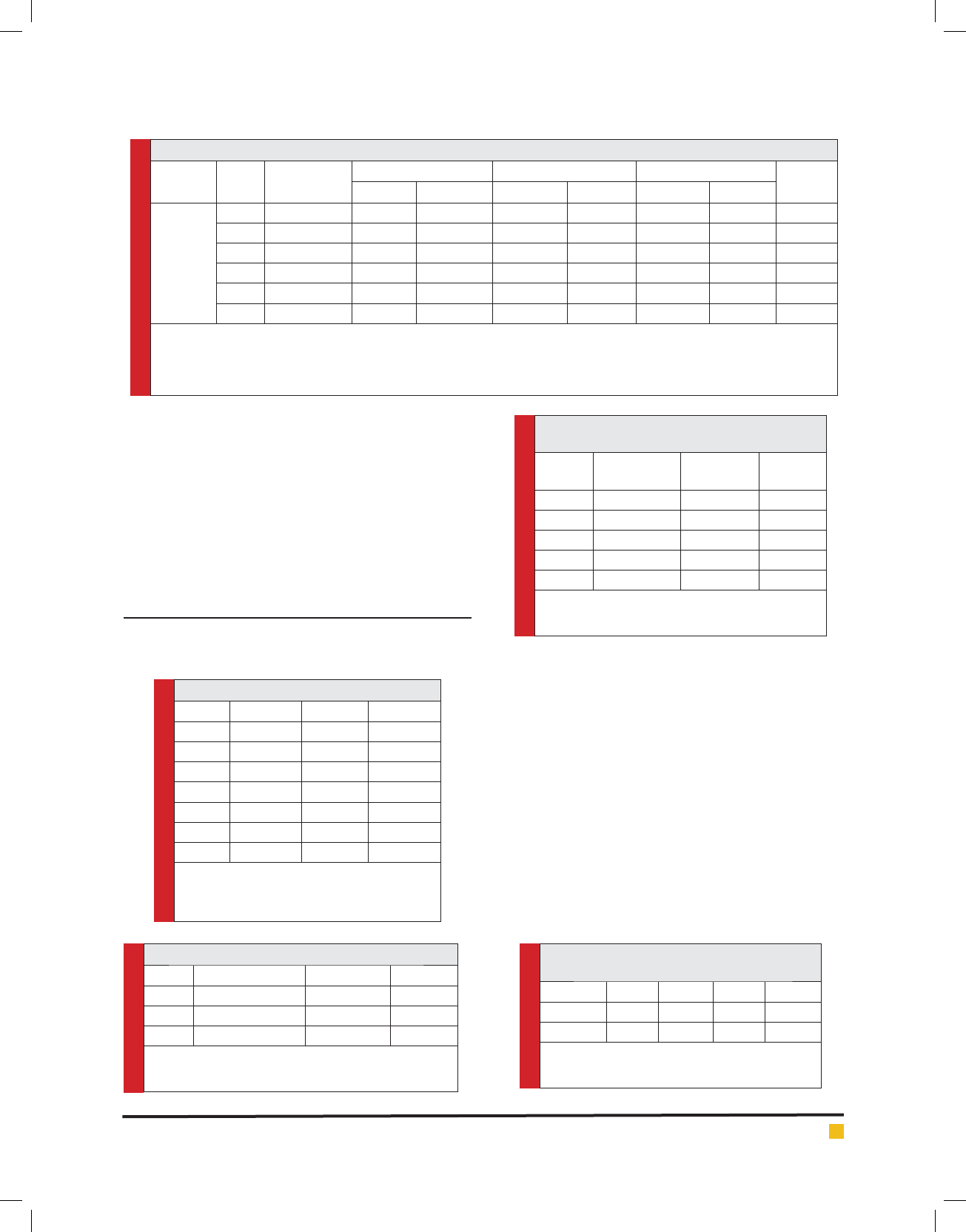
Table1. Synthesis of some new 4, 4’-(phenylene) bis tetrahydropyrimidines 4a-4f
Product
c
RAr
HCl
a
H
3
[PMo
12
O
40
]
b
H
14
[NaP
5
W
30
O
110
]
b
M.P. (ºC)
Time(hr) Yield (%)
d
Time(min) Yield (%) Time(min) Yield (%)
4a Me 1,4-phenylene 4 84 30 86 60 84 315 dec.
4b Et 1,4-phenylene 4 87 30 90 60 86 310 dec.
4c PhCH
2
1,4-phenylene 5 90 30 92 60 88 294-296
4d Me 1,3-phenylene 6 85 30 88 60 80 279-281
4e Et 1,3-phenylene 4 80 30 85 60 84 289-291
4f PhCH
2
1,3-phenylene 5 85 30 85 60 82 300 dec.
a
Reaction conditions : Urea 1 (2 mmol), a dialdhyde 2a-2b (1 mmol), an acetoacetate 3a-3c (2 mmol), Ethanol (6 ml), Hydrochloric acid (4-5 drops), re ux.
b
Reaction conditions : Urea 1 (2 mmol), a dialdhyde 2a-2b (1 mmol), an acetoacetate 3a-3c (2 mmol), Ethanol (6 ml), Hydrochloric acid (4-5 drops),
H
3
[PMo
12
O
40
] or H
14
[NaP
5
W
30
O
110
] (0.1gr), temperature room.
c
All the products were characterized according to their spectral and microanalytical data.
d
Isolated yields.
Table 2. Effect of Solvent on the yields of 4a
a
Entry Solvent Time(hr) Yield (%)
1H
2
O3 38
2 DMSO 3 32
3 DMF 3 35
4C
6
H
5
CH
3
3 Trace
5CH
2
Cl
2
3 Trace
6 CHCl
3
3 Trace
7 EtOH 30(min) 86
a
Reaction conditions : Urea 1 (2 mmol),
Terephthaldialdhyde 2a (1 mmol), methyl acetoacetate
3a (2 mmol), solvent (6 ml), H
3
[PMo
12
O
40
] (0.1gr),
Hydrochloric acid (4-5 drops), temperature room.
Table 3. Effect of temperature on the yields of 4a
a
Entry Temperature (ºC) Product (gr) Yield (%)
1 Room 0.356 86
2 50 0.228 55
3 110 0.141 34
a
Reaction conditions : Urea 1 (2 mmol), Terephthaldialdhyde 2a (1
mmol), methyl acetoacetate 3a (2 mmol), solvent (6 ml), H
3
[PMo
12
O
40
]
(0.1gr), Hydrochloric acid (4-5 drops).
Table 4. Effect of the amounts of Keggin
H
3
[PMo
12
O
40
] on the yields of 4a
a
Entry amounts of
Keggin(gr)
Time (min) Yield (%)
1 0.05 30 28
2 0.07 30 68
3 0.10 30 86
4 0.12 30 63
5 0.15 30 52
a
Reaction conditions : Urea 1 (2 mmol), Terephthaldialdhyde
2a (1 mmol), methyl acetoacetate 3a (2 mmol), solvent (6 ml),
Hydrochloric acid (4-5 drops), temperature room.
Table 5. Synthesis of 4a with recycled Keggin
H
3
[PMo
12
O
40
]
a
.
1
st
run 2
nd
run 3
rd
run 4
th
run
Time (min) 30 30 40 50
Yield (%) 86 78 76 70
a
Reaction conditions : Urea 1 (2 mmol), Terephthaldialdhyde
2a (1 mmol), methylacetoacetate 3a (2 mmol), solvent (6 ml),
H
3
[PMo
12
O
40
] (0.1gr), Hydrochloric acid (4-5 drops).
The effect of varying the reaction duration was stud-
ied for the synthesis of 4a by reaction of Urea 1, Tere-
phaldialdhyde 2a, and methyl acetoacetate 3a.
The effect of solvent on the synthesis of 4a was stud-
ied on solvents including C
6
H
5
CH
3
, DMF, DMSO, H
2
O,
CH
2
Cl
2
, CHCl
3
, and EtOH. Ethanol proved to be the best
in terms of yield (Table 2).
The effects of temperature, and the amounts of heter-
opolyacid, had showed respectively Tables 3 and 4.
CONCLUSION
Melting points were recorded on a Stuart SMP3 melt-
ing point apparatus. The IR spectra were obtained using
a Bruker Tensor 27 spectrophotometer using KBr discs.
The
1
H NMR (300 MHz) was recorded with Bruker-300
MHz spectrometers. The
13
C-NMR was recorded with
Bruker-300 MHz spectrometers at 75 MHz frequencies.
The mass spectra were scanned on an Agilent Technolo-
gies instrument at 70 eV. Elemental analysis was per-
formed on a Thermo Finnigan Flash EA microanalyzer.
Synthesis of bis-1, 2, 3, 4-tetrahydropyrimidines
4a-4f; (general procedure). A mixture of urea 1 (2
mmol), an aldehyde 2a-2b (1 mmol), -ketoester 3a-3c
(2 mmol) and conc. HCl (4-6 drops) in EtOH (6mL) was
heated under re ux for 4-6 hours. After the completion
of the reaction, the solvent was evaporated in vacuo.
The crude product was collected and re-crystallized from
ethanol to give compounds 4a-4f in 80-90% yields.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SYNTHESIS OF 4, 4’-(1, 3 AND 1, 4-PHENYLENE) BIS 115

Haniyeh et al.
Synthesis of bis-1, 2, 3, 4-tetrahydropyrimidines
4a-4f in presence of heteropolyacids; (general proce-
dure). To a mixture of urea 1 (2 mmol), a dialdehyde
2a-2b (1 mmol), and -ketoester 3a-3c (2 mmol), a
catalytic amount of heteropolyacid (0.1 gr) was added
and the resulting mixture was stired in solvent (6 mL).
The progress of the reaction was monitored by TLC. On
completion, the catalyst was ltered off, the solvent was
evaporated and the pure product was collected and re-
crystallized from ethanol to give compounds 4a-4f in
85-92 % yields.
Dimethyl 4, 4’-(1, 4-phenylene) bis (6-methyl-2-
oxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylate)
(4a). Yield 84-86%, yellow powder, mp 315ºC >decom-
posed, IR spectrum, , cm
-1
: 3430(N-H), 3028 (arom-
CH), 2943 (aliph-CH), 1697(C=O), 1661(C=O), 1433 and
1385 (C=C), 1238(C-N), 1094(C-O).
1
H NMR spectrum
(DMSO-d
6
), , ppm (J, Hz): 2.250(s, 6H, -CH
3
); 3.541(s,
6H, -CH
3
); 5.109(s, 2H, -CH); 7.189(s, 4H, C
6
H
4
); 7.736(s,
2H, N-H exchange with D
2
O), 9.231(s, 2H, N-H exchange
with D
2
O).
13
C NMR (DMSO-d
6
), , ppm: 18.30(CH
3
),
51.32(CH
3
), 53.99(CH), 99.40(C), 126.78(Ar), 144.25(Ar),
149.15(C), 152.62(C=O), 166.28(C=O). Mass spectrum (EI,
70 eV), m/z (I
rel
, %): 414.1[M]
+
(10), 413.1[M-H]
+
(15),
375.1(40), 311.1(20), 260.1(20), 182.9(20), 131.0(25),
97.1(45), 71.0(43), 43.1(100). Elemental Analysis: Found,
%: C 55.43; H 5.21; N 12.89; C
20
H
22
N
4
O
6
. Calculated, %:
C 57.97; H 5.35; N 13.52.
Diethyl 4, 4’-(1, 4-phenylene) bis(6-methyl-2-
oxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylate)
(4b). Yield 86-90% , white powder, mp 310ºC decom-
posed, IR spectrum, , cm
-1
: 3308(N-H), 3019 (arom-
CH), 2931 (aliph-CH), 1703(C=O), 1660(C=O), 1453 and
1372 (C=C), 1235(C-N), 1085(C-O).
1
H NMR spectrum
(DMSO-d
6
), , ppm (J, Hz): 1.067-1.128(t, 6H, CH
3
);
2.242(s, 6H, -CH
3
); 3.949-4.018(q, 4H, -CH
2
);5.115(s, 2H,
-CH); 7.190(s, 4H, C
6
H
4
); 7.704(s, 2H,N-H exchange with
D2O), 9.197(s, 2H, N-H exchange with D
2
O).
13
C NMR
(DMSO-d
6
), , ppm: 14.53(CH
3
), 22.88(CH
3
), 54.06(CH),
59.69(CH
2
), 99.69(C), 126.78(Ar), 144.40(Ar), 148.76(C),
152.53(C=O), 165.77(C=O). Mass spectrum (EI, 70 eV),
m/z (I
rel
, %): 442.3[M]
+
(15), 441.2[M-H]
+
(22). Elemen-
tal Analysis: Found, %: C 58.46; H 5.68; N 12.07;
C
22
H
26
N
4
O
6
. Calculated, %: C 59.72; H 5.92; N 12.66.
Dibenzyl 4, 4’-(1, 4-phenylene)bis(6-methyl-2-
oxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylate)
(4c). Yield 88- 92%, white powder, mp 294-296 ºC, IR
spectrum, , cm
-1
: 3356(N-H), 3107 (arom-CH), 2958
(aliph-CH), 1693(C=O), 1641(C=O), 1453 and 1380
(C=C), 1223(C-N), 1090(C-O).
1
H NMR spectrum (DMSO-
d
6
), , ppm (J, Hz): 2.281(s, 6H, -CH
3
); 5.028(s, 2H, -CH);
5.148(s, 4H, -CH
2
); 7.127-7.331(m, 14H, C
6
H
4
and C
6
H
5
);
7.754(s, 2H, N-H exchange with D
2
O), 9.301(s, 2H,
N-H exchange with D
2
O).
13
C NMR (DMSO-d
6
), , ppm:
18.36(CH
3
), 54.09(CH), 65.31(CH
2
), 99.21(C), 126.85(Ar),
128.06(Ar), 128.20(Ar), 128.76(Ar), 128.78(Ar), 136.96
(Ar), 144.28(Ar), 149.74(C), 152.53(C=O), 165.52 (C=O).
Mass spectrum (EI, 70 eV), m/z (I
rel
, %): 566.3[M]
+
(5),
259.0(20), 183.0(18), 90.9(100), 44.0(95). Elemental
Analysis: Found, %: C 66.34; H 5.04; N 9.13; C
32
H
30
N
4
O
6
.
Calculated, %: C67.83; H5.34; N 9.89.
Dimethyl 4, 4’-(1,3-phenylene)bis(6-methyl-2-
oxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylate)
(4d). Yield 80-88%, white powder, mp 279-281 ºC, IR
spectrum, , cm
-1
: 3408(N-H), 3031 (arom-CH), 2950
(aliph-CH), 1696(C=O), 1646(C=O), 1436 and 1318 (C=C),
1233(C-N), 1092(C-O).
1
H NMR spectrum (DMSO-d
6
),
, ppm (J, Hz): 2.249(s, 6H, -CH
3
); 3.550(s, 6H, -CH3);
5.124(s, 2H, -CH); 7.142-7.169(m, 3H, C
6
H
4
); 7.280-
7.332(m, 1H, C
6
H
4
); 7.769(s, 2H,N-H exchange with
D
2
O), 9.249(s, 2H, N-H exchange with D2O).
13
C NMR
(DMSO-d
6
), , ppm: 18.24(CH
3
), 51.19(CH
3
), 54.29(CH),
99.56(C), 124.43(Ar), 125.63(Ar), 129.04(Ar), 145.51(Ar),
149.01(C), 152.65(C=O), 166.18(C=O). Mass spectrum (EI,
70 eV), m/z (I
rel
, %): 414.2[M]
+
(4), 169.0(100), 137.0(50),
42.1(40). Elemental Analysis: Found, %: C 57.24; H
5.12; N 12.98; C
20
H
22
N
4
O
6
. Calculated, %: C 57.97; H5.35;
N 13.52.
Diethyl 4, 4’-(1, 3-phenylene)bis(6-methyl-2-
oxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxy-
late) (4e). Yield 80-85%, yellow powder, mp 289-291
ºC, IR spectrum, , cm
-1
: 3360(N-H), 3118 (arom-CH),
2978 (aliph-CH), 1699(C=O), 1649(C=O), 1461 and
1385 (C=C), 1225(C-N), 1093(C-O).
1
H NMR spectrum
(DMSO-d
6
), , ppm (J, Hz): 1.048-1.095(t, 6H, -CH
3
);
2.227(s, 6H, -CH
3
); 3.913-3.998(q, 4H, -CH
2
); 5.101(s,
2H, CH); 7.118-7.138(m, 3H, C
6
H
4
); 7.256-7.309(m, 1H,
C
6
H
4
); 7.761(s, 2H,N-H exchange with D
2
O), 9.182(s,
2H, N-H exchange with D
2
O).
13
C NMR (DMSO-d
6
), ,
ppm: 14.55(CH
3
), 18.18(CH
3
), 54.41(CH), 59.60(CH2),
99.70(C), 124.55(Ar), 125.78(Ar), 128.93(Ar), 145.53(Ar),
148.80(C), 152.52(C=O), 165.69(C=O). Mass spectrum
(EI, 70 eV), m/z (I
rel
, %): 442.1[M]
+
(6), 441.0[M-H]+(20).
Elemental Analysis: Found, %: C 58.91; H 5.38; N 11.96;
C
22
H
26
N
4
O
6
. Calculated, %: C 59.72; H 5.92; N 12.66.
Dibenzyl 4, 4’-(1, 3-phenylene)bis(6-methyl-2-
oxo-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylate)
(4f). Yield 82-85%, white powder, mp 300ºC decom-
posed, IR spectrum, , cm
-1
: 3369(N-H), 3048 (arom-
CH), 2933 (aliph-CH), 1697(C=O), 1644(C=O), 1451 and
1382 (C=C), 1227(C-N), 1084(C-O).
1
H NMR spectrum
(DMSO-d
6
), , ppm (J, Hz): 2.260(s, 6H, -CH
3
); 4.990(s,
2H, CH); 5.179 (s, 4H, -CH
2
); 7.153-7.276(m, 3H, C
6
H
4
);
7.454(m, 1H, C
6
H
4
); 7.835(s, 2H,N-H exchange with
D
2
O), 9.310(s, 2H, N-H exchange with D
2
O).
13
C NMR
(DMSO-d
6
), , ppm: 18.32(CH
3
), 54.26(CH), 65.23(CH
2
),
99.30(C), 124.61(Ar), 125.79(Ar), 128.79(Ar), 129.57(Ar),
137.03(Ar), 145.39(Ar), 148.83(Ar), 149.75(C), 152.55
116 SYNTHESIS OF 4, 4’-(1, 3 AND 1, 4-PHENYLENE) BIS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Haniyeh et al.
(C=O), 165.49(C=O). Mass spectrum (EI, 70 eV), m/z (I
rel
,
%): 565.9[M]
+
(3), 565.0[M-H]+(20), 220.9(15), 137.0(10),
97.1(65), 91.1(100), 43.6(85) . Elemental Analysis:
Found, %: C 64.82; H 5.11; N 9.06; C
32
H
30
N
4
O
6
. Calcu-
lated, %: C 67.83; H 5.34; N 9.89.
REFERENCES
Biginelli, P. Ber. Dtsch. Chem. Ges. (1891): 24, 1317. Chwegler,
M. A.; Bekkum, H.; Van Munck, N. (1991): Appl Catal, 74, 191.
Coronado, E.; Gomez-Garcia, C. J (1998): Chem Rev, 98, 273.
Da Silva, D.L.; Reis, F.S.; Muniz, D.R.; Ruiz, A.L.T.G.; Carvalho,
J.E.; Sabino, A.A.; Modolo, L.V.; De Fatima, A. (2012): Bioorg
Med Chem, 20, 2645.
Davoodnia, A.; Bakavoli, M.; Barakouhi, G.; Tavakoli-
Gulten, S. J (2013): Heterocyclic Chem, 50, 391.
Harikrishnan, P.S.; Rajesh, S.M.; Perumal, S.; Almanson, A.I.
(2013): Tetrahedron Lett, 54, 1076.
Hu, E.; Sidler, D.R.; Dolling, U.H. J (1998): Org Chem, 63, 3454.
Kappe, C.O. (1999): Synthesis, 10, 1799.
Kumar, B.R.P.; Sankar, G.; Baig, R.B.N.; Chandrashekaram, S.
(2009): Eur J Med Chem, 44, 4192.
Kumar, K.A.; Kasthuraiah, M.; Reddy, C.S.; Reddy, C.D. (2001):
Terahedron Lett, 42, 7873.
Lu, J.; Bai, Y.; Wang, Z.; Yang, B.; Ma, H. (2000): Tetrahedron
Lett, 41, 9075.
Lu, J.; Ma, H.; (2010): Synlett, 1, 63.
Müller, A.; Peters, F.; Pope, M. T.; Gatteschi, D. (1998): Chem
Rev, 98, 239.
Okuhara, T.; Mizuno, N.; Misono, M. (1996): Adv Catal, 41,
221.
Pope, M. T.; Müller, A. (2001): Polyoxometalate Chemistry from
Topology via Self-Assembly to Applications; Kluwer Academic
Publishers: Netherlands.
Radha, Rani; V.; Srinivas, N.; Radha, K.M.; Kulkarni, S.J.;
Raghavan, K.V. (2001): Green Chem, 3, 305.
Ramalinga, K.; Vijayalakshmi, P.; Kaimal, T.N.B. (2001): Syn-
lett, 6, 0863.
Ranu, C. B.; Hajra, A.; Jana, U. J (2000): Org Chem, 65, 6270.
Russowsky, D.; Canto, R.F.S.; Sanches, S.A.A.; D’Oca, M.G.M.;
De Fatima, A.; Pilli R.A.; Kohn, L.K.; Antonio, M.A.; De Car-
vallho, J.E. (2006): Bioorg Chem, 34, 173.
Salehi, H.; Guo, Q.X. (2004): Synth Commun, 34, 171.
Singh, K.; Arora, D.; Falkowski, D.; Liu, Q.; Moreland, R.S. J
Org Chem (2009): 19, 3258.
Studer, A.; Jeger, P.; Wipf, P.; Curran, D.P. J (1997): Org Chem,
62, 2917.
Yadav, J.S.; Subba, R.B.V.; Srinivas, R.; Venugopal, C.; Ramal-
ingam, T. (2001): Synthesis, 9, 1341.
Zhang, Y.; Wang, B.; Zhang, X.; Huang, J.; Liu, C. (2015): Mol-
ecules, 20, 3811.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SYNTHESIS OF 4, 4’-(1, 3 AND 1, 4-PHENYLENE) BIS 117