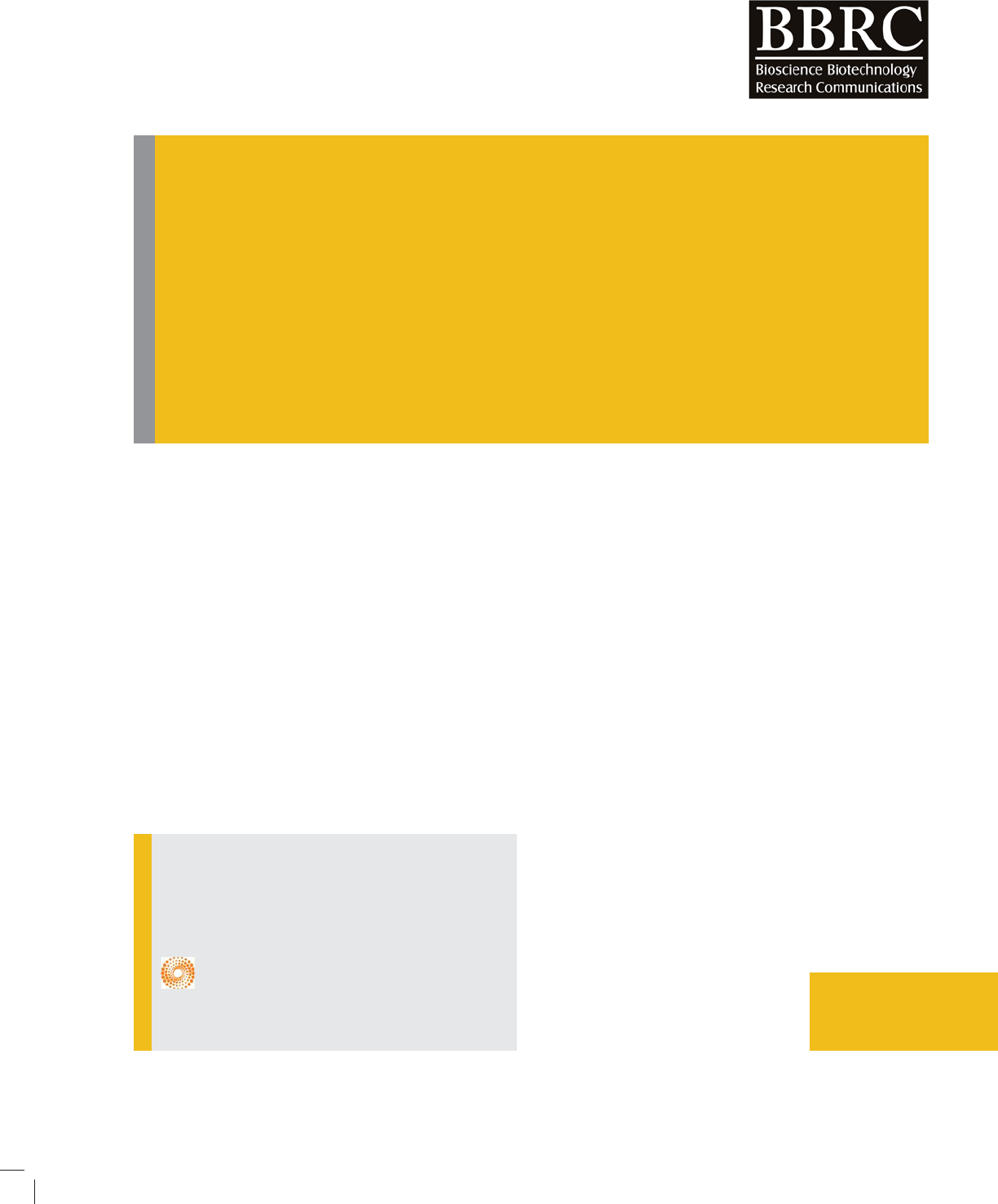
Investigation of photocatalytic degradation of diazinon
using titanium dioxide (TiO2) nanoparticles doped
with iron in the presence of ultraviolet rays from the
aqueous solution
MohammadMehdiBaneshi
1
, Soheila Rezaei
1
, Abdolmohammad Sadat
1
, Ali Mousavizadeh
2
,
Mansour Barafrashtehpour
3
and Hamid Hekmatmanesh
1
*
1
Social Determinants of Health Research Center, Yasuj University of Medical Sciences, Yasuj Iran
2
Department of Biostatistics, Yasuj University of Medical Sciences, Yasuj, Iran
3
Department of Environment Health, Isfahan University of Medical Sciences, Isfahan, Iran
ABSTRACT
Diazinon is one of organophosphate pesticides which it is classi ed as a relatively dangerous substance (Class II by
World Health Organization). The aim of this study was to determine the ef ciency of photocatalytic degradation of
diazinon using titanium dioxide (TiO2) nanoparticles doped with iron in the presence of ultraviolet light in aqueous
solution. This cross-sectional study was conducted at the laboratory scale in a 2 L reactor. The nanoparticles were
synthesized by sol-gel method. The degradation of diazinon was evaluated in various amounts of pH, time, nanopar-
ticles dosage, and the concentration of diazinon. The obtained results were analyzed using Excel2007 and SPSS V.16
software and regression test. The results showed that the increasing pH, reducing the concentration of diazinon and
increasing contact time can lead to increase the removal ef ciency. The optimum pH was obtained to be in neutral
range and at the pH=7. In addition, the optimum amount for contact time, nanoparticle dosage and diazinon concen-
tration was found to be 60 min, 100mg/L and 50mg/L, respectively. The maximum removal ef ciency was 98.58%.
Photocatalytic processes have high capacity in removing of diazinon and can effectively mineralized this compound.
Furthermore, the TiO2 nanoparticles are not toxic and they can be used to remove the pollutants in various industries.
Thus, doped nanoparticles due to the small amount of nano-materials, low energy consumption and high ef ciency
can be used as a good alternative to the removal of diazinon.
KEY WORDS: DIAZINON, PHOTOCATALYTIC DEGRADATION, DOPING, TIO2
60
ARTICLE INFORMATION:
*Corresponding Author: hamidhekmat60@yahoo.com
Received 27
th
Nov, 2016
Accepted after revision 27
th
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
Biosci. Biotech. Res. Comm. Special Issue No 1:60-67 (2017)

MohammadMehdiBaneshi et al.
INTRODUCTION
A variety of methods such as degradation by ultrasonic
waves (Mahmoodi et al. 2007), biodegradation (Danesh-
var, 2007), optical degradation ( olovi et al. 2011), ozo-
nation (Wu, 2009; Yuk Sing and Chongyu, 2007), deg-
radation by gamma rays (Yuk Sing and Chongyu, 2007),
Fenton (Wang and Lemley, 2002), treatment with UV /
H2O2 (APHA and WEF, 2005) and photocatalytic degra-
dation (Li et al. 2002, Kansal et al. 2007) have been used
to remove the diazinon.
The problems of these methods are including process
complexity, high cost and high consumption of chemi-
cals. However, the nano photocatalytic method has
rarely applied. In general, common physical techniques
such as occulation, aeration, adsorption on activated
carbon and reverse osmosis may effectively remove the
contaminants; however, these methods are not able to
destroy the pollutants and contaminants from water
and they only can transfer these pollutants to another
phase and this is led to the formation of a secondary
environmental pollution which it is casused to require
retreatment and increase total cost. Photocatalyst is an
advanced oxidation technology with bright future and
it has been utilized in Usa, Europe and Japan in order
to purify the water of pollutants (Ugurlu and Karaoglu,
2009). Advanced oxidation processes produce a strong
oxidizing agent (hydroxyl radicals) that they destroy
the pollutants in wastewater completely (Mesgari et al.
2012).
One of the chief wastewater treatment process tech-
nologies is the photocatalysts and semiconductor that
have been shown to be potentially useful for the treat-
ment of wastewater contaminants (Liu et al. 2005;
Mekprasart, 2011). Among the various semiconductor
materials (oxides, sul des ), TiO2 has gained more popu-
larity and attention due to high Photocatalytic activity,
chemical stability, resistant against optical corrosion,
economic acceptability, cost-effectiveness and lack of
toxicity (Zhou et al. 2006; Sun et al. 2009). Although
the ef ciency of TiO2 with relatively high energy band
gap (3.2eV) has limited, but various methods such as
increasing surface to volume ratio, connecting TiO2
to other semiconductor particles, splashing of various
types of TiO2 into the zeolite pores and doping the metal
and non-metal ions with TiO2 have been developed to
increase the photocatalysis activity of TiO2 particles (Zhu
et al. 2006). The conductive ion metals can lead to the
formation of doped energy level between the conduc-
tion and valence bands of TiO2 which it has identi ed
as an effective way for increasing of the Photocatalytic
activity of TiO2. Moreover, the doped ions may be act
as electrons or holes traps and they boost the catalytic
activity of TiO2 (Liu and Chen, 2009). Previous studies
have clari ed that the transition metal ions, e.g Fe+3
can be utilized to increase the Photocatalytic activity
(
Rezaei kalanteri et al. 2014; Fadaei and Sadeghi, 2013).
Metal ions Fe+3 can be easily accommodated among
TiO2 network due to half- lled electron con gurations
and with an ionic radius close to the ionic radius of Ti+4
(Sorouri Zanjani et al. 2009) and it is caused to increase
the photocatalytic activity in the visible light region. In
addition, Fe+3 ions can create a surface trap on TiO2
network for electrons and holes arising from radiation,
thereby it can increase Kvantayy ef ciency and pho-
tocatalytic activity by reducing the recombination of
generated electrons and holes. Thus, Fe+3 ions are con-
sidered as a striking doping factor (
Rezaei kalanteri et al.
2014, Samadi et al. 2010).
For many years, the mankind uses the various types of
chemicals to eliminate the pets. This material has brought
severe and irreparable damage to nature, environmental
health, balance and stability of ecosystems and living
creatures (Balschmiter et al. 1983). A part of pollutants
such as organic material are often degraded through the
biological processes but other materials such as pesti-
cides are resistant against degradation and remain in
the aquatic environment for a long time (EsmailiSari,
2001). Organophosphates, as a group of pesticides, were
replaced with organochlorine a few decades ago due
to their lesser resistant and stability (Girón-Pérez et al.
2007). These poisons are capable to create serious effect
on non-target animals such as invertebrates, mammals,
birds and sh due to widespread distribution in the
aquatic environment (Vandergeest et al. 1997; Castano
et al. 1986). The exposure of shes with fatal doses of
diazinon is caused to anemia (Anees et al. 1978), reduc-
tion of DNA, RNA and protein in the liver (Ansari, 1988),
effect on the nervous system, the anomaly in the gills,
increasing the amount of macrophages and the effect
on the reproductive behavior (Dutta and Maxwell,
2003). Diazinon is partially soluble in water (40 mg/L
at 25°C); non-polar and resistant against degradation
in soil (APHA and WEF, 2005) which its characteristics
are given in Table 1. Unlike to chlorinated pesticides,
they have not accumulative nature in the body and are
faster degraded in the environment (Shemer and Linden,
2006).
Diazinon is one of the Organophosphate insecticides
which are classi ed as relatively dangerous materials
(Class II by the World Health Organization). It makes
toxicity for aquatic organisms at a concentration of
350 ng/l (Li et al. 2002), and its LC50 for sh is 4.4
ppm (Zhang and Pehkonen, 1999). More than 13 mil-
lion pounds of diazinon are annually used in the United
States (10). Thus, the releasing of this compound into
the groundwater is one of the major concerns. The toxic
effect of Diazinon, like other organic phosphorus pesti-
C
ˇ
ˇ
c
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INVESTIGATION OF PHOTOCATALYTIC DEGRADATION OF DIAZINON USING TITANIUM DIOXIDE 61
MohammadMehdiBaneshi et al.
FIGURE 1. Schematic of reactor used in the process
(1) Trans of lamp 150 watt, (2) glass reactor, (3) cool-
ing water, (4) Magnet (5) magnetic stirrer (6) reac-
tor doors, (7) UV lamp 150 watt medium pressure (8)
covering quartz, (9) pump, (10) the water tank (11)
pipes for water.
cides, is to stop the acetylcholinesterase (Li et al. 2002,
Zhang and Pehkonen, 1999). It was also reported that
diazinon has a negative effect on the immune system
(Immunotoxic), cells (Cytotoxic) and genes (Genotoxic)
(Mahmoodi et al. 2007).
Since the diazinon is the most widely use and danger-
ous pesticides for the environment, especially aquatic
organisms, thus, the aim of this study was to determine
the ef ciency of photocatalytic degradation of diazinon
using titanium dioxide nanoparticles doped with iron in
the presence of ultraviolet light is the aqueous medium.
The most conducted studies in Iran have revealed
that the concentration of diazinon in the water is more
than standard levels. Shaeghi et al has found that the
diazinon concentration in Gharehsou and Gorgan riv-
ers in Golestan province was 22.4ppm and 6.74ppm,
respectively which it was higher than the standard lev-
els (
Daneshvar, 2007). Khazaeii and colleagues has also
observed that the concentration of diazinon in a number
of water samples was higher than standard levels (Wu,
2009
).
MATERIALS AND METHODS
In this study, a reactor made of Pyrex with the overall
volume of 2.7 liters was used. The diameter and height
of this reactor was 14 cm and 18 cm, respectively. A
medium pressure Lamp UV (125 watt, length of 12 cm
and a diameter of 1 cm and coating quartz with exter-
nal diameter of 2.5cm, internal diameter of 2 cm and a
length of 12.5 cm) has been installed in the middle of the
reactor lid made of multilayer aluminum foil. There was
another hole on the reactor lid which it was for sampling
and it was covered during the process. The maximum
wavelength emitted by the UV lamps was 247.3 nm and
in UV-C range. The reactor was covered by aluminum
foil to protect against the radiation. Mixing in the reac-
tor was carried out using a magnetic stirrer and magnet.
Free height of 5.5 cm inside the reactor was intended
to move the magnet. Samples with different concentra-
tions of diazinon, which was prepared by diluting the
diazinon 60 percent, were entered into the reactor and
the samples were taken under different conditions and
at different times.
The detection of diazinon level was performed by
reverse phase method of high performance liquid chro-
matography (HPLC). Chromatographic conditions were
as following: mobile phase of methanol + water was
applied at a ratio of 70:30 and C18 was used as the col-
umns. The determination of dizazinon level was per-
formed with a UV detector at 220 nm. Diazinon was
prepared from Sigma-Aldrich CO, USA. A hanger radi-
ometer instrument (ECL-X model) which it was to meas-
ure the intensity of light in UV-C range was utilized to
determine the intensity of the lamp UV125 watt medium
pressure used in reactors radiation. Intensity of lamps
were measured and controlled in half the diameter of the
reactor (about 7 cm) at different times.
The formula for calculating the radiation intensity:
D=L ×T T: time (s) L: radiation intensity (mw/s/cm2)
The diazinon removal percentage is calculated by fol-
lowing equation:
Where C0 and C are the initial and nal concentration of
Diazinon, respectively.
Test method and statistical analysis
Samples were taken in different states from the reac-
tor and were centrifuged at 4000 rpm for 30 minutes
and then were ltered with a 0.23μm lter to remove
particles of TiO2. The DX8 and SPSS V.16 software and
the ANOVA test were applied for design of experiments,
drawing graphs and statistical analysis of results and
LSD POST HOC was used to distinguish between differ-
ent modes. Also, DX8 software was used to determine
the optimal mode and model.
Nanoparticle characterization
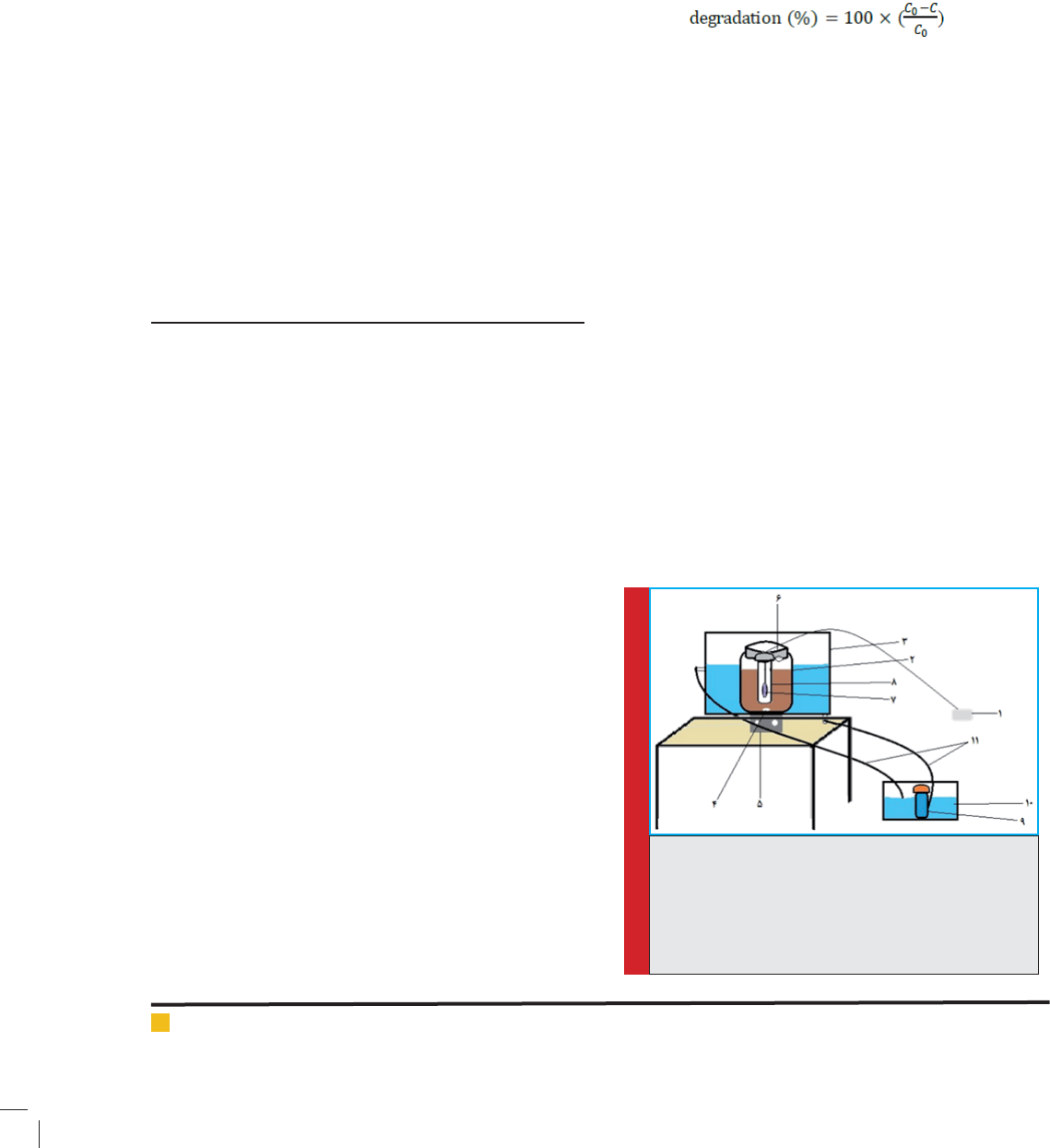
Fig 2&3 depict the SEM images and diameter distri-
bution of TiO2 nanoparticles before doping with Fe,
respectively. The diameter measurement of nanoparticles
was performed with the Measurement software and their
average diameter was determined and it is approved that
they are nanoparaticles. In this case, the average diam-
eter of the nanoparticles was determined to be 42.4 nm.
62 INVESTIGATION OF PHOTOCATALYTIC DEGRADATION OF DIAZINON USING TITANIUM DIOXIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
MohammadMehdiBaneshi et al.
FIGURE 2. SEM images of nanoparticles
FIGURE 3. The diameter distribution of nanoparticle
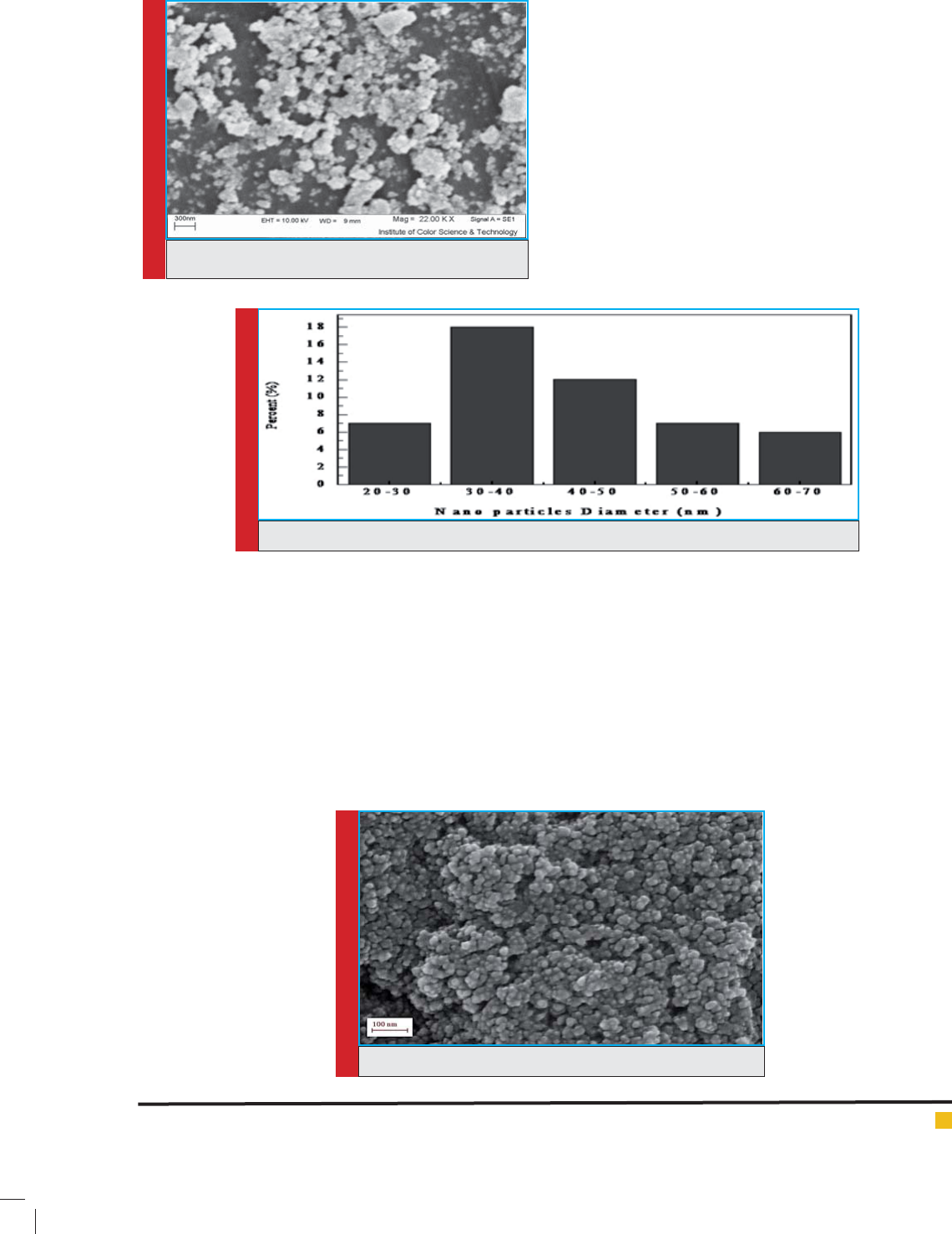
In addition, Fig 4&5 are related to the SEM images
and diameter distribution of the doped TiO2 nanoparti-
cles with Fe, respectively. The average diameter of nano-
particle was obtained to be 37.89 nm. The comparison
of the Fig 2 and Fig 4 show that the doped nanoparti-
cle structure is smaller while it was bulky before dop-
ing. Furthermore, it was clari ed that the average size
of doped nanoparticles (Fig5) is smaller than un-doped
nanoparticles (Fig 3).
FIGURE 4. SEM images of Fe-TiO2 nanoparticles
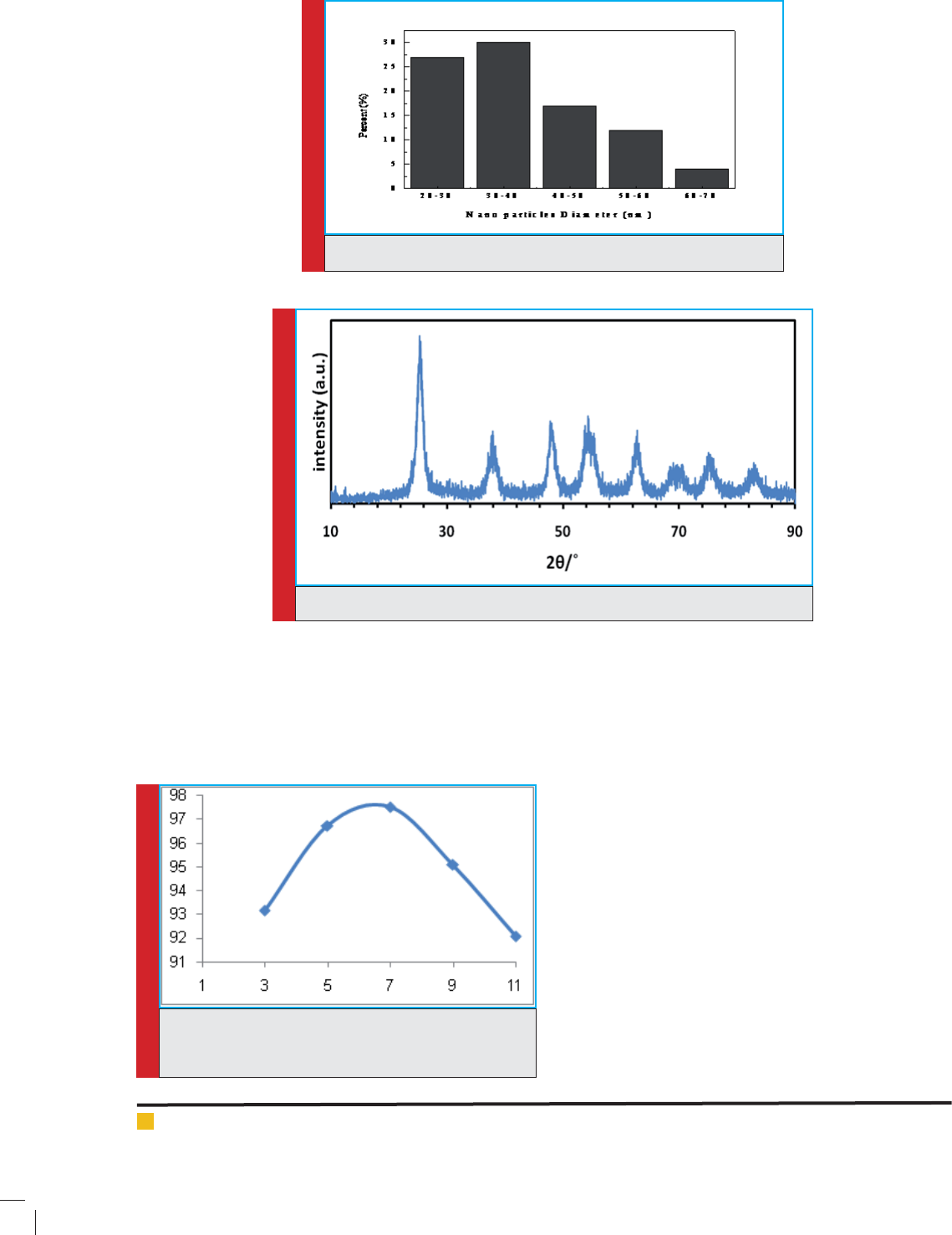
To obtain the optimum pH, the experiments were
conducted in different pH (3, 5, 7, 9, 11) while other
parameters including time, Diazinon concentration and
nanoparticle dose were kept constant. The results are
presented in g 7. The results clarify that the maximum
removal ef ciency was achieved to be 97.52% at pH=7
and therefore, this pH was considered as optimum pH for
next experiments. It is also observed that the removal
ef ciency was decreased at the pH<5 and pH>9.
The X-ray diffraction pattern (XRD) for doped TiO2
nanoparticles with Fe is represented in Fig 6. The
observed peaks (maximum peak at 250) in XRD pattern
indicates that TiO2 doped with Fe has Anatase structure.
Strong peaks at 27, 36 and 55 degrees are indica-
tive of TiO2 in the rutile phase. On the other hand
strong peaks at 25 and 48 degrees is represented TiO2
is in anatase. The Fig 6 shows that highest amounts are
related to anatase phase while rutile phase exists with
anatase phase, heterogeneously. Titanium dioxide can be
observed in 3 forms including Anatase, Rutile and Bru-
cite which anatase and rutile have light catalytic activ-
ity. The Anatase shows more light activity than Rutile;
thus, it is more applicable.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INVESTIGATION OF PHOTOCATALYTIC DEGRADATION OF DIAZINON USING TITANIUM DIOXIDE 63
MohammadMehdiBaneshi et al.
FIGURE 5. The diameter distribution of Fe-TiO2 nanoparticle
FIGURE 6. X-ray diffraction pattern for TiO2 nanoparticles doped with Fe
FIGURE 7. The effect of pH on diazinon removal ef ciency
(time= 60 min, diazinon concentration = 50 mg/L, nano-
particle dosage=100 mg/L)
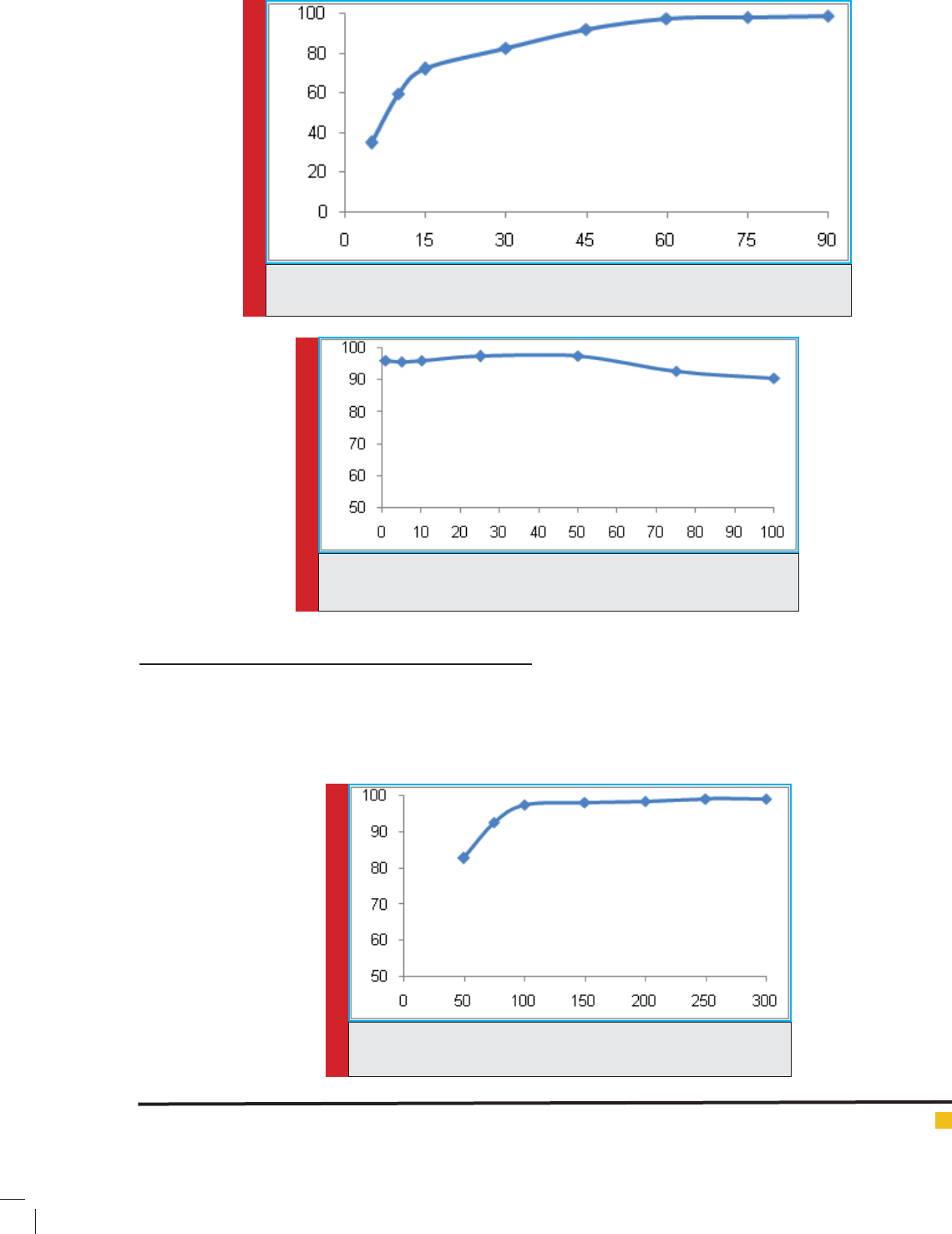
To determine the optimum time, the studied param-
eter were kept constant except the time. It was varied
from 5 to 90 min. it was observed that best removal ef -
ciencies was obtained in 60, 75 and 90 min. the removal
ef ciency percentage in 60, 70 and 90 min was obtained
to be 98.57%, 98.06% and 98.66%, respectively. In
this study, 60 min was selected as optimum time. The
obtained results were presented in g 8.
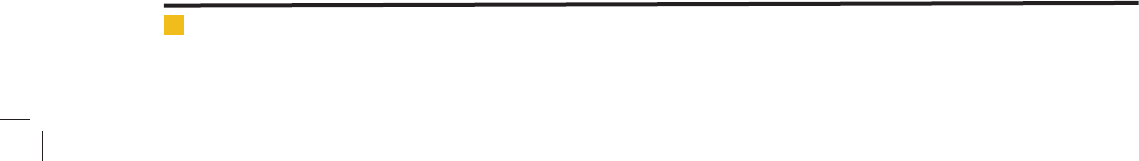
The optimum diazinon concentration was determined
by keeping constant of the pH, time and nanoparticle
dose and the varying of the diazinon concentration in
range of 1-100 mg/L. The highest removal ef ciency was
related to diazinon concentration of 50 mg/L (97.52%)
and 25mg/L (97. 40%). It is clear that the removal
ef ciency for 50 mg/L is slightly more than 25mg/L;
thus the 50 mg/L was accepted as optimum diazinon
concentration. The results of this section are shown
in Fig 9.
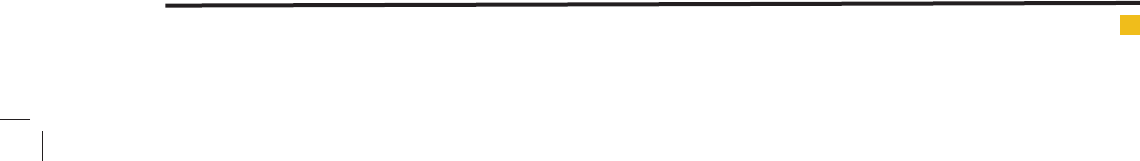
The optimum dose of nanoparticle was determined
by varying the nanoparticle doses between 50-300
mg/L and keeping constant of other parameters. The
best results were observed in the dosage of 100 mg/l
(97.52%), 150mg/L (98.16%) and 200 mg/L (98.58%).
Since there is no signi cance difference in diazinon
removal ef ciency in mentioned concentrations; there-
fore, there the nanoparticle dosage of 100 mg/L were
selected as optimum dose. The results are shown in
g 10.
64 INVESTIGATION OF PHOTOCATALYTIC DEGRADATION OF DIAZINON USING TITANIUM DIOXIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
MohammadMehdiBaneshi et al.
FIGURE 8. The effect of time on diazinon removal ef ciency (pH=7, diazinon concentration
= 50 mg/L, nanoparticle dosage=100 mg/L)
FIGURE 9. The effect of diazinon concentration on diazinon removal ef -
ciency (time= 60, pH=7, nanoparticle dosage=100 mg/L)
FIGURE 10. The effect of nanoparticle dosage on diazinon removal
ef ciency (time = 60, pH=7, diazinon concentration = 50mg/L)
DISCUSSION AND CONCLUSION
In the present study, photocatalytic decomposition of
diazinon using TiO2 nanoparticles doped with iron in the
presence of ultraviolet rays from the aqueous medium
was studied. In addition, the effect of different param-
eters including pH, dose of TiO2 nanoparticles doped
with iron, reaction time and concentration of diazinon
was discussed.
The results indicated that the increasing of pH,
decreasing of the diazinon concentration and increas-
ing of time is resulted in higher removal ef ciencies.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INVESTIGATION OF PHOTOCATALYTIC DEGRADATION OF DIAZINON USING TITANIUM DIOXIDE 65
MohammadMehdiBaneshi et al.
Best pH for diazinon removal is obtained to be in neu-
tral range and t pH of 7 which it is in accordance with
Merabat study (2009); in his study, the photocatalytic
decomposition of Indole was evaluated and the optimum
ph was found to be in range of 6-7.
Daneshvar et al (2007) has investigated the photo-
catalytic decomposition of diazinon with UV-C/ZNO
and the results indicated that the 80 min is required
to remove 80% of diazinon; it shows that the results
of present work is better than their results because the
optimum time of present work is 60 min. furthermore,
the results showed that the best dosage of nanoparti-
cles to obtain the highest removal rate was 100 mg/L in
addition, 50 mg/L of diazinon was selected as optimum
concentration. Zhang et al. (2011) has neeb reported that
TiO2 is effective on photocatalytic removal process that
it is agreed with the results of present study. According
to the results, the TiO2 dosage and contact time have
positive role on diazinion removal ef ciency.
Bazrafashan et al (2007) has found that higher elec-
trical potential or contact time is needed to remove the
higher concentration of diazinon. For any speci ed time,
the removal ef ciency has signi cantly increased by
increasing the voltage. The highest Electrical potential
(40 V) was led to fastest treatment with an over 99% of
removal ef ciency of diazinon after 60 minutes which it
is consistent with present study. The removal ef ciency
was 97.52% after optimum time of 60 min.
Diazinon is one of widely used as well as most
dangerous pesticides for environment and especially
aquatic organisms. The photocatalytic processes have
high capacity in removal and effective mineralization of
diazinon. Besides, the TiO2 nanoparticles can effectively
use to remove the toxic pollutants in various industries
because they are not toxic; thus, the doped nanoparti-
cle can be used as a suitable alternative to remove the
diazinon due to small amounts of nano-materials, low
energy consumption and high ef ciency.
REFERENCES
Esmail iSari. A. (2001): Pollutants, Health and standard in envi-
ronment: Naghsh Mehr.
Balsch miter, K., H. Buchert, C. Scholz and M. Zell. (1983): Base-
line studies of the global pollution by chlorinated hydrocarbons
in the Caspian Sea. Fresenius Z Anal Chem. 1983;316:242-6.
Girón- Pérez, M.I., Santerre, A., Gonzalez-Jaime, F., Casas-
Solis, J., Hernández-Coronadoa, M., Peregrina-Sandoval,
J., Takemura, A., Zaitseva, G., (2007): Immunotoxicity and
hepatic function evaluation in Nile tilapia (Oreochromis niloti-
cus) exposed to diazinon. Fish and Shell sh Immunology 23,
760-769.
Vander geest H.G., Studijfzand S.C., Kraak M.H.S. and Admiraal
W. (1997): Impact of diazinon calamity in 1996 on the aqautic
macroinvertebrates in the river mesue. The Netherlands. Jour-
nal of Aquatic Ecolology, 30:327-330.
Castan o, A., Bols, N.C., Braunbeck, T., Dierick, P., Halder, M.,
Isomaa, B., Kawahara, K., Lee, L. E. J., Mothersill, C., Pärt, P.,
Repetto, G., Sintes, J.R., Ru i, H., Smith, R., Eisler, R., (1986):
Diazinon hazards to sh, wildlife, and invertebrates: a synop-
tic review. U.S. Fish and Wildlife Service, U.S., 85,1–38.
Anees, M.A., (1978): Hepatic pathology in a freshwater teleost
Channa punctatus (Bloch) exposed to sublethal and chronic
levels of three organophosphorous insecticides. Bulletin Envi-
ronmental Contamination and Toxicology. 19, 524–527.
Ansari , B.A., Kumar, K., (1988): Diazinon toxicity on protein
and nucleic acid metabolism in the liver of Zebra sh, Brachy-
danio rerio (Cyprinidae). Scienti c Total Environment. 76,
63–68.
Dutta, H.M., Maxwell, L., (2003): Histological examination of
sublethal effects of Diazinon on ovary of Bluegill, Lepomis
macrochirus. Environmental Pollution. 121, 95–102.
APHA, A., WEF (2005): Standard Methods For the examination
of water and wastewater. 21st ed. Washington, DC, American
Public HealthAssociation.
Shemer H, Linden KG. (2006): Degradation and by-product
formation of diazinon in water during UV and UV/H 2 O 2
treatment. Journal of hazardous materials. 136(3):553-9.
Li P, Swanson E, Gobas F. (2002): Diazinon and its degrada-
tion products in agricultural water courses in British Columbia,
Canada. Bulletin of environmental contamination and toxicol-
ogy. 69(1):59-65.
Zhang Q, Pehkonen SO. (1999): Oxidation of diazinon by aque-
ous chlorine: kinetics, mechanisms, and product studies. Jour-
nal of agricultural and food chemistry. 47(4):1760-6.
Mahmoo di NM, Arami M, Limaee NY, Gharanjig K. (2007):
Photocatalytic degradation of agricultural N-heterocyclic
organic pollutants using immobilized nanoparticles of titania.
Journal of hazardous materials. 145(1):65-71.
Danesh var N, Aber S, Dorraji MS, Khataee A, Rasoulifard M.
(2007): Photocatalytic degradation of the insecticide diazinon
in the presence of prepared nanocrystalline ZnO powders
under irradiation of UV-C light. Separation and puri cation
Technology. 58(1):91-8.
C
ˇ
olovi MB, Krsti
c
´ DZ, Uš
c
´umli
c
´ GS, Vasi
c
´ VM. (2011): Sin-
gle and simultaneous exposure of acetylcholinesterase to
diazinon, chlorpyrifos and their photodegradation products.
Pesticide Biochemistry and Physiology. 100(1):16-22.
Wu J, Lan C, Chan GYS. (2009): Organophosphorus pesticide
ozonation and formation of oxon intermediates. Chemosphere.
76(9):1308-14.
Yuk Si ng G, Chongyu L. (2007): food and chemical toxicol-
ogy.45(10):2057-63.
Wang Q , Lemley AT. (2002): Oxidation of diazinon by anodic
Fenton treatment. Water research.36(13):3237-44.
Kansal S, Singh M, Sud D. (2007): Studies on photodegrada-
tion of two commercial dyes in aqueous phase using different
photocatalysts. Journal of hazardous materials.141(3):581-90.
66 INVESTIGATION OF PHOTOCATALYTIC DEGRADATION OF DIAZINON USING TITANIUM DIOXIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
MohammadMehdiBaneshi et al.
Ugurlu M, Karaoglu MH. (2009): Removal of AOX, total nitro-
gen and chlorinated lignin from bleached Kraft mill ef uents
by UV oxidation in the presence of hydrogen peroxide utiliz-
ing TiO 2 as photocatalyst. Environmental Science and Pollu-
tion Research. 16(3):265-73.
Mesgar i Z, Gharagozlou M, Khosravi A, Gharanjig K. (2012):
Spectrophotometric studies of visible light induced photo-
catalytic degradation of methyl orange using phthalocya-
nine-modi ed Fe-doped TiO
2
nanocrystals. Spectrochimica
Acta Part A: Molecular and Biomolecular Spectroscopy. 92:
148-53.
Liu G, Zhang X, Xu Y, Niu X, Zheng L, Ding X. (2005): The
preparation of Zn2+-doped TiO2 nanoparticles by sol–gel and
solid phase reaction methods respectively and their photocata-
lytic activities. Chemosphere. 59:1367–71.
Mekpra sart W, Pecharapa W. (2011): Synthesis and characteri-
zation of nitrogen-doped TiO2 and its photocatalytic activ-
ity enhancement under visible light. Energy Procedia. 9:
509-14.
Zhou M , Yu J, Cheng B. (2006): Effects of Fe-doping on the
photocatalytic activity of mesoporous TiO2 powders prepared
by an ultrasonic method. Journal of hazardous materials.
137(3):1838-47. .
Sun L, Li J, Wang CL, Li SF, Chen HB, Lin CJ. (2009): An elec-
trochemical strategy of doping Fe3+ into TiO2 nanotube array
lms for enhancement in photocatalytic activity. Solar Energy
Materials and Solar Cells. 93:1875-80. .
Zhu J , Zheng W, He B, Zhang J, Anpo M. (2004): Characteriza-
tion of Fe-TiO2 photocatalysts synthesized by hydrothermal
method and their photocatalytic reactivity for photodegrada-
tion of XRG dye diluted in water. Journal of Molecular Cataly-
sis A: Chemical. 216(1):35-43. .
Liu S , Chen Y. (2009): Enhanced photocatalytic activity of TiO2
powders doped by Fe unevenly. Catalysis Communications.
10(6):894-9.
Rezaei kalanteri R, Dadban shahamat Y, Farzadkia M, Esraf-
ily A. (2014): Investigation of Photocatalytic Degradation of
Diazinon in Synthetic Wastewater Using Nano -TiO2/UV. Jour-
nal of Guilan University of Medical Sciences. 22(88):32-41.
Fadaei A, Sadeghi M. (2013): Ef cacy study on Advanced
Oxidation Processes (AOPs) application for pesticides removal
from water with emphasis on their cost aspects. Journal of
Shahrekord Uuniversity of Medical Sciences. 15(5):80-9.
Sorour i Zanjani R, Mir-Esmaili SM, Lati AM, ValiPour E.
(2009): Isolation and identi cation of a type strain bacteria
with the highest ability to produce organophosphorus acid
anhidrase. Journal of Mazandaran University of Medical Sci-
ences. [Research(Original)]. 18(68):19-26.
Samadi MT, Khodadadi M, Rahmani AR, Allahresani A, Saghi
MH. (2010): Comparison of the ef ciency of simultaneous
application of UV/O3 for the removal of organophosphorus
and carbamat pesticides in aqueous solutions. Water Waste-
water. 73:69-75.
c
´
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INVESTIGATION OF PHOTOCATALYTIC DEGRADATION OF DIAZINON USING TITANIUM DIOXIDE 67