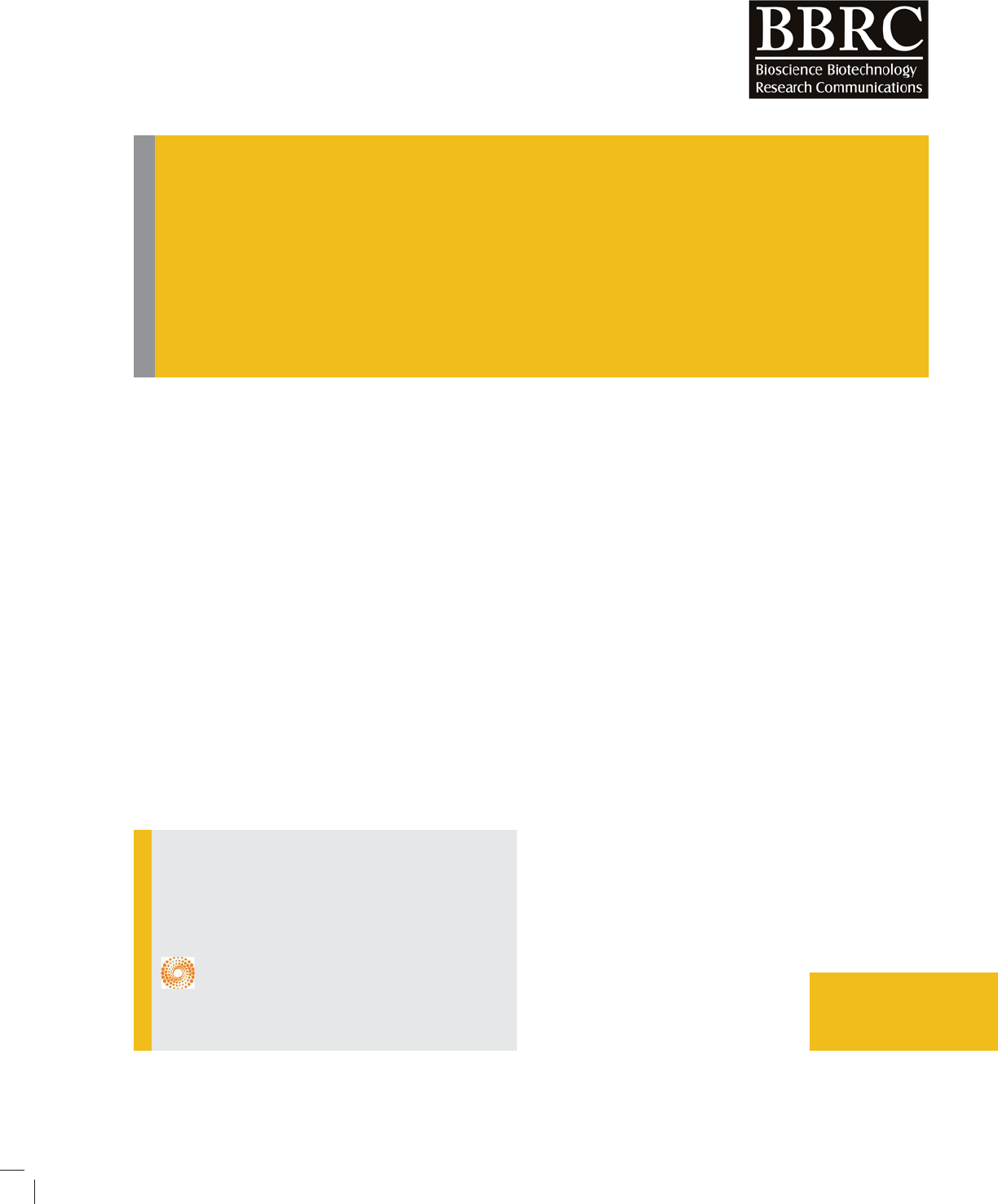
Enhanced re retardancy of poly methyl methacrylate
by combination with aluminium hydroxide and
magnesium hydroxide
A. Ebdam
1
, S. Jameh-Bozorghi
2
, M. Youse
3
* and A. Niazi
1
,
1Department of Chemistry, Arak Branch, Islamic Azad University, Arak, Iran
2Department of Chemistry, Hamedan Branch, Islamic Azad University, Hamedan, Iran
3Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran
ABSTRACT
The use of polymeric materials in life, due to their unique properties such as low weight and easy process, signi -
cantly increased. But polymers are relatively high combustibility and most of them produce smoke and toxic and
corrosive gases during burning. As a result of modi cation of the polymer behavior against re is the main challenge
to make them more useful. Retardant additives reduce the risk of re and release of it. The performance of this class
of materials is by increase in combustion time, improve in in ammability of polymer, reduce in heat release rate and
prevent of dripping during burning. In this study, it is tried to improve thermal resistance of polymethyl methacrylate
PMMA by synthesis and characterization of nanoparticles of magnesium hydroxide and aluminum hydroxide nano-
particles by SEM and XRD and use it as llers for polymers, which is highly used in Industry. 4 composite samples
with ratios of 10/90, 20/80, 30/70 and 40/60 of PMMA and Mg(OH)2 and 4 composite samples with ratios of 5/5/90,
10/10/80, 15/15/70 and 2/20/40 of PMMA and Mg(OH)2 and Al(OH)3 were prepared and their thermal behavior was
studied. The results show that increasing the percentage of magnesium hydroxide in the composites, heat resistance
of polymer and the melting temperature and the percentage of residual mass in composite increased. The addition of
aluminum hydroxide to composite increased the thermal resistance and increased the percentage of residual mass in
some of them.
KEY WORDS: FIRE RETARDANCY, NANO ALUMINUM HYDROXIDE, POLY(METHYLMETHACRYLATE), NANO MG(OH)2, NANO COMPOSITE
54
ARTICLE INFORMATION:
*Corresponding Author: Myouse 50@hotmail.com
Received 27
th
Nov, 2016
Accepted after revision 31
th
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
Biosci. Biotech. Res. Comm. Special Issue No 1:54-59 (2017)

Ebdam et al.
INTRODUCTION
The research and developments on new engineering
materials belong to the important elds of material sci-
ence. One can see the continuous competition between
the traditional inorganic engineering materials and pol-
ymers. Since polymeric materials (including composites)
are promising, due to their economic versatile applica-
bility, they are widely used in many applications, such
as housing materials, transport and electrical engineer-
ing (Almeras et al. 2003; Anna et al. 2002; Bourbigot
et al. 1996a; Bourbigot et al. 1996b). These commercial
polymers are easily ammable. The ame retardant can
act in various ways i.e. physically or chemically. Many
types of ame retardants are used in consumer prod-
ucts (Troitzsch, 1990; Sain, 2004; Zhu, 2003). They are
mainly phosphorus, antimony, chlorides and bromides,
magnesium and boron-containing compounds (Seymour
et al. 1978).
Al(OH)3can act also as a reinforcing agent and smoke
suppressant additive with low or zero emissions of toxic
or hazardous substances. The main advantages of poly-
meric materials over many metal compounds are high
toughness, corrosion resistance, low density and ther-
mal insulation. Improvement of the ame retardancy
and thermal stability of polymers is a major challenge
for extending their use for most applications. The higher
level of ame retardancy of nanoparticles is due to their
bigger surface to volume fractions which let them dis-
perse into the polymeric matrix homogeneously, and
hence leads to formation of a compact char during the
combustion (Grigsby et al. 2005; Wang et al. 2007; Kul-
janin et al. 2006).
Poly(methyl methacrylate) (PMMA) isa highly am-
mable polymer, improving its thermal stability is very
important. There are several re retardants available in
the market. Although they improve the re resistance of
PMMA, they have a lot of disadvantages. Many addi-
tives are effective only at high loadings, generally from
10 wt% to 40 wt%, which changes the physical proper-
ties of the polymer. Most ame retardants additivesare
halogenated compounds (Bundersek et al. 2012a). On
the other hand, metaloxides are halogen-free products,
which can also be used for improving thermal stabil-
ity and re properties of PMMA. Aluminum hydrox-
ide (Al(OH)3) and magnesium hydroxide (Mg(OH)2) are
environmentally benign, butneed to be added in high
concentrations to be effective (Beyer, 2002). Trans-
parency of PMMA in the visible region is reason that
PMMA is an importantthermoplastic for numerous uses
(Beyer, 2005; Beyer, 2002; Chiang et al. 2009; Bundersek
et al. 2012b).
Inorganic compound magnesium hydroxide [Mg(OH)
2] as a smoking- and toxic-free additive has been exten-
sively used in halogen-free ame-retardant polymeric
materials. However, its fatal disadvantages are low
ame-retardant ef ciency and thus very large usage
amount, which lead the mechanical properties of a ame-
retardant polymeric material to drop down sharply. The
surface modi cation of magnesium hydroxide in order
to increase the compatibility between Mg(OH)2 particles
and polymers can enhance the mechanical strength of
composites (Wang et al. 2001).
Finally, to improve the properties of composites
made of PMMA, some changes in proposed structures
are necessary. Therefore, in this study, Nano-magnesium
hydroxide and aluminum hydroxide nanoparticles as
mineral retardant llers are synthesized and identi ed
by XRD and SEM. Then, composites of PMMA with dif-
ferent ratios of Mg (OH) 2 were built DSC-TGA and LOI
test were carried out on them. At the end, composites
composed of PMMA with different ratios of Mg(OH)2
and Al(OH)3 were made and the same tests with the
previous composites were done on them and thermal
behavior of composites containing aluminum hydrox-
ide and composites lacking aluminum hydroxide were
compared.
MATERIALS AND METHODS
All materials and solvents used in the synthesis of Nano
magnesium hydroxide and Nano aluminum hydroxide
were purchased from Sigma Aldrich and Merck and used
without any further puri cation. the melting points (ºC)
of the complexes were recorded on a Kruss instrument
and TG/DSC curves were obtained from a Diamond TGA
PerkinElmer 60 Hz. Poly methyl methacrylate (PMMA)
was obtained from I Tech polymer company Iran with
melt mass ow rate (MFR) of 0.9 – 27 g/10 min at 125ºC
and density 1.15 ‐ 1.19 g/cm3.. Infrared spectra were
recorded as KBr disks on Tensor 27 Bruker spectropho-
tometer. The evaluation of Al(OH)3oxide and synthe-
sized Nano composites were monitored by powder X-ray
diffraction Philips PW 1800 diffractometer with Cu K
radiation. Atomic force microscopy was carried out on
a Denmark Dual scope/Raster scope C26, DME micro-
scope. Scanning electron microscopy measurements was
performed on a VEGA\\TESCAN at an accelerating volt-
age of 15 kV. The LOI values were measured using a ZRY
type instrument (made in China) on the sheets of120 · 60
·3 mm3 according to ASTM D2863-77 standard.
2.1 Synthesis of Nano magnesium hydroxide
To prepare magnesium hydroxide nanoparticles, rst 5
g (MgSO4.7H2O) was solved in 40 ml of deionized water
and 5 ml of sodium hydroxide was added to it. Then, the
obtained solution was stirred with 1600 rpm for 1 hour
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ENHANCED FIRE RETARDANCY OF POLY METHYL METHACRYLATE 55
Ebdam et al.
in 60 ºC. The obtained solution was passed from Nano
lter paper by Buchner funnel and then was rinsed with
deionized water in order to completely rinse the Ammo-
nia. The remaining sediment was put in an oven with
105 ºC for 8 hours and nally magnesium hydroxide
nanoparticles will be synthesized.
2.2 Synthesis of Nanoaluminum hydroxide
1g Al(NO3)2 9H2O were dissolved 33 ml of water. Then
various precipitation agents ethylenediamine, propylene
diamine, triethylenetetramine, tetraethylenepentamine
were added until the pH of the solution adjust to 8. A
white precipitate is obtained con rming the synthesis
of Al(OH)3. The white precipitate was centrifuged and
washed with distilled water to removing the surfactant,
and later dried at 70°C for 24h in a vacuum dryer.
Preparation of PMs composites
In this study, 4 samples with different percent of mag-
nesium hydroxide and PMMA were prepared to study
thermal properties that were named as follows:
To prepare the composite samples, a two-armed mixer
device was used and its temperature and speed were
adjusted respectively as 110ºC and 60 rpm and then 10
minutes was considered for the formation of composites.
2.4 Preparation of PMAs composites
To study the effect of aluminum hydroxide nanoparti-
cles in retardaning PMs composite samples, 4 samples of
PMAs composite were prepared with different percent of
aluminum hydroxide, magnesium hydroxide and PMMA
and then were named as follows:
To prepare the composite samples, a two-armed mixer
device was used and its temperature and speed were
adjusted respectively as 110ºC and 50 rpm and then 15
minutes was considered for the formation of composites.
Thermal gravimetric
The tests related to differential thermal analysis were
performed by TGA device under ASTM-E1131 standard.
Thermal analysis, changes in the sample weight (los-
ing the weight of the sample that is controlled under
a heating program) is considered as the basis of Ther-
mogravimetry analysis (TG) and test conditions are
similar for all produced composites and the temperature
increases to 10 centigrade per minute and the tempera-
ture range from ambient conditions is to 600 ºC.
Characterization of Mg(OH)2 Nanoparticlesand of
Al(OH)3Nanoparticles
The typical powder XRD pattern of Mg(OH)2 nano-
particlesis given in Fig. 1. All diffraction peaks canbe
indexed as the hexagonal structure of Mg(OH)2 with the
lattice constants comparable to the values of JCPDS7-
239. No XRD peaks arising from impurities such as NaCl
and MgO were detected. Moreover, the peaks of the sam-
ples are signi cantly broadened. This indicates that the
Mg(OH)2 particles have a very small grain size, which
can be calculated from the broadened XRD peaks by
means of Scherrer formula (Staudenmaier, 1898).
XRD pattern of Al(OH)3 nanoparticles is shown in
Fig. 2. The pattern of as prepared Al(OH)3 nanopar-
ticles is indexed as a pure monoclinic phase (space
group:P21/n) which is very close to the literature values
(JCPDS No. 33-0018), the narrow sharp peaks indicate
that Al(OH)3 nanoparticles are well crystallized.
The crystallite size measurements were also carried
out using the Scherrer equation, Dc = K/Cos, Where
b is the width of the observed diffraction peak at its half
maximum intensity (FWHM), K is the so-called shape
factor, which usually takes a value of about 0.9, and is
the X-ray wavelength (CuK radiation, equals to 0.154
nm). The estimated crystallite size is about 8 nm.
Table 1. The properties of PMs composite samples
Composite Compounds
PM-10PMMA 90% - Mg(OH)210%
PM-20PMMA80% - Mg(OH)220%
PM-30PMMA70% - Mg(OH)230%
PM-40PMMA60% - Mg(OH)240%
Table 2. The properties of the produced composite
samples
Composite Compounds
PMA-10PMMA90% - Mg(OH)25%-Al(OH)35%
PMA-20PMMA80% - Mg(OH)210%-Al(OH)310%
PMA-30PMMA70%- Mg(OH)215%-Al(OH)315%
PMA-40PMMA60% - Mg(OH)220%-Al(OH)320%
FIGURE 1. Effect of vemicompost and azotobacter on
kernel weight
56 ENHANCED FIRE RETARDANCY OF POLY METHYL METHACRYLATE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Ebdam et al.
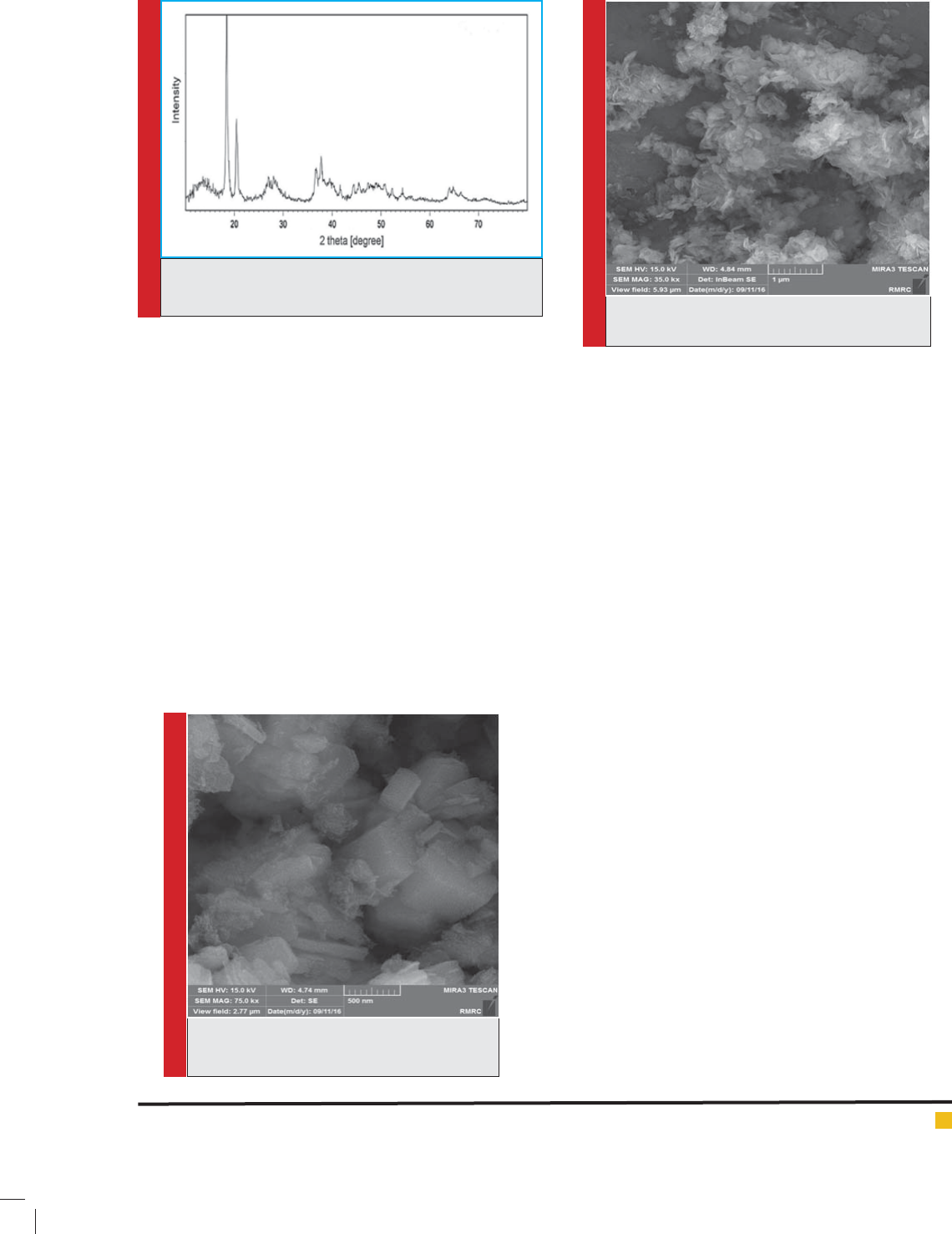
Scanning electron microscopic analysis (SEM) on
magnesium hydroxide nanoparticles was shown in g-
ure 3.and Scanning electron microscopic analysis (SEM)
on aluminum hydroxide nanoparticles was shown in
gure 4. As is clear, the magnesium hydroxide nano-
particles like aluminum hydroxide nanoparticles have a
good and uniform distribution.
Thermal analysis
The results of the thermal behavior of composites PMs in
Table 4 and the results of the thermal behavior of PMAs
composites are shown in Table 5. Degradation began in
PMs series of composite with increasing Mg(OH)2 nan-
oparticles in three samples are almost identical an at
the nal sample due to changes in the physical struc-
ture of PMMA it was decreased. The peak temperature
of degradation and thermal resistance of composites in
Table 4, increases from top to bottom that shows the effect
of Nano-Mg(OH)2 nanoparticles in burning behavior of
composites this series. In Table 5, thermal behavior of
composites of PMAs series is provided and the differ-
ence over PMs series is the nanoparticles of Al(OH)3 in
produced composites structure. It is well shown that the
temperature composites. Degradation temperature in
PMAs series is not much different from PMs compos-
ite series but peak temperature of degradation in PMAs
composite series improved by average of 7 ° C in PMs
series.
Analysis of the residual mass of the composite material
The results of the composite residual mass after thermal
analysis is provided in Table 6 for composites of PMs
Series and in Table 7 it is provided for composites of
PMAs. It is well shown that by increasing the amount of
retardant in the composite structure, a smaller percent-
age of them disappears. By comparing Tables 6 and 7 it
clear that apart from PMA-20 composite that its percent-
age of residual mass compared to composite of PM-20 is
signi cantly improved, the percentage of residual mass
of other composites in Tables 6 and 7 are almost the
same in comparison.
Limited Oxygen Index (LOI) analysis
LOI test results of PMs composites and PMAs compos-
ites series are respectively shown in Figure 5 in Figure
6. It is well shown that by increasing the percentage of
Mg(OH)2, the amount of oxygen required for combus-
tion of samples has increasing trend. LOI of pure PMMA
sample was 17 % and with an increase of Mg(OH)2 in
PMMA it had a rising trend so that LOI of PM-40 that
40% of composite products is Mg(OH)2 is 30. In the case
of Figure 6, the same trend is visible and LOI of PAM-
FIGURE 2. Effect of vemicompost and azotobacter on ker-
nel weight
FIGURE 3. Effect of vemicompost and azotobac-
ter on kernel weight
FIGURE 4. Effect of vemicompost and azoto-
bacter on kernel weight
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ENHANCED FIRE RETARDANCY OF POLY METHYL METHACRYLATE 57

Ebdam et al.
40 composite is 31.5 that 20% of produced sample is
Mg(OH)2 and 20% is Al(OH)3.
By comparing Figures 5 and 6, the close test results of
LOI between PMAs and PMs series composites is realized
and it is clear that except PMA-20composite, which had
about 22.5 % favorable performance than PM-20 com-
posites, other sample results are almost the same.
RESULTS AND DISCUSSION
Given the importance and high consumption of poly
methyl methacrylate (PMMA) in different industries
including construction, automotive, electronics, etc.
in this project we tried to improved thermal resistance
of PMMA using magnesium hydroxide and aluminum
hydroxide nanoparticles as additives. Although the
addition of llers weakens the mechanical and physical
properties and behavior of the polymer. According to
Table 3. Thermal behavior of PMs composites Series
Sample
First Temperature of
demolition ºC
Peak Temperature of
demolition ºC
End Temperature of
demolition ºC
PM-10199301425
PM-20198300430
PM-30200330442
PM-40190420490
Table 4. Thermal behavior of PMAs composites Series
Sample
First Temperature
of demolition ºC
Peak Temperature
of demolition ºC
End Temperature of
demolition ºC
PMA-10200298430
PMA-20200301436
PMA-30220331450
PMA-40188410495
Table 5. Amount of residual mass
after the thermal Analysis in PMs
composites Series
Sample
The Remaining
Percentage
PM-103.22
PM-208.70
PM-3024.67
PM-4049.33
Table 6. Amount of residual mass
after the thermal Analysis in
PMAs composites Series
Sample
The Remaining
Percentage
PMA-104.38
PMA-2021.05
PMA-3026.56
PMA-4049.19
FIGURE 5. Effect of vemicompost and azotobacter
on kernel weight
FIGURE 6. Effect of vemicompost and azotobacter on ker-
nel weight
58 ENHANCED FIRE RETARDANCY OF POLY METHYL METHACRYLATE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Ebdam et al.
the results, resistance of composite made from magne-
sium hydroxide particles against re increased and melt
rate increased as well. Increase in the amount of mag-
nesium hydroxide in composite improved the residual
mass of composite. The LOI test results show that com-
posite had achieved a good resistance against re and
smoke of process of burning is reduced. By comparing
the degradation temperature magnesium hydroxide and
aluminum hydroxide nanoparticles it can be concluded
that the degradation temperature was between 180° C
-200 ° C and given the degradation reaction reduced
the ammability of polymer and produced Al2O3 pro-
vides a thermal insulation coating and the resulting
water vapor diluted the ammable gases and provided
a thin protective gas layer. However, due to lower deg-
radation point compared to magnesium hydroxide,
when it was used in PMAs composites it decreased the
peak degradation temperature of the composite com-
pared to degradation temperature of PMs series. It is
because of Magnesium hydroxide nanoparticles degra-
dation temperature, which is about 350° C. The residual
mass percentage of composite and LOI test results indi-
cated an improvement in polymer properties of PMAs
series.
Generally, by comparison of eight composites, aver-
age degradation temperature is more favorable in PMAs
series and according to initial thermal resistance against
the destruction of the best examples of PAM-30 is made
of composite.
REFERENCES
Almeras X, Le Bras M, Hornsby P, Bourbigot S, MarosiGy,Keszei
S. (2003): Effect of llers on the re retardancy of intumescent
polypropylene compounds. Polymer Degradation and Stabil-
ity; 82(2):317-23.
Anna P, MarosiGy, Bourbigot S, Le Bras M, Delobel R. (2002):
Intumescent ame retardant system of modi ed rheology. Pol-
ymer Degradation and Stability; 77:243-7.
Beyer G., (2002): Plast. Additive. Compound, 4, 22.
Beyer G., (2005): Plast. Additive. Compound, 7, 32.
Bourbigot S, Le Bras M, Breant P, Tremillon JM, Delobel R.
(1996): Zeolite new synergistic agents for intumescent re
retardant thermoplastic formulations criteria for the choice of
the zeolite. Fire and Materials; 20(3).
Bourbigot S, Le Bras M, Delobel R, Decressain R, Amourex JP.
(1996): Synergistic effect of zeolite in an intumescent process
study of the carbonaceous structure usıng solid-state NMR.
Faraday Transactions; 92(1):149-58.
Bundersek A., B. Japelj, B. Music, N. Rajnar, and P. Krajnc,
(2012a): Midem, 48thInternational Conference on Microelec-
tronics, Devices and Materials, Otocec.
Bundersek A., B. Japelj, B. Music, N. Rajnar, and P. Krajnc,
Midem, (2012b): 48th International Conference on Microelec-
tronics, Devices and Materials, Otocec.
Chiang C.L. and Chiu S.L., (2009): J. Polym. Res., 16, 637.
Grigsby W. J., Ferguson C. J., Franich R. A., Russell G.T., Int. J.
(2005): Adhes. Adhes. 25:127–137.
Kuljanin J., M. I. Comor, V. Djokovic, and J. M. Nedeljkovic.
(2006): Mater. Chem. Phys. 95: 67–71.
Sain M, Park SH, Suhara F, Law S. (2004): Flame retardant and
mechanical properties of natural bre-PP composites contain-
ing magnesium hydroxide. Polymer Degradation and Stability:
83:363-7
Seymour RB. (1978): Additives for plastic. New York: Aca-
demic Press.
Staudenmaier L., (1898): Ber Dtsch Chem Ges. 31:1481.
Troitzsch J. (1990): International plastics ammability hand-
book. New York: Hanser Pub.
Wang H., Fang P., Chen Z., Wang S., (2007): Appl. Surf. Sci.
Synthesis and characterization of CdS/PVA nanocomposite
lms. 253:8495–8499.
Wang ZZ, Qu BJ, Fan WC, Huang P. (2001): Combustion char-
acteristics of halogen-free ame-retarded polyethylene con-
taining magnesium hydroxide and some synergists. J Appl
Polym Sci; 81(1):206–14.
Zhu S, Shi W. (2003): Thermal degradation of a new ame
retardant phosphate methacrylate polymer. Polymer Degrada-
tion and Stability; 80:217-22.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ENHANCED FIRE RETARDANCY OF POLY METHYL METHACRYLATE 59