The role of probiotics in nosocomial infections
Z. Mahmmudi
1
and A.A. Gorzin*
2
1
M.Sc. in Biology, Kazeroun Branch, Islamic Azad University, Kazeroon, Iran
2
Assistant Professor of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences,
Shiraz, Iran
ABSTRACT
There is an increasing scienti c and commercial interest in the use of bene cial microorganisms, or “probiotics,” for
the prevention and treatment of disease. The microorganisms most frequently used as probiotic agents are lactic-acid
bacteria such as Lactobacillus rhamnosus GG (LGG), which has been extensively studied in recent literature. Multiple
mechanisms of action have been postulated, including lactose digestion, production of antimicrobial agents, competi-
tion for space or nutrients, and immunomodulation. We have reviewed recent studies of probiotics for the treatment
and control of infectious diseases. Studies of pediatric diarrhea show substantial evidence of clinical bene ts from
probiotic therapy in patients with viral gastroenteritis, and data on LGG treatment for Clostridium dif cile diarrhea
appear promising. However, data to support use of probiotics for prevention of traveler’s diarrhea are more limited.
New research suggests potential applications in vaccine development and prevention of sexually transmitted dis-
eases. Further studies are needed to take full advantage of this traditional medical approach and to apply it to the
infectious diseases of the new millennium.
KEY WORDS: INTENSIVE CARE UNITS; PEDIATRIC; CROSS INFECTION; BACTEREMIA; PNEUMONIA; URINARY TRACT INFECTION; PROBIOTICS
48
ARTICLE INFORMATION:
*Corresponding Author:
Received 27
th
Nov, 2016
Accepted after revision 29
th
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Despite marked improvements in antimicrobial ther-
apy and critical care technology, nosocomial infection
remains a signi cant cause of morbidity and mortality
in critically ill patients (Salminen et al. 1998; Savaiano
et al, 1984; DeVrese et al. 2001; Kim and Gilliland, 1983).
Because the nal common pathway of Gram-negative
bloodstream infection, ventilator-associated pneumo-
nia, and urinary tract infection (UTI) involves patho-
genic enteric organisms, recent interest has emerged in
how to suppress the growth of these organisms. Mul-
tiple studies have demonstrated that the colonization
of the bowel with nonpathogenic commensal bacteria
Biosci. Biotech. Res. Comm. Special Issue No 1:48-53 (2017)
Mahmmudi and Gorzin
(probiotics) competitively inhibits the attachment of
these pathogenic organisms (Kolars et al. 1984; Allen et
al. 2003; Guandalini et al. 2000). In addition, probiotics
have been shown to augment the local gut immunity by
enhancing immunoglobulin (Ig)-A–speci c responses to
enteric pathogens (Shornikova et al. 2003; Pant et al.
1996). Probiotics also are thought to produce a variety
of antimicrobial substances that may interfere with the
growth of pathogenic bacteria (Raza et al. 1995; Sepp
et al. 1995; Szajewska et al. 2001; Mastretta et al. 2002).
Finally, probiotics have been shown in numerous animal
models to reduce intestinal permeability and decrease
the bacterial translocation of pathogenic bacteria (Ober-
helman et al. 1999; Shornikova et al. 1997; Cetina-Sauri
and Sierra Basto, 1994).
Moreover, probiotics also have been shown to non-
speci cally stimulate the systemic immune system. Pro-
biotic bacteria have been shown in several studies to
enhance the phagocytic ability of neutrophils (Ho¨chter
et al. 1990; Arvola et al. 1999). Multiple trials also have
demonstrated an improvement in natural killer cell
activity following the administration of various probi-
otic agents (Vanderhoof et al. 1999). Probiotic intake also
has been shown to modulate production of interleukin-6
and -10, as well as tumor necrosis factor- (Armuzzi et
al. 2001a). Speci c stimulation of the systemic immune
system also has been shown using probiotic bacteria as
vehicles for vaccines with resultant increases in antigen
speci c T-cell and immunoglobulin G responses (Cre-
monini et al. 2002; Armuzzi et al. 2001b).
As a result of these studies demonstrating stimulation
of local and systemic immune defenses and a reduction in
bacterial translocation, there has been a rapidly growing
interest in the clinical applications of probiotics. A few
small clinical trials in intensive care settings have begun
looking at the incidence of nosocomial infections with
probiotic use and have demonstrated promising results
(Siitonen et al. 1990). Therefore, the purpose of this study
was to evaluate the hypothesis that the administration of
probiotics in infants and children admitted to a pediatric
intensive care unit setting would reduce the incidence of
nosocomial infection, bloodstream infection, pneumonia,
tracheobronchitis, and UTI.
MATERIAL AND METHODS
Episode occurring after 48 hours of hospitalization,
resulting in a positive blood, CSF, or urine culture.4 Hos-
pital-acquired bloodstream infection: clinical signs of
sepsis occurring after 48 hours of life and followed by a
positive blood culture drawn after 48 hours of life. If cul-
ture was positive for a coagulasenegative Staphylococ-
cus species, an additional positive culture with the same
organism was required for con rmation and treatment.
Nosocomial pneumonia: development of respiratory dis-
tress after 48 hours of hospitalization evidenced by rapid,
noisy, or dif cult breathing, respiratory rate .60 breaths
per minute, chest retractions or grunting, and con rmed
with a chest radiograph, a blood culture, or additional
blood work. If the chest radiograph was suggestive of
pneumonia and the blood culture was negative, clini-
cal signs of sepsis or laboratory tests were required for
diagnosis (Duke28 modi ed de nition). Chest radiograph
suggestive of pneumonia: presence of nodular or coarse
patchy in ltrate, diffuse haziness, or granularity, or lobar
or segmental consolidation. Clinical signs of sepsis: pres-
ence of lethargy, recurrent apnea, hypothermia (axillary
temperature ,37°C) or hyperthermia (.38°C).
Laboratory tests suggestive of sepsis: a leukocyte
count out of the reference range (neutropenia ,5000 or
neutrophilia .25 000), a ratio of immature to total neu-
trophilic forms .0.2 or an elevated C-reactive protein. Uri-
nary tract infection: clinical signs of sepsis and a positive
urine culture with .104 organisms of a single pathogen
obtained by the use of standard sterile technique and ure-
thral catheterization.4 Meningitis: clinical signs of sepsis
with a CSF white blood cell count .29/mm3 and neutro-
phil count .60%, or a positive CSF Gramstain, culture, or
polymerase chain reaction for bacterial antigens.4 Feed-
ing intolerance: any of the following: recurring emesis,
gastric residuals with 50% or more of the previous feed
volume, abdominal distension, or the presence of macro-
scopic blood in stools. Necrotizing enterocolitis: modi -
cation of Bells criteria for stage II29 based clinical and/
or radiographic data: (1) pneumatosis or portal vein gas,
(2) localized pneumatosis, xed dilated bowel loops, or
pneumoperitoneum AND 2 GI signs/ symptoms and 1 sys-
temic sign/ symptom, or (3) thickened bowel loops AND
an abnormal gas pattern AND 2 GI and 2 systemic signs/
symptoms. GI signs: abdominal distension or tenderness,
feeding intolerance, erythema of the abdominal wall,
and decreased bowel sounds. Systemic signs: lethargy,
increased frequency or severity of apnea, temperature
instability, new-onset metabolic acidosis, hemodynamic
instability, and disseminated intravascular coagulation or
thrombocytopenia.
RESULTS AND DISCUSSION
DOCUMENTATION OF THE HEALTH EFFECTS
OF PROBIOTICS FOR HUMAN DISEASES AND
DISORDERS
Lactose malabsorption. A large number of people, as
they age, experience a decline in the level of lactase
(bgalactosidase) in the intestinal brush border mucosa.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE ROLE OF PROBIOTICS IN NOSOCOMIAL INFECTIONS 49
Mahmmudi and Gorzin
This decline causes lactose to be incompletely absorbed,
resulting in atus, bloating, abdominal cramps, and
moderate-to-severe (watery) diarrhea. This results in
a severe limitation in consumption of dairy products
among the elderly population. There have been several
studies that have demonstrated that, during the fermen-
tative process involved in the production of yogurt,
lactase is produced, which can exert its in uence in
the intestinal tract (Savaiano et al, 1984; DeVrese et al.
2001; Kim and Gilliland, 1983; Kolars et al. 1984). The
organisms commonly used for the production of yogurt
are Lactobacillus bulgaricus and Streptococcus sali-
varius subsp. thermophilus. Kim and Gilliland (Kim and
Gilliland, 1983) found that feeding lactose-intolerant
individuals yogurt caused a signi cant reduction in the
level of breath hydrogen compared with that in subjects
who were fed milk. The level of hydrogen in the breath is
an indication of the extent of lactose metabolism in the
large bowel. Kolars et al. (Kolars et al. 1984) observed
that the ingestion of 18 g of lactose in yogurt caused the
production of 67% less hydrogen in the breath compared
with that produced by a similar dose of lactose deliv-
ered in milk. Analysis of aspirates obtained from the
duodenum 1 h after the consumption of yogurt showed
signi cant levels of lactase (Kolars et al. 1984). These
studies indicate that the delivery of lactase to the intes-
tine via the consumption of lactase-producing probiotics
is a practical approach for treatment of lactose malab-
sorption. Acute diarrhea. There are at least 12 studies
that have reported the use of probiotics to either treat
or prevent acute diarrhea (Allen et al. 2003; Guandalini
et al. 2000; Shornikova et al. 2003; Pant et al. 1996; Raza
et al. 1995; Sepp et al. 1995; Szajewska et al. 2001; Mas-
tretta et al. 2002; Oberhelman et al. 1999; Shornikova
et al. 1997; Cetina-Sauri and Sierra Basto, 1994; Ho¨chter
et al. 1990). The majority of these studies were done
with infants or children, the etiologic agent was either
rotavirus or unknown, and the probiotic used was Lacto-
bacillus rhamnosus strain GG (Lactobacillus GG) (ATCC
53103) (Guandalini et al. 2000; Shornikova et al. 2003;
Pant et al. 1996; Raza et al. 1995; Sepp et al. 1995; Sza-
jewska et al. 2001; Mastretta et al. 2002; Oberhelman
et al. 1999). Other probiotics that have shown positive
results for the treatment of acute gastroenteritis include
Lactobacillus reuteri and Saccharomyces boulardii
(Shornikova et al. 1997; Cetina-Sauri and Sierra Basto,
1994; Ho¨chter et al. 1990). The European Society for
Pediatric Gastroenterology, Hepatology, and Nutrition
conducted the most extensive trial using Lactobacillus
GG for the treatment of moderate-to-severe diarrhea in
children (Guandalini et al. 2000). The study included
287 children aged 1–36 months from 10 countries. The
patients were randomized to be given either placebo or
Lactobacillus GG along with the standard treatment, oral
rehydration solution. Patients who received Lactobacil-
lus GG had decreased severity and shorter duration of
illness and a shorter hospital stay and were found to
have a decreased likelihood of persistent diarrheal illness
(Guandalini et al. 2000).
A similar study was conducted with 137 children
aged 1–36 months who were admitted to the hospital
with diarrhea and were randomized to receive placebo
or Lactobacillus GG plus oral rehydration solution. Chil-
dren given Lactobacillus GG had a signi cantly shorter
duration of illness (Shornikova et al. 2003). A study of
26 children in Thailand with watery diarrhea showed
a signi cantly shorter duration of symptoms for those
who received treatment with Lactobacillus GG (Pant
et al. 1996). A similar investigation involving 40 chil-
dren that was conducted in Pakistan found that those
who received treatment with Lactobacillus GG were less
likely to have persistent diarrhea and had fewer episodes
of vomiting, compared with the placebo group (Raza
et al. 1995). In a preventive study of 81 children aged
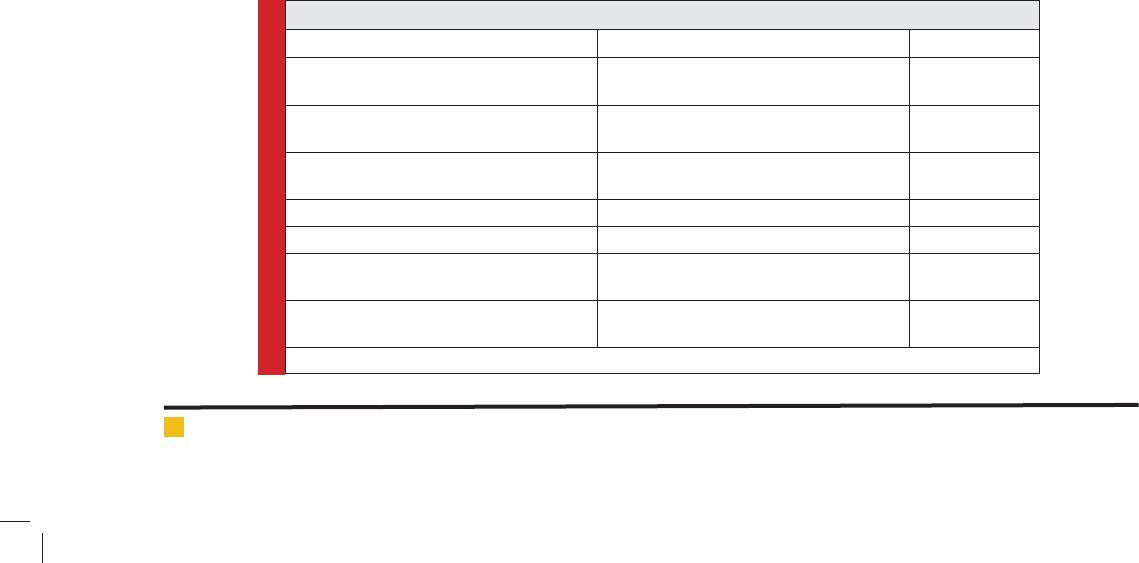
Table 1. Medical applications in humans for different classes of probiotics
Medical condition Class(es) of probiotic Reference(s)
Lactose maldigestion
LAB and Streptococcus salivarius subsp.
Thermophiles
[2–5]
Gastroenteritis Acute diarrhea
LAB, Bi dobacterium species, or
Saccharomyces boulardii
[6–17]
Antibiotic-associated diarrhea LAB or S. boulardii
[18–24]
Traveler’s diarrhea LAB [25, 26]
Clostridium dif cile–induced colitis LAB [32–34]
Dental caries
LAB [35]
Intestinal in ammation in children with
cystic brosis
LAB [36]
NOTE. LAB, lactic acid bacteria.
50 THE ROLE OF PROBIOTICS IN NOSOCOMIAL INFECTIONS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mahmmudi and Gorzin
1–36 months who were hospitalized for illnesses other
than diarrhea, symptoms of hospital-acquired rotavirus
gastroenteritis were prevented by administration of Lac-
tobacillus GG (Szajewska et al. 2001). In another preven-
tion study conducted in Peru, 204 children aged 6–24
months who were undernourished were randomized to
receive placebo or Lactobacillus GG. There was a signi -
cant decrease in the rate of incidence of diarrhea among
the children who received Lactobacillus GG who were
not being breast-fed (Oberhelman et al. 1999). In one
study, Lactobacillus reuteri was shown to shorten the
duration of diarrhea in children (Shornikova et al. 1997).
In a clinical trial involving 130 children, S. boulardii
was found to be effective for the treatment of acute diar-
rhea in children (Cetina-Sauri and Sierra Basto, 1994),
and, in another study of 92 adults, a similar nding was
reported (Ho¨chter et al. 1990).
Probiotic Use and Safety Probiotics are widely consid-
ered to be safe for human oral and vaginal use and there
is a long history of the use of fermented milk products
with minimal recorded reported side effects. The number
of probiotic products available on the world market is
estimated to be over 2000 (Shornikova et al. 2003), but
the industry remains largely unregulated and unstand-
ardized—making comparative studies dif cult. To begin
lling this void, scientists have formalized groups such
as the International Scienti c Association for Probiotics
and Prebiotics (ISAPP), a nonpro t founded in 2002 to
raise the scienti c credibility of the eld by working
with experts and conducting meetings on high qual-
ity research. By providing an objective, science-based
voice, ISAPP hopes to bene t the end users of these
products by helping them make informed choices (Pant
et al. 1996). ISAPP has endorsed the guidelines set by
the World Health Organization (WHO) and the United
Nations Food and Agriculture Organization (FAO) for
evaluation of probiotics—governing, strain designation,
ef cacy/effectiveness and safety (Kim and Gilliland,
1983; Raza et al. 1995). For example, new strains and
products should be proven safe in human studies amend
those bearing some limitations, (such as use of S. bou-
lardii [S. cerevisiae]) in patients with a leaky gut or other
risks) should be clearly labeled (Sepp et al. 1995). In
the United States, probiotics are currently classi ed as —
dietary supplements, (not —drugs) and as such, the Food
and Drug Administration (FDA) only requires premar-
ket noti cation, with no demonstrations of safety and
ef cacy required (Szajewska et al. 2001). Due to their
overall safety, guidelines for use of probiotics in the
hospital are generally lacking, although some caution is
advised for use in certain disease states (e.g., severe coli-
tis, bowel leaks, neutropenia) where the potential exists
for the probiotic to enter the blood or peritoneum (Mas-
tretta et al. 2002). Likewise, special care should be taken
by healthcare personnel who handle both probiotic cap-
sules and venous catheters in order to avoid transfer to
the bloodstream (Szajewska et al. 2001). Of more recent
interest and concern are safety considerations relating to
transferable genetic elements that may confer antibiotic
resistance from the probiotic to pathogenic strains, or
even to the commensal ora (Oberhelman et al. 1999).
In a mouse model have demonstrated a possible role
for these agents in the prevention or treatment of graft-
versus-host disease in transplant recipients (ksanen
et al. 1990).
FUTURE DIRECTIONS
The following are some of the future possibilities for
these biological products in the eld of infectious dis-
eases.The use of LAB as live vectors for oral immuniza-
tion appears to be an exciting approach, on the basis of
their safety, ability to persist within the indigenous ora,
adjuvant properties, and low intrinsic immunogenicity.
Medaglini et al. [38] have recently developed a genetic
system for the expression of heterologous antigens from
human papillomavirus and HIV type 1 (HIV-1) in the
surface of the human commensal Streptococcus gordoo-
nii and L. casei. Local and systemic immune responses
were detected in BALB/c mice and Cynomolgus mon-
keys after vaginal colonization with the aforementioned
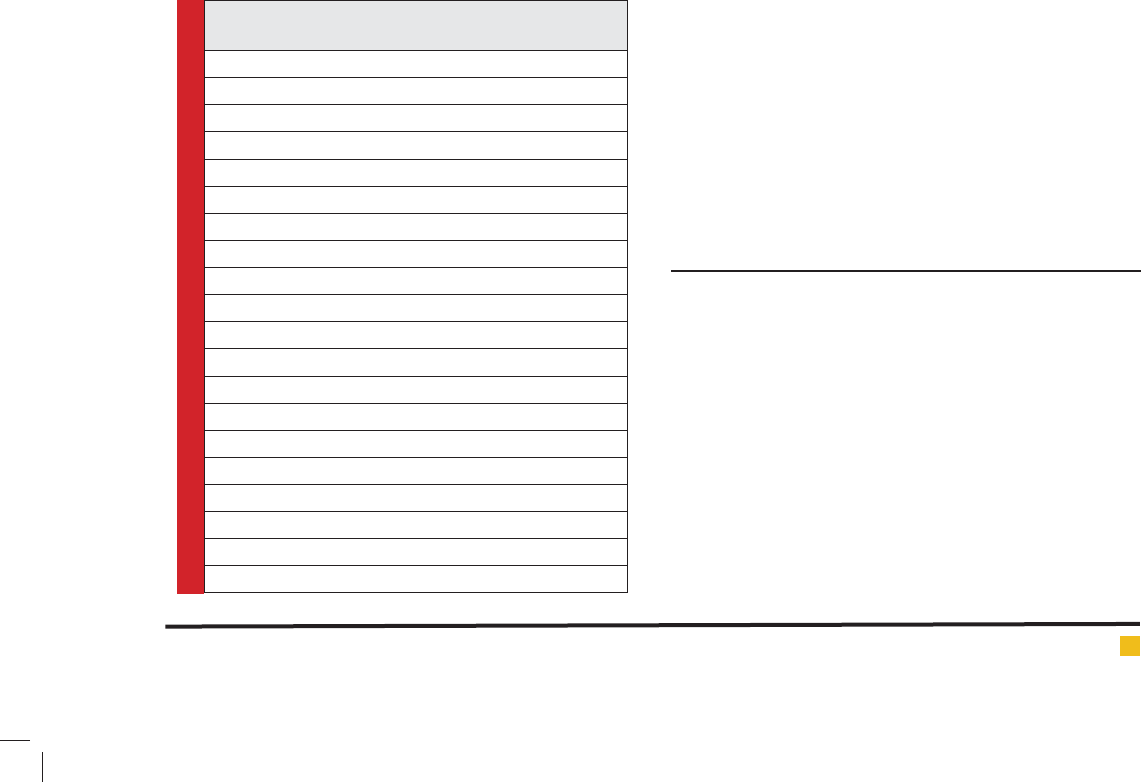
Table 2. Present and future clinical applications of
probiotics, by level of evidence of ef cacy
Applications with strong evidence
Gastroenteritis
Acu
Antibiotic associated
Applications with substantial evidence of ef cacy
Allergic reactions, speci cally atopic dermatitis
Applications that have shown promise
Childhood respiratory infection
Dental caries
Nasal pathogens
Relapsing Clostridium dif cile-induced
Gastroenteritis (prevention)
In ammatory bowel disease
Potential future applications
Rheumatoid arthritis
Irritable bowel syndrome
Cancer (prevention)
Ethanol-induced liver disease
Diabetes
Graft-versus-host disease
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE ROLE OF PROBIOTICS IN NOSOCOMIAL INFECTIONS 51
Mahmmudi and Gorzin
recombinant strains. Both macrophage activation and
IL-12/g-IFN pathway stimulation are promising areas of
research with regard to resistance to intracellular patho-
gens by enhancement of mucosal and systemic immu-
nity (Malchow et al. 1997; Guslandi et al. 2000). More
experimental and clinical studies are needed to clarify
the role of probiotics as immunomodulators, not only in
infectious diseases of the GI tract, but also for in amma-
tory and allergic conditions.
CONCLUSIONS
The current and proposed uses of probiotics cover a wide
range of diseases and ailments. An attempt has been made
to classify the quality of evidence that supports these var-
ious applications (Nase et al. 2001). These classi cations
are based on existing studies, most of which are cited in
this article, and not on an exhaustive review of the entire
literature on probiotics. The broad classi cations include
(table 2) applications with proven bene ts, applications
with substantial evidence that require additional sup-
port, promising applications that need substantial addi-
tional evidence, and proposed future applications. Proven
bene ts of probiotics include the treatment of acute and
antibiotic associated diarrhea; applications with substan-
tial evidence include the prevention of atopic eczema and
traveler’s diarrhea; promising applications include the
prevention of respiratory infections in children, preven-
tion of dental caries, elimination of nasal pathogen car-
riage, prevention of relapsing C. dif cile– induced gastro-
enteritis, and treatment of in ammatory bowel disease;
and proposed future applications include the treatment
of rheumatoid arthritis, treatment of irritable bowel syn-
drome, cancer prevention, prevention of ethanol-induced
liver disease, treatment of diabetes, and prevention or
treatment of graft versus-host disease.
The mechanisms of action of probiotics are strain
speci c but can be summarized mainly in three areas:
changes of gut ecology, modulation of gut mucosal bar-
rier and regulation of the immune response through
interaction with gut-associated immune system (Sava-
iano et al, 1984). Several studies regarding the supple-
mentation of probiotics in nosocomial infections have
been conducted mainly in adult population. Among
pediatric studies major ndings have been observed in
treatment of acute gastroenteritis, primarily caused by
Rotavirus (DeVrese et al. 2001; Kim and Gilliland), and
in the prevention of antibiotic associated diarrhea (AAD)
(Kolars et al. 1984). Supplementation with probiotics has
proven useful even in the treatment of Clostridium dif -
cile disease (CDD), the most common pathogen involved
in AAD (1983Allen et al. 2003). Data from meta-analysis
and cochrane review on the prevention of necrotizing
enterocolitis (NEC) show an overall bene t of probi-
otic supplementation (Guslandi et al. 2000). The limita-
tions of the above cited studies are mainly related to
heterogeneity in terms of strain, dosage and duration
of treatment and the lack of studies on extremely low
birth weight preterm infants. Data on nosocomial pneu-
monia and ventilatorassociated pneumonia in neonatal
and pediatric age is scanty. In a large randomized, dou-
ble-blind placebo controlled study, Hojsak et al demon-
strated that supplementation with Lactobacillus GG sig-
ni cantly decreased the risk of nosocomial respiratory
tract infections (Shornikova et al. 1997). On the other
hand, the data from adult studies have been con icting,
with a tendency towards the demonstration of probiotic
ef cacy in reducing the incidence of ventilatorassoci-
ated pneumonia (Pant et al. 1996). Meticillin-resistant
Staphylococcus aureus is a multidrug-resistant nosoco-
mial pathogen; a recent review of literature (Raza et al.
1995) showed that many probiotic strains inhibit MRSA
growth in vitro. Furthermore, this review describes that
there is little published clinical data on the use of pro-
biotics in prophylaxis or treatment of MRSA-mediated
infections (Nase et al. 2001).
The use of probiotics in medical practice is rapidly
increasing, as are studies that demonstrate the ef cacy
of probiotics. A note of caution should be applied: nega-
tive ndings are being reported, as would be expected as
more studies are being performed and as more applica-
tions are being sought for the use of probiotics. Overall,
probiotics appear to be here to stay as part of the phy-
sician’s armamentarium for the prevention and treat-
ment of disease; however, more evidence-based research
is required to rmly establish medical areas of use and
areas in which probiotics are not applicable.
REFERENCES
Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. (2003):
Probiotics for treating infectious diarrhea. Cochrane Database
Syst Rev; 2: CD003048.
Armuzzi A, Cremonini F, Bartolozzi F. (2001b): The effect of
oral administration of Lactobacillus GG on antibiotic-asso-
ciated gastrointestinal side effects during Helicobacter pylori
eradication therapy. Aliment Pharmacol Ther; 15:163–9.
Armuzzi A, Cremonini F, Ojetti V. (2001a): Effect of Lactobacil-
lus GG supplementation on antibiotic-associated gastrointesti-
nal side effects during Helicobacter pylori eradication therapy:
a pilot study. Digestion; 63:1–7.
Arvola T, Laiho K, Torkkeli S. (1999): Prophylactic Lactobacil-
lus GG reduces antibiotic-associated diarrhea in children with
respiratory infections: a randomized study. Pediatrics; 104:e64.
Cetina-Sauri G, Sierra Basto G. (1994): Evaluation therapeu-
tique de Saccharomyces boulardii chez des enfants souffrant
de diarrhee aigue. Ann Pediatr; 41:397–400.
52 THE ROLE OF PROBIOTICS IN NOSOCOMIAL INFECTIONS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mahmmudi and Gorzin
Cremonini F, Di Caro S, Covino M. (2002): Effect of different
probiotic preparations on anti-Helicobacter pylori therapy-
related side effects: a parallel group, triple blind, placebo-con-
trolled study. Am J Gastroenterol; 97:2744–9.
DeVrese M, Stegelmann A, Richter B, Fenseau S, Love C,
Schrezenneir J. (2001): Probiotics compensation for lactose
insuf ciency. Am J Clin Nutr; 73:421S–9S.
Guandalini S, Pensabene L, Zikri MA. (2000): Lactobacillus
GG administered in oral rehydration solution to children with
acute diarrhea: a multicenter European trial. J Pediatr Gastro-
enterol Nutr; 30: 54–60.
Guslandi M, Mezzi G, Sorghi M, Testoni PA. (2000): Saccharo-
myces boulardii in maintenance treatment of Crohn’s disease.
Dig Dis Sci; 45: 1462–4.
Ho¨chter W, Chase D, Hegenhoff G. (1990): Saccharomyces
boulardii in treatment of acute adult diarrhoea: ef cacy and
tolerance of treatment. Mu¨nch Med Wochen; 132:188–92.
Kim HS, Gilliland SE. (1983): Lactobacillus acidophilus as
dietary adjunct for milk to aid lactose digestion in humans. J
Dairy Sci; 66:959–66.
Kolars JC, Levitt MD, Aouj M, Savaino DA. (1984): Yogurt—an
antidigesting source of lactose. N Engl J Med; 310:1–3.
Malchow HA. (1997): Crohn’s disease and Escherichia coli:
a new approach in therapy to maintain remission of colonic
Crohn’s disease? J Clin Gastroenterol; 25:653–8.
Mastretta E, Longo P, Laccisaglia A. (2002): Effect of Lacto-
bacillus GG and breast-feeding in the prevention of rotavirus
nosocomial infection. J Pediatr Gastroenterol Nutr; 35:527–31.
Nase L, Hatakka K, Savilahti E. (2001): Effect of long-term
consumption of a probiotic bacterium, Lactobacillus rhamno-
sus GG, in milk on dental caries and caries risk in children.
Caries Res; 35: 412–20.
Oberhelman RA, Gilman RH, Sheen P. (1999): A placebo-con-
trolled trial of Lactobacillus GG to prevent diarrhea in under-
nourished Peruvian children. J Pediatr; 134:15–20.
Oksanen PJ, Salminen S, Saxelin M. (1990): Prevention of
travellers’ diarrhea by Lactobacillus GG. Ann Med; 22:53–6.
Pant AR, Graham SM, Allen SJ. (1996): Lactobacillus GG and
acute diarrhea in young children in the tropics. J Trop Pediatr;
42:162–5.
Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA.
(1995): Lactobacillus GG promotes recovery from acute non-
bloody diarrhea in Pakistan. Pediatr Infect Dis J; 14:107–11.
Salminen S, Gibson C, Bouley MC. (1998): Gastrointestinal
physiology and function: the role of prebiotics and probiotics.
Br J Nutr; 80(Suppl 1):S147–71.
Savaiano DA, Abou EA, Smith DE, Levitt MD. (1984): Lac-
tose malabsorption from yogurt, sweet acidophilus milk, and
cultured milk in lactosede cient individuals. Am J Clin Nutr;
40:1219–23.
Sepp E, Tamm E, Torm S, Lutsar I, Mikelsaar M, Salminen S.
(1995): Impact of a Lactobacillus probiotic on the faecal micro-
ora in children with shigellosis. Microecol Ther; 23:74–80.
Shornikova AV, Cosas I, Mykkanen H, Salo E, Vesikari T.
(1997): Bactiotherapy with Lactobacillus reuteri in rotavirus
gastroenteritis. Pediatr Infect Dis J; 16:1103–7.
Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesi-
kari T. (1997): A trial in the Karelian Republic of oral rehydra-
tion and Lactobacillus GG for treatment of acute diarrhea. Acta
Paediatr; 86:460–5.
Siitonen S, Vapaatalo H, Salminen S. (1990): Effect of Lac-
tobacillus GG yoghurt in prevention of antibiotic associated
diarrhea. Ann Med; 22:57–9.
Szajewska H, Kotowska M, Murkowicz JZ, Armanska M,
Mikolajczyk W. (2001): Ef cacy of Lactobacillus GG in preven-
tion of nosocomial diarrhea in infants. J Pediatr; 138:361–5.
Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo
JV, Young RJ. (1999): Lactobacillus GG in the prevention
of antibiotic-associated diarrhea in children. J Pediatr; 135:
564–8.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE ROLE OF PROBIOTICS IN NOSOCOMIAL INFECTIONS 53