
Experimental protection of ESBL producing
Salmonella
typhi
bacteremic induced mice model by GRCST; a
therapeutic approach
Rahul Narasanna,
1
Manjunath Chavadi,
1
Liyakat Ahmed,
2
Syed Sannauallah
2
and Kelmani
Chandrakanth
1
*
1
Department of Biotechnology Gulbarga University Kalaburagi
2
Luqman College of Pharmacy, Gulbarga, Karnataka 585101, India
ABSTRACT
Salmonella typhi speci c bacteriophage i.e. GRCST exhibited potential bacteriolytic activity against n=4, ESBL produc-
ing S. typhi isolates in vitro. The GRCST possesses an icosahedral head with 50 nm size and contractile tail belongs
to Myoviridae Vi01-like family. The experimental outcome of in vivo studies in BALB/c mice induced with S. typhi
bacteraemia treated with 1.5×10
7
PFU GRCST showed 100% survival with zero causality was recorded. On contrary,
only 67% and 83% survival rate was observed in the group of mice which received standard antibiotic cipro oxacin.
The IgG and IgM titres of anti-phage GRCST antibodies were detected, with increased 4100 fold, 600 fold respectively.
This result demonstrates that the antibodies elicited by GRCST are non-neutralizing.
KEY WORDS: BALB/C MICE, ESBL, GRCST, IN VIVO, IGG, IGM, SALMONELLA TYPHI
17
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 12(1): 17-25 (2019)
INTRODUCTION
The Viruses or Bacteriophage which infects bacteria
were discovered in 1915 by Frederick Twort. The era of
“bacteriophage” was begun with the seminal publication
by Felix D’Herelle in 1917, demonstrating “un bacterio-
phage obligatoire” means “a bacteriophage mandatory”.
Total of 13 microbiologists worked together integrate
the applications of phages in the eld of medicine. Till
date, over 6000 various bacteriophages were discovered,
which includes 6196 bacterial and 88 archaeal viruses,
identi ed morphologically and classi cation was
accomplished (Ackermann et al., 2012). Morphologically,
the majority of these phages consisting contractile tail
ARTICLE INFORMATION:
Corresponding Author: ckelmani@gmail.com
Received 4
th
Jan, 2019
Accepted after revision 20
th
March, 2019
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
NAAS Journal Score 2019: 4.31 SJIF: 4.196
© A Society of Science and Nature Publication, Bhopal India
2019. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/12.1/3

Rahul Narasanna et al.
18 EXPERIMENTAL PROTECTION OF ESBL PRODUCING SALMONELLA TYPHI BACTEREMIC INDUCED MICE MODEL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
with polyhedral, lamentous or pleomorphic head types.
The classi cation of phages so far has been achieved
signi cantly based on their genetic content (DNA vs.
RNA), their morphology, and their limited host range i.e.
speci c host (Deghorain et al., 2012).
Increasing case studies of antimicrobial resistance and
relented discoveries and development have propelled the
researchers to search for an alternative therapy has led
to the revitalization of bacteriophage (phage) studies in
the Western world. Recently, WHO Listed, a global pri-
ority pathogens consist of 12 bacterial species catego-
rized into critical, high and medium priority based on
their level of resistance and availability of therapeutics
pathogens (Tacconelli et al., 2018). While this is a con-
tentious gure (De Kraker et al., 2016), it nonetheless
highlights the serious problem we face regarding thera-
peutic options for multi-drug resistant (MDR) bacterial
infections (Bassetti et al., 2017).
Phage therapy; obligatory lytic phages were employed
to kill the speci c bacterial hosts, without causing dam-
age to human host cells and nullifying the impact on
commensal bacteria. Rapid evolving of phage therapy
has resulted in resolving life-threatening clinical cases.
Currently, antibiotic alternative facing the regulations
and policies surrounding clinical use and application
beyond compassionate cases (Furfaro et al., 2018).
In the year 1919, phage therapy was the rst time
practiced in human beings at the hospital des Enfants
Malades in Paris, France, when D’Herelle successfully
treated many children’s who were suffering from severe
dysentery by using phages as a therapeutics, he has iso-
lated these infective phages in Pasteur Institute, from
stools of soldiers (Sulakvelidze et al., 2005).
Salmonella bacteria are often health hazards, asso-
ciated with a million food borne illnesses per year in
the US. Bacteriophages have been speci cally used to
identify Salmonella species and may also be useful in
therapy and prophylaxis of Salmonella infections. The
phage FelixO1 was rst used in 1943 byFelix and Cal-
lowas part of a “phage-typing” system for theidenti -
cationSalmonella typhi (Anderson et al., 1953).
First commercial phage produced by Theodore
Mazure, in which contains, cocktails—Bacté-Coli-Phage,
Bacté-Intesti-Phage, Bacté-Dysentérie-Phage, Bacté-
Pyo-Phage and Bacté-Rhino-Phage (Abedon et al.,
2011). Henri de Montclos, chief clinical microbiologist at
Pasteur Institute of Lyon, for 10 years, his research team
has produced rst anti-staphylococcal vaccines and
therapeutic phages in the year early 1990s. The bacterio-
phages were administered to treat the “acute colitis” due
to infections of Shigella or Salmonella in Georgia (Mike-
ladze et al., 1936). Potential administration of Bacté-
Pyo-Phage and Bacté-Intesti-Phage, undiluted resulted
in drastic reduction of mortality rate from 85% to 20%.
Therapeutic application of bacteriophage started in
Eastern Europe and the former Soviet Union, currently
it’s been applied widespread as a part of health care
systems. However, the ef ciency of phage therapy is
investigated according to rigorous scienti c standards
and presented a list of key criteria for consideration and
reporting of phage therapy studies (Kutter et al., 2010;
Abedon, 2017; Villarroel et al., 2017). Information criti-
cal to the success of clinical trials includes the adequate
characterization and selection of phages as well as of the
subjects (humans) and the target bacteria., in addition to
that, the choice of appropriate disease targets for phage
therapy (Harper, 2018). On the other hand, it may be that
broad-host range phages are more common than is cur-
rently believed, due in part to biases in phage isolation
methods (De Jonge et al., 2018); this disparity deserves
much further research.
However, recent research and its outcomes suggest
that bacteriophage therapy is the appropriate treatment
to cure Salmonella associated infections. Majorly typhoid
fever was treated with bacteriophages by Tsouloukidze
et al., 1936 (Tsulukidze et al., 1936); who successfully
treated twenty patients suffering from peritonitis due
to intestinal perforations in typhoid fever (Abedon et
al., 2011). There are some published reports of success-
ful treatment against Salmonella-associated disease with
prophylactic phage therapy in treating Russian soldiers
suffering from dysentery during and after World War II
(Kutter et al., 2009). The reports suggest that, it has been
already practiced in broiler chickens. The bacteriophages
were able to reduce S. enteritidis counts on chicken
skin at refrigeration temperature and short contact time
(Atterbury et al., 2007). In addition, the decrease of S.
enteritidis count on arti cially-contaminated chicken
skin after phage treatment corresponded to the reduc-
tion achieved by chemical agents commonly used in the
poultry industry. A signi cant breakthrough is, bacte-
riophages were used as biocontrol agents in Pigs to con-
trol the infection, according to the study conducted by
Albino et al., 2014; and the outcome of the study was a
signi cant reduction in the colonization of Salmonella
in pigs administered with pool of bacteriophages (Albino
et al., 2014)
MATERIALS AND METHODS
Phage Isolation, Production, and Titration
ESBL resistant strain S. typhi BST 51 was used to speci c
host isolate bacteriophage from raw sewage samples.
The sewage sample was collected from various places
of Kalaburagi. The sample was ltered with sewage was
ltered with lter paper, and subsequently 40 ml of sew-
age was added to the 10 ml of 10X LB broth, inoculated

Rahul Narasanna et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EXPERIMENTAL PROTECTION OF ESBL PRODUCING SALMONELLA TYPHI BACTEREMIC INDUCED MICE MODEL 19
with S. typhi BST 51 strain and incubated for 18-24 hr.
The media was centrifuged at 10000 rpm for 10 min and
the supernatant was collected and subsequently ltered
using a 0.2 μm syringe lter (Melo et al., 2014b).
Screening of GRCST (G: Gulbarga, R: Rahul, C:
Chandrakanth Kelmani, S: Salmonella, T: Typhi) bacte-
riophage was accomplished by plaque assay method i.e.
agar overlay technique. The LB agar plates were pre-
pared, 0.1 ml of supernatant was serially diluted in 0.9
ml of LB media from 10
1
-10
10
in 1.5 ml eppendorf tubes
0.5 ml of test culture S. typhi BST 51 with 0.1 O. D was
equally distributed to another set of 10 eppendorf and
labelled for each dilution tubes subsequently 0.1 ml seri-
ally diluted ltrate was added to the respective tubes
containing 0.5 ml of S. typhi BST51 bacterial culture
labelled with respective dilution, incubated for 10-15
min. Each labelled tube was taken and uniformly mixed
with LB soft agar containing 0.6% agar in a molten state
at a temperature of 40
0
C- 45
0
C. Thereafter soft agar was
overlaid on LB hard agar plates containing 2% agar and
kept for incubation at 18-24 hr. Plaque formation on
agar plates indicates bacteriophage positive (Mazzocco
et al., 2009; Kropinski et al., 2009).
Phage puri cation and storage
An isolated colony of S. typhi BST 51 strain was inocu-
lated into the LB broth, the culture was allowed to attain
an OD of 0.1, and then infected with GRCST of 2X10
7
PFU, the culture was co-cultivated for 18 hr at 37
0
C in a
shaking incubator (240 rpm). Polyethylene glycol-8000
(PEG) or NaCl was added to the lysate to a nal concen-
tration of 20% or 0.5 M respectively and incubated at 1
hr at 4
0
C. After centrifugation at 10,000 rpm (16 min at
4
0
C) in a sorvall RC5B centrifuge, polyethylene glycol
(PEG-8000) was added to the supernatant to a nal con-
centration of 10%. The lysate was incubated overnight
at 4
0
C with gentle stirring. Polyethylene glycol-precip-
itated phage was collected by centrifugation at 15,000
rpm for 20 min. The resulting pellets were resuspended
in 3 ml of SM phage buffer (20 mM Tris-HCL [PH 7.4],
100 mM NaCl, 10 mM MgSO
4
), ltered through 0.2 μm
bacterial lters and phage ltrate was recovered and
dialyzed against phage buffer. Puri ed phage GRCST
was stored in aliquots of phage buffer at- 20
0
C (Sam-
brook and Russell, 2001)).
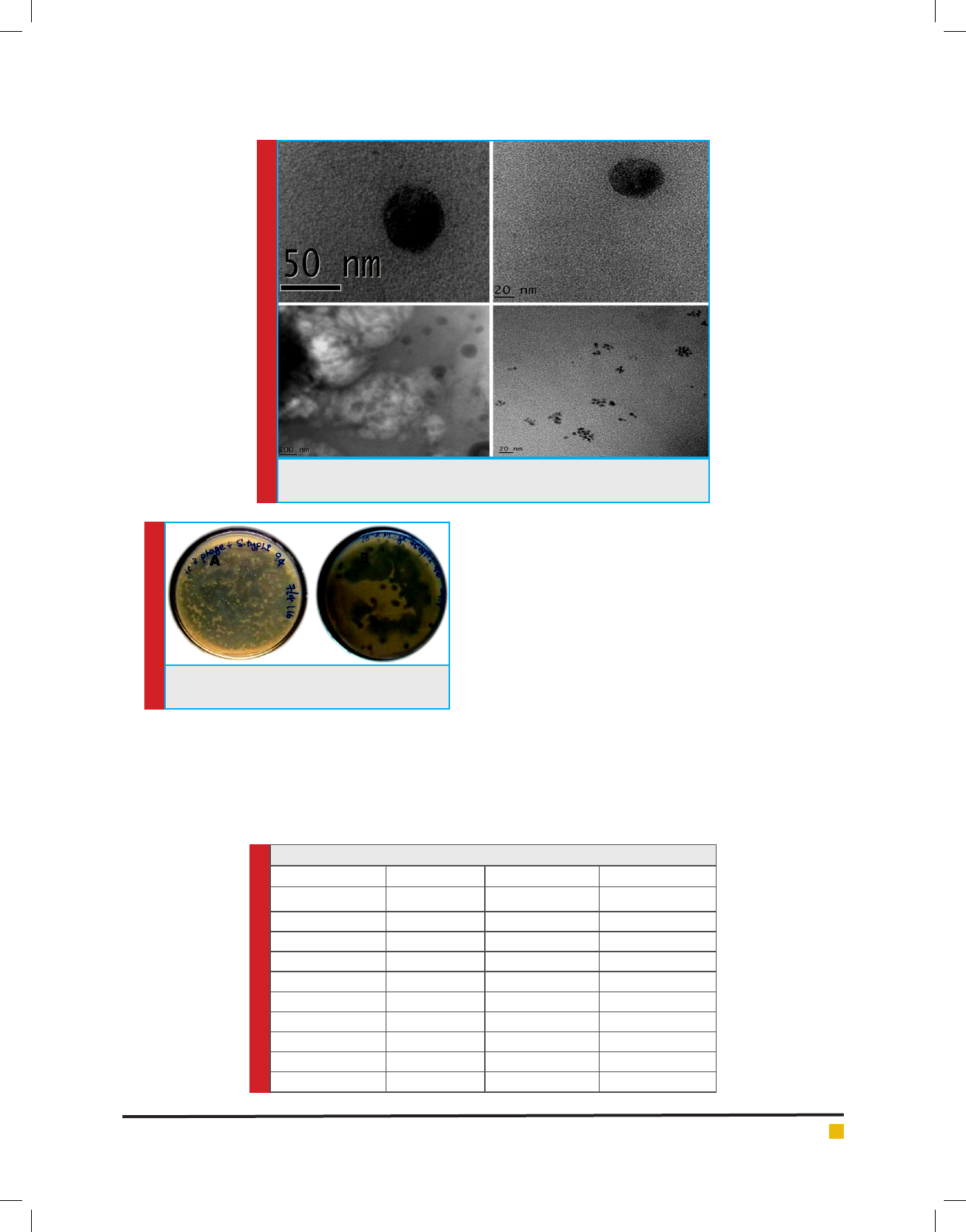
Transmission Electron Microscopy
The morphology of GRCST particles was observed
by transmission electron microscopy, as previously
described (Melo et al., 2014b). A drop of Puri ed phage
GRCST suspension was xed with xative. Samples
were dehydrated with series of ethanol series, passed
through a “transition solvent” such as propylene oxide
and then in ltrated and embedded in a liquid resin such
as epoxy and LR White resin. The processed suspension
was applied to a Farmvar carbon coated grid for 5 min;
subsequently stained with 2% uranyl acetate. The grids
were examined in a Transmission Electron Microscope
at 200kv (2000X – 1500000 X) (Ayache et al., 2010).
ANIMAL EXPERIMENT
Selection of Animals
Disease free, healthy and active BALB/c mice breed were
selected for in vivo studies. Both female and male mice
were chosen for the experimental purpose with ani-
mals weighing in the range of 20-30 gm. Animals were
obtained from Sri Venkateswara Enterprises, Bangalore,
approved by the institute of Animal Ethics Committee
(237/99/CPCSEA). Animals were nourished under con-
trolled climate conditions and fed with standard pel-
let (VRK Nutrition and Solutions, Sangli, Maharashtra,
India Ltd.), and provided suf cient amount of potable
water for drinking. Animals were kept for 10 days before
experimentation to acclimatize for laboratory condi-
tions. The animals were housed and the entire experi-
ment was carried out in Luqman Pharmacy College,
Kalaburagi).
Selection of pathogen and induction of bacteraemia
Salmonella typhi BST 51 (Blood Salmonella typhi 51)
selected for induction of typhoid fever in experimental
mice. S. typhi BST 51 strain has been chosen based on
its resistance power to n=7, antibiotics and exhibited
a high range of MIC to cefetoxime and also capable of
producing ESBL. The selected pathogen was inoculated
in LB broth, after 8-12 hr incubation, growth reached
0.2 O.D. Thereafter it was serially diluted in 0.1M PBS
and CFU (Colony forming units) was calculated. Subse-
quently, 10
7
-10
9
CFU was administered to experimental
mice intraperitoneal to determine the MLD (Minimum
Lethal Dose).
Ef cacy of bacteriophage in challenged BALB/c mice
Experimental animals (BALB/c) mice were divided into
six groups and each group consist of 6 animals each. The
doses were xed and prepared in PBS and administered
intraperitoneal (i.p). Mice from the group I received only
PBS as a control, Group II animals administered with S.
typhi BST 51 (2×10
9
CFU) diluted in PBS, Group III ani-
mals administered with only GRCST phage (1.5×10
7
PFU)
to check the lethality of phage on Mice. Group IV ani-
mals (Mice) represents (Test group), Group V and VI ani-
mals represent (Standard) challenged with S. typhi BST
51 (2×10
9
CFU) by intraperitoneal injection to induce
typhoid. Thereafter, 20 mins induction, Group IV (Test)
Mice received a GRCST (1.5×10
7
PFU), and similarly
Group V and VI animals received standard cipro oxacin

Rahul Narasanna et al.
20 EXPERIMENTAL PROTECTION OF ESBL PRODUCING SALMONELLA TYPHI BACTEREMIC INDUCED MICE MODEL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
antibiotic substituting bacteriophage. In Group VI cip-
ro oxacin were administered in multiple doses, daily up
to 7 days. All the six groups were kept in hygienic con-
dition with a continuous supply of food and water for 14
days. The signi cant observation made and results were
recorded (Table 1).
Determination of immunologic response against GRCST
in mice
Introduction of bacteriophage in human body as a ther-
apeutic agent cause signi cant stimulation of humoral
immunity subsequently leads to production of antibod-
ies. According to previous reports it is a potent anti-
gen causes no toxic effect on health of humans. Dur-
ing experiment BALB/c mice were treated with GRCST
(1.5×10
7
PFU) through i.p injection. At various time
point, mice blood was collected from optic vein and sub-
sequently subjected for ELISA (Enzyme Linked Immu-
nosorbent Assay) for the detection of antibody titres of
IgG and IgM antibody in serum of experimental mice
described by Biswas et al., 2002.
ELISA is a semi-quantitative method used to determine
the concentration of primary antibody in serum in antigen
coated wells. In ELISA detection was done based on posi-
tive enzyme-substrate reaction makes change in colour.
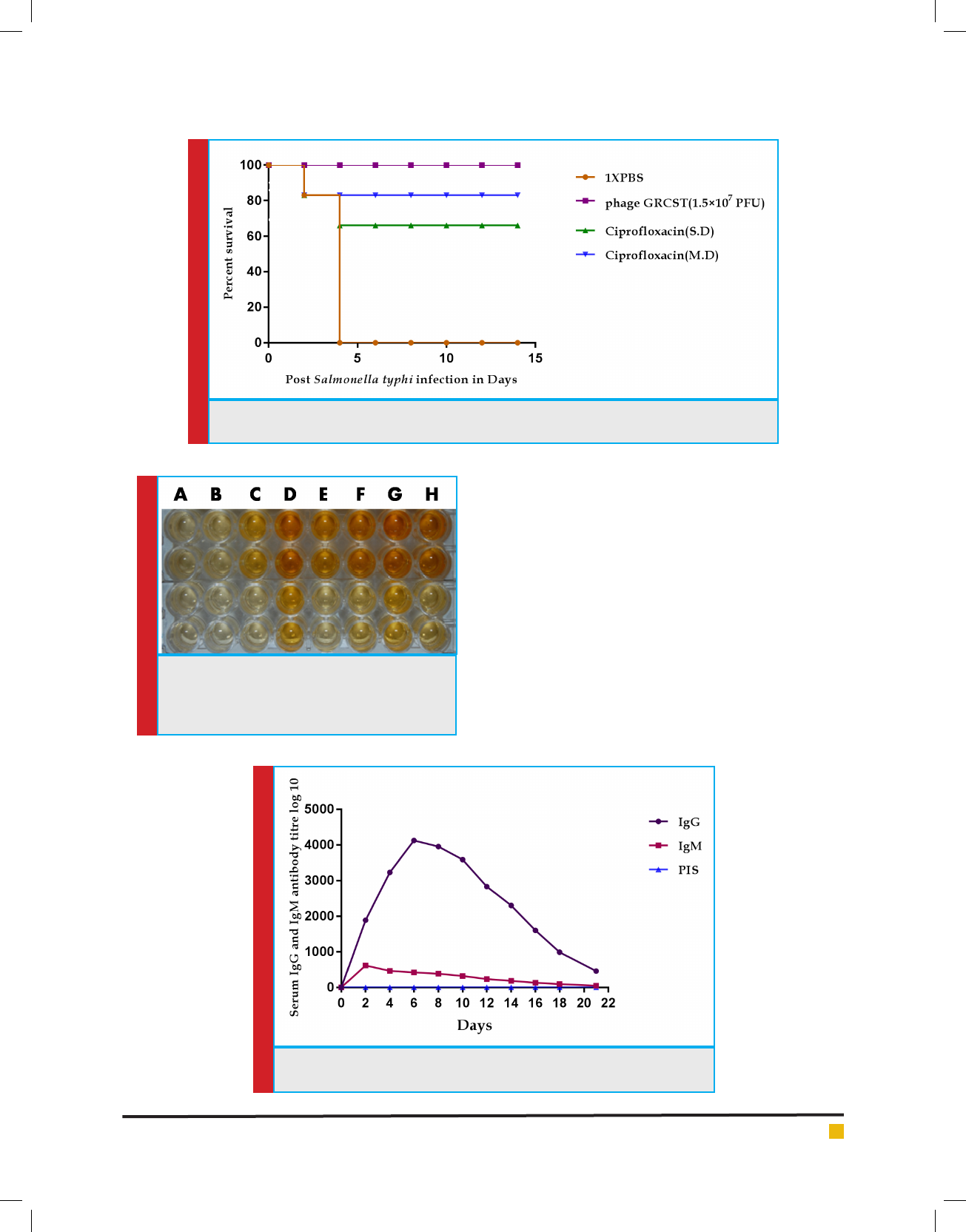
RESULTS
Isolation and Morphology of Salmonella typhi speci c
GRCST
The plaque formation indicates the presence of S. typhi
speci c bacteriophage i.e. GRCST (Fig 1). TEM results
revealed that GRCST (G: Gulbarga, R: Rahul, C: Chan-
drakanth Kelmani, S: Salmonella, T: Typhi) possesses an
icosahedral head with 50 nm size and contractile tail as
shown in gure belongs to Myoviridae Vi01-like family
(Fig 2).
Broad Host Range Screening
In order to investigate the broad host speci city of
GRCST exhibited potential bacteriolytic activity
against n=4 (BST 43, BST 94, BST 130, BST 141), ESBL
producing S. typhi isolates among n=9 selected isolates.
The plaque formation was observed against (n=4) tested
isolates (Fig 3 and Table 2)
ANIMAL EXPERIMENTS
Experimental induction of
S. typhi
BST 51 strain in
BALB/c mice and determination of Minimum Lethal dose
(MLD)
No causality was reported in the rst group mice, which
received 1XPBS and were proactive and healthy. Con-
sequently, only 83 % of mice survived in group II mice
which received until the 7
th
day of experimentation.
However, we observed the 100% mortality in III group
on day 7 but in contrast, 100% mortality was recorded
on 4
th
day itself in group IV. Based on the observation
2×10
9
CFU was determined as MLD (Fig 4)
Treatment and rescue of experimentally challenged
BALB/c mice with GRCST
The comparative study was carried out to evaluate the
ef cacy of phage GRCST with Standard antibiotic (cip-
Table 1. Group wise distribution of mice with intraperitoneal administration with various
inducing agents
Groups
Control Group I Mice+PBS
Control GroupII Mice + S. typhi BST 51 (2×10
9
CFU )
Control Group III Mice + phage GRCST (1.5×10
7
PFU)
Test Group IV
Mice + S. typhi BST 51 (2×10
9
CFU) + GRCST (1.5×10
7
PFU)
Standard Group V Mice + S. typhi BST 51 (2×10
9
CFU) + Cipro oxacin (1mg/ml)
Standard (Multiple doses) Group VI Mice + S. typhi BST 51 (2×10
9
CFU) + Cipro oxacin (1mg/ml)
FIGURE 1. Plaques of GRCST against S. typhi
BST 51 isolate
Rahul Narasanna et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EXPERIMENTAL PROTECTION OF ESBL PRODUCING SALMONELLA TYPHI BACTEREMIC INDUCED MICE MODEL 21
FIGURE 2. Transmission Electron Microscopic images of GRCST Scale
bars represent 50 nm.
FIGURE 3. Plates showing the clear lysis of MDR
S. typhi BST 94 isolate by GRCST
Table 2. Bacteriolytic activity of GRCST against MDR S. typhi isolates
S. typhi
isolates Bacteriophage Plaque formation Growth inhibition
BST 42 GRCST Negative Negative
BST 43 GRCST Positive Positive
BST 48 GRCST Negative Negative
BST 51 GRCST Positive Positive
BST 72 GRCST Negative Negative
BST 94 GRCST Positive Positive
BST 103 GRCST Negative Negative
BST 107 GRCST Negative Negative
BST 130 GRCST Positive Positive
BST 141 GRCST Positive Positive
ro oxacin). The observation made on daily basis up
to 14 days and data was recorded and represented in
the graph. According to the observation made, there
was 100% rescue was accomplished challenged BALB/c
mice treated with 1.5×10
7
PFU GRCST, comparatively
67% and 83% survival rate was observed in the group
of mice which received standard antibiotic cipro oxa-
cin in a single dose and in multiple doses for treatment.
The multiple doses were given on daily basis and results
obtained were represented in statistical graph (Fig 5).
The host immune response against phage GRCST in
BALB/c mice
The blood was collected from the optical vein of BALB/c
mice (n=6) and serum was separated, both IgG, IgM
titres of anti-phage GRCST antibodies were detected,
subsequently increased with 4100 fold, 600 fold respec-
tively in both the cases (Fig 6 and Fig 7). Incubation of
phage GRCST with an excess of mice anti-phage GRCST
antibodies did not interfere with phage’s capacity to
lyse susceptible bacteria. This result demonstrates that
the antibodies elicited by GRCST are non-neutralizing.

Rahul Narasanna et al.
22 EXPERIMENTAL PROTECTION OF ESBL PRODUCING SALMONELLA TYPHI BACTEREMIC INDUCED MICE MODEL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 4. Determination of the MLD of S. typhi BST 51 in BALB/c mice n=6 in each group
were infected intraperitoneal (I.p) with serially diluted bacterial suspensions of S. typhi
BST 51. The percentage Survival was determined upto 7 days.
No signi cant difference was found in BALB/c mice IgG
and IgM titres against GRCST. The anaphylactic reac-
tions were negative, and no changes in animal behav-
iour, no signi cant changes in the body temperature or
no other side effects were observed in both the groups.
DISCUSSION
Over the years, application of phages as therapeutic
alternatives or complements to antibiotic therapy has
been evaluated extensively (Viertel et al., 2014) and has
even been listed by the US National Institute of Allergy
and Infectious Diseases as one important approach to
combat antibiotic resistance (Reardon, 2014)
In the present study, the isolated GRCST effectively
infective to MDR S. typhi BST 51 strain (ESBL producing
isolate) from sewage sample. In vitro experiments showed
remarkable antibacterial activity against the S. typhi BST 51;
plaque formation indicates the presence of bacteriophage.
The broad host range study of GRCST, demonstrated the
ef ciency of GRCST potentially lysed n=4 (44.44%), ESBL
producing S. typhi isolates (Table 2). The positive plaque
formation was observed in all (n=4) S. typhi strains (Fig 2).
Similarly, host range screening was carried out by Wang et
al., 2016; with Phage 5460 potentially lysed 12 out of 18P.
mirabilisstrains (67%), three out of sixP. vulgarisstrains
and one testedP. penneristrain; while phage 5461 killed all
(100%) of theProteusspp. tested.
Transmission Electron Microscopic (TEM) study of
GRCST revealed that our phage belongs to Myoviridae
family. The phages which exhibit tail were classi ed in
the order Caudavirales (dsDNA) (Ackermann et al., 2006).
This study was concentrated on the bacteriophage in the
eld of therapeutics, which act against MDR S. typhi,
the characteristic feature GRCST possesses an icosahe-
dral head with size 50 nm with contractile tails consist-
ing of a sheath with a central tube (Fig 2); it belongs
to Myoviridae Vi01-like family of phages containing S.
typhi-speci c Vi01 (Hooton et al., 2011).
In vivo studies conducted in the BALB/c mice model
(weighing from 20-30 gms) for the experimental exami-
nation of the ef cacy of GRCST. The mice models were
showed the effective of prevention of infection caused
by antibiotic-resistant bacteria (Wang et al., 2006; Cap-
parelli et al., 2007; Vinodkumar et al., 2008). In the pre-
sent study, successfully experimented the in vivo ef -
cacy of GRCST against S. typhi BST 51 infected mice
model and obtained moderate results.
The minimum lethal dose (MLD) was determined by
intraperitoneal administration of 2×10
7
CFU, 2×10
8
CFU
and 2×10
9
CFU bacterial dose. Total of 100% survival
rate was recorded in 1X PBS induced mice. Only 83%
survival rate was observed in mice received 2×10
7
CFU
bacterial dosage. The comparative study was carried out
to evaluate the ef cacy of GRCST with standard antibi-
otic (cipro oxacin). The 100% rescue was accomplished
in challenged BALB/c mice treated with 1.5×10
7
PFU
phage GRCST, comparatively, 67% and 83% survival
rate was observed in the group of mice which received
standard antibiotic cipro oxacin in a single dose and in
multiple doses for treatment. The multiple doses were
given on daily basis (Fig 4). Relatively, the similar kind
of experiment was conducted on S. paratyphi B infected
mice and successfully rescued by treating with phage 1
(Wang et al., 2006).
The therapeutic effect of GRCST was successfully
achieved in vivo, the given phage (1.5×10
7
PFU) adminis-
tered along with saline showed no side effects on health
Rahul Narasanna et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EXPERIMENTAL PROTECTION OF ESBL PRODUCING SALMONELLA TYPHI BACTEREMIC INDUCED MICE MODEL 23
FIGURE 7. The raised antibody titre level (IgG and IgM) against GRCST in
BALB/c mice
FIGURE 5. The protection ef cacy of phage therapy with cipro oxacin (single and multiple doses)
treatment of BALB/c mice.
FIGURE 6. ELISA of IgG antibody titre in GRCST
immunized mice from S. typhi. A: Serum sam-
ple taken from control induced with 1X PBS B-F:
Serum of mice treated with phage GRCST
and behavior of the experimental animals. Thus, phage
rescue experiments could be conducted by without bias
(Uchiyama et al., 2008).
The immunology of phages has been a subject debate
over the years. The potential induced phages in; in vivo,
subsequently lead to the humoral immune responses ulti-
mately results in the inactivation of phage virion parti-
cles. There were some early assumptions; Kucharewica-
Krukowska and Slopek, 1987, phage therapy in both
animals and patients subsequently affect the patients
immunity, by stimulating the immune system and sub-
sequent production of anti-phage antibodies production
of neutralizing antibodies, rapid emergence of phage-
resistant bacterial strains (Stent, 1963; Lederberg, 1996;
Cairns et al., 2009) and ef cacy of phages only when

Rahul Narasanna et al.
24 EXPERIMENTAL PROTECTION OF ESBL PRODUCING SALMONELLA TYPHI BACTEREMIC INDUCED MICE MODEL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
administered shortly after bacterial infection (Bull et
al., 2002); are the most frequent criticisms of the clini-
cal use of phages. Recently, similar kind of studies were
conducted by Wang et al., (2016); comparatively results
obtained were very much similar to the activity of Phage
SLPW showed a broad host range and high ef ciency
of plating against various types of S. aureus. Phage
SLPW remained stable under a various temperatures or
pH range. Further, it ef ciently lysed MRSA strainsin
vitroandin vivo. Intraperitoneal phage administration
at 1 h post-infection cured the mice and reduced the
bacterial expression of in ammatory cytokines in mice
(Wang et al., 2016).
Titre of IgG and IgM was well measured BALB/c mice
after a single dose of GRCST induction, the background
titre was increased signi cantly with 4100 fold, and 600
fold respectively (Fig 6 and Fig 7). Incubation of phage
GRCST with an excess of mice anti-phage GRCST anti-
bodies did not interfere with phage’s capacity to lyse
susceptible bacteria. This result demonstrates that the
antibodies elicited by phage GRCST are non-neutral-
izing. The similar kind of immune response study was
conducted in phage1 against Salp572 (S. paratyphi B)
with an elevated level of mice anti-1 antibodies did not
interfere with phage’s capacity to lyse phage-suscepti-
ble bacteria. This result demonstrates that the antibod-
ies elicited by phage1 are non-neutralizing (Capparelli
et al., 2010). This study clearly emphasizes that mice
does produce antibodies against induced phages but
they are non-neutralizing. Indeed, Gorski et al., 2006
(Górski, et al., 2007); have provided enough evidence
of a positive impact of phages on immune system func-
tioning and have explored potential phage anti-tumour
properties mediated through observed shifts in levels
of various cytokines as a consequence of interactions
between extra decorative head proteins with surface
proteins of certain immune-system cells (Budynek et al.,
2010). The immune response to GRCST was not associ-
ated with anaphylaxis or other adverse immunological
reactions. The anaphylactic reactions were negative, and
no changes in animal behavior, no signi cant changes
in the body temperature or no other side effects were
observed in both the group
CONCLUSION
The bacteriophage therapy will serve for better perspec-
tive, with minimum side effects. The present investiga-
tion attempted to nd and characterize a bacteriophage
infective against the multidrug resistant and ESBL pro-
ducing S. typhi. Our explored phage was lytic against
many MDR S. typhi isolates, it’s in vivo ef cacy proved
as an excellent therapeutic agent. The signi cant out-
come of our conducted study a single dose of 1.5×10
7
PFU of phage GRCST successfully eliminated bacteria
from mice circulatory system without in uencing the
host immune system and rescued infected mice, compare
to antibiotics failed to rescue all infected mice. Hence,
we conclude that our explored GRCST is clinically
more ef cient than earlier reported; further characteri-
zation like whole genome sequencing and identi cation
and cloning of genes coding for bacterial lysis of the
GRCST may prove as an excellent alternative therapeu-
tic agent. Based on our observations of this study, phage
therapy can be used as an alternative therapy for those
patients not responding to antibiotic treatment.
CONFLICT OF INTEREST
The authors declare of no con ict of interest in conduct-
ing this study.
REFERENCES
Abedon, S. T., Kuhl, S. J., Blasdel, B. G., & Kutter, E. M. (2011).
Phage treatment of human infections.Bacteriophage,1(2), 66-85.
Ackermann, H. W. (2012). Bacteriophage electron micros-
copy.Adv. Virus Res,82, 1-32.
Ackermann, H.W. (2006). Classi cation of bacteriophages. In
The Bacteriophages, Ed.Calendar R, Oxford University Press,
ISBN 0-19-514850-9, New York, USA, pp. 8–16
Albino, L. A., Rostagno, M. H., Húngaro, H. M., & Mendonça,
R. C. (2014). Isolation, characterization, and application of
bacteriophages for Salmonella spp. biocontrol in pigs. Food-
borne pathogens and disease, 11(8), 602-609.
Anderson, E. S., & Felix, A. (1953). The Vi type-determining
phages carried by Salmonella typhi.Microbiology,9(1), 65-88.
Atterbury, R. J., Van Bergen, M. A. P., Ortiz, F., Lovell, M. A., Harris,
J. A., De Boer, A., & Barrow, P. A. (2007). Bacteriophage therapy to
reduce Salmonella colonization of broiler chickens.Applied and
environmental microbiology,73(14), 4543-4549.
Ayache, J., Beaunier, L., Boumendil, J., Ehret, G., & Laub, D.
(2010).Sample preparation handbook for transmission electron
microscopy: techniques(Vol. 2). Springer Science & Business
Media.
Bassetti, M., Poulakou, G., Ruppe, E., Bouza, E., Van Hal, S.
J., and Brink,A. (2017). Antimicrobial resistance in the next
30 years, humankind,bugs and drugs: a visionary approach.
Intensive Care Med. 43, 1464–1475. doi: 10.1007/s00134-017-
4878-x
Biswas, B., Adhya, S., Washart, P., Paul, B., Trostel, A. N., Pow-
ell, B., ... & Merril, C. R. (2002). Bacteriophage therapy res-
cues mice bacteremic from a clinical isolate of vancomycin-
resistant Enterococcus faecium.Infection and immunity,70(1),
204-210.
Budynek, P., Dabrowska, K., Skaradzinski, G., Górski, A. (2010).
Bacteriophages and cancer.Arch.Microbiol.192:315–320.
Bull, J. J., Levin, B. R., DeRouin, T., Walker, N., & Bloch, C. A.
(2002). Dynamics of success and failure in phage and anti-

Rahul Narasanna et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EXPERIMENTAL PROTECTION OF ESBL PRODUCING SALMONELLA TYPHI BACTEREMIC INDUCED MICE MODEL 25
biotic therapy in experimental infections.BMC microbiology,
2(1), 1.
Cairns, B. J., Timms, A. R., Jansen, V. A., Connerton, I. F., &
Payne, R. J. (2009). Quantitative models of in vitro bacteri-
ophage–host dynamics and their application to phage ther-
apy.PLoS Pathog,5(1), e1000253.
Capparelli, R., Nocerino, N., Iannaccone, M., Ercolini, D., Par-
lato, M., Chiara, M., & Iannelli, D. (2010). Bacteriophage ther-
apy of Salmonella enterica: a fresh appraisal of bacteriophage
therapy.Journal of Infectious Diseases,2011(1), 52-61.
Capparelli, R., Parlato, M., Borriello, G., Salvatore, P., & Ian-
nelli, D. (2007). Experimental phage therapy against Staphylo-
coccus aureus in mice.Antimicrobial agents and chemother-
apy,51(8), 2765-2773.
Chanishvili, N., Sharp, R.A. (2009). Literature Review of the
Practical Application of Bacteriophage Research. Tbilisi, Geor-
gia: Eliava Institute.
De Jonge, P. A., Nobrega, F. L., Brouns, S. J. J., and Dutilh, B.
E. (2018). Molecular and evolutionary determinants of bacte-
riophage host range. Trends Microbiol.7:1352. doi: 10.1016/j.
tim.2018.08.006
De Kraker,M. E., Stewardson, A. J., and Harbarth, S. (2016).
Will 10 million people die a year due to antimicrobial resist-
ance by 2050? PLoS Med. 13:e1002184. doi: 10.1371/journal.
pmed.1002184
Deghorain M, Bobay LM, Smeesters PR, Bousbata S, Ver-
meersch M, Perez-Morga D, Drèze PA, Rocha EP, Touchon
M, Van Melderen L (2012) Characterization of novel phages
isolated in coagulase-negative staphylococci reveals evolu-
tionary relationships with Staphylococcus aureus phages. J
Bacteriol 194:5829–5839
Furfaro, L.L., Payne, M.S. and Chang, B.J., 2018. Bacteriophage
therapy: Clinical trials and regulatory hurdles.Frontiers in cel-
lular and infection microbiology,8.
Górski, A., Borysowski, J., Miedzybrodzki, R., & Weber-Dab-
rowska, B. (2007).Bacteriophages in medicine (pp. 125-158).
Caister Academic Press.
Harper, D. R. (2018). Criteria for selecting suitable infectious
diseases for phage therapy. Viruses 10:E177. doi: 10.3390/
v10040177
Hooton, S. P., Timms, A. R., Rowsell, J., Wilson, R., & Con-
nerton, I. F. (2011). Salmonella Typhimurium-speci c bacte-
riophage SH19 and the origins of species speci city in the
Vi01-like phage family.Virology journal,8(1), 1.
Kropinski, A. M., Mazzocco, A., Waddell, T. E., Lingohr, E., and
Johnson, R. P. (2009). Enumeration of bacteriophages by dou-
ble agar overlay plaque assay.Methods Mol. Biol.501, 69–76.
doi: 10.1007/978-1-60327-164-6_7
Kutter EM. Bacteriophage therapy: past and present. In: Schae-
cter M, editor.Encyclopedia of Microbiology.Oxford: Elsevier;
2009. pp. 258–266.
Lederberg, J. (1996). Smaller eas... ad in nitum: therapeutic
bacteriophage redux.Proceedings of the National Academy of
Sciences,93(8), 3167-3168.
Mazzocco, A., Waddell, T. E., Lingohr, E., & Johnson, R. P.
(2009). Enumeration of bacteriophages by the direct plating
plaque assay.Bacteriophages: Methods and Protocols, Volume
1: Isolation, Characterization, and Interactions, 77-80.
Melo, L. D., Sillankorva, S., Ackermann, H. W., Kropinski, A.
M., Azeredo, J., and Cerca, N. (2014b). Isolation and characteri-
zation of a new Staphylococcus epidermidis broad-spectrum
bacteriophage.J. Gen. Virol.95(Pt 2), 506–515. doi: 10.1099/
vir.0.060590-0
Mikeladze, C., Nemsadze, E., Alexidze, N., Assanichvili, T.
(1936). On the treatment of typhoid fever and acute colitis by
d’Herelle bacteriophage.La Médecine.1936; 17:33–38.(Fre).
Reardon, S. (2014). Phage therapy gets revitalized.Nature510,
15–16. doi: 10.1038/510015a
Sambrook, J., and Russell, D. W. (2001). Molecular Cloning:
A Laboratory Manual, 3rd Edn. New York, NY: Cold Spring
Harbor Laboratory Press
Stent, G. S. (1963). Molecular biology of bacterial
viruses.Molecular biology of bacterial viruses.
Sulakvelidze, A., Barrow, P. (2005). Phage therapy in animals
and agribusiness. In: Kutter E, Sulakvelidze A, eds. Bacterio-
phages: Biology and Application. Boca Raton, FL: CRC Press,
335-80.
Tacconelli, E., Carrara, E., Savoldi, A., Harbarth, S., Mendel-
son, M., Monnet, D.L., et al. (2018). Discovery, research, and
development of new antibiotics: the WHO priority list of anti-
biotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 18,
318–327. doi: 10.1016/S1473-3099(17)30753-3
Tsulukidze, A. (1936). Sur l’application du bacteriophage dans
la peritonite par perforation au cours de la evre typhoide. La
Médecine, 17(Suppl), 41-2.
Uchiyama, J., Rashel, M., Maeda, Y., Takemura, I., Sugihara, S.,
Akechi, K., ... & Matsuzaki, S. (2008). Isolation and characteri-
zation of a novel Enterococcus faecalis bacteriophage EF24C
as a therapeutic candidate.FEMS Microbiology letters,278(2),
200-206.
Vinodkumar, C. S., Kalsurmath, S., & Neelagund, Y. F.
(2008). Utility of lytic bacteriophage in the treatment of
multidrug-resistant Pseudomonas aeruginosa septicemia in
mice. Indian Journal of Pathology and Microbiology, 51(3),
360.
Vinogradov, E., and Perry, M. B. (2000). Structural analysis
of the core region of lipopolysaccharides fromProteus mira-
bilisserotypes O6, O48 and O57.Eur. J. Biochem.267, 2439–
2446. doi: 10.1046/j.1432-1327.2000. 01262.x
Wang, J., Hu, B., Xu, M., Yan, Q., Liu, S., Zhu, X., & Li, Q. Q.
(2006). Use of bacteriophage in the treatment of experimen-
tal animal bacteremia from imipenem-resistant Pseudomonas
aeruginosa.International journal of molecular medicine,17(2),
309-318.
Wang, Z., Zheng, P., Ji, W., Fu, Q., Wang, H., Yan, Y., & Sun, J.
(2016). SLPW: A virulent bacteriophage targeting methicillin-
resistant Staphylococcus aureus in vitro and in vivo. Frontiers
in microbiology, 7, 934.