Biotechnological
Communication
Biosci. Biotech. Res. Comm. 10(4): 680-688 (2017)
Genome-wide comparative analysis of the codon usage
pattern in
Flaviviridae
family
Anjusha Mune
1
, Ajay Pandey
2
and Khushhali Menaria Pandey
1
*
1
Department of Biological Science and Engineering, MANIT Bhopal (M.P.) India
2
Department of Applied Mechanics, MANIT Bhopal (M.P.) India
ABSTRACT
Flaviviridae family is a group of viruses that cause several deadly diseases like Dengue Fever Virus, Zika Virus, Japa-
nese Encephalitis Virus, and Hepatitis C Virus etc. The codon usage analysis can serve as a tool to understand about
the molecular evolution and regulation of viral gene expression. The objective of this study is to nd the key deter-
minants of codon usage in the family. In this study, the codon usage pattern for 114 genomes of Flaviviridae family
(with four genus Flavivirus, Hepacivirus, Pegivirus, Pestivirus) was analysed through codon usage indices (like NC,
RSCU, ENC, PCA) and multivariate statistical methods. Our results show that among the four genus Flavivirus and
Pestivirus show similarity in preferred base on the count of being AG rich. On the other hand, Pegivirus and Hepacivi-
rus show similarity in preferred base on the count of being GC rich. The overall codon usage bias in the entire family
is slightly biased. RSCU analysis showed that Flavivirus and Pestivirus prefer AG ending codons, whereas Pegivirus
and Hepacivirus show preference to GC ending codons. Many unclassi ed members show similarity with members
of genus Flavivirus in choices of codon. The ENC -GC3 plot show that mutation pressure is dominating evolutionary
driving force in making codon usage preferences. The study represents comprehensive analysis of codon usage pat-
tern and help to better understand the mechanism of codon usage bias.
KEY WORDS: EFFECTIVE NUMBER OF CODON, MUTATION PRESSURE, NUCLEOTIDE CONTENTS, PRINCIPAL COMPONENT ANALYSIS, RELATIVE
SYNONYMOUS CODON USAGE
680
ARTICLE INFORMATION:
*Corresponding Author: kmpbiomanit@gmail.com
Received 25
th
Oct, 2017
Accepted after revision 19
th
Dec, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF: 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at:
http//www.bbrc.in/
DOI: 10.21786/bbrc/10.4/11
INTRODUCTION
Flaviviridae family is composed of fast evolving
RNA viruses. The members of the family are positive
single stranded RNA viruses that are causative agents
for number of neglected tropical diseases in humans and
animals. Flaviviridae family is mainly classi ed into four
genera: Flavivirus, Hepacivirus, Pegivirus and Pestivirus
(Lobo et al., 2009; Lu et al., 2017). The viral genomes
vary from 9 to 13 Kb and contain a single known ORF

Anjusha Mune etal.
that codes for a polyprotein, which is processed co- and
post-translationally by host and viral proteases into at
least 10 functional, individual polypeptides (Blitvich and
Firth, 2015; Brand etal., 2017).
Flavivirus genus comprises arthropod born viruses
(arboviruses) which are transmitted to host by vectors
(mosquitoes or ticks) via blood feeding. Birds and mam-
mals are usual primary hosts. Members of this genus are
further classi ed into groups on the basis of vector. They
are mosquito born, tick born, and non-known arthropod
vector (NKV) (Velazquez-Salinas etal., 2016). Flavivirus
includes viruses which cause several diseases includ-
ing: Dengue fever, Japanese encephalitis, Murray valley
encephalitis, West Nile fever, Zika fever. These viruses
are distributed worldwide but individual species are
restricted to particular epidemic areas (Moosavi et al.,
2011; Huang etal., 2014; Zhang etal., 2017).
Hepacivirus are the group of viruses mainly trans-
mitted by blood contact in mammals (horses, rodents,
bats, cows and primates); its best species being Hepati-
tis C virus. Genus Pestivirus infect mammal’s members
of family Bovidae (cattle, sheep, and goats) and suidae
family (various species of swine). Compared to the other
viruses in thefamily Pestivirus encode two unique gene
products, namely N
pro
and E
rns
. These unique proteins are
involved in repression of the host type I IFN response.
The genus Pegivirus commonly causes persistent infec-
tion in a broad range of mammals (humans, non-human
primates, pigs, horses and a range of rodent and bat
species).Less information is available on transmission of
these viruses in different host species (Theze etal., 2015;
Zhou etal., 2012; Tautz etal., 2015).
Many new viruses have been documented but their
relationship with other virus, mode of transmission
and vector association is not clear, hence they are not
assigned under any genus and we consider them unclas-
si ed. The genetic code comprises 64 codons that can
be divided into 20 groups. Each group corresponds to
each of the standard amino acids and consists of one
to six codons (Butt etal., 2013; Chen, 2013; Gu etal.,
2003). Alternative codons within the same group cod-
ing for the same amino acid are often termed ‘synony-
mous’ codons. Most amino acids can be translated by
more than one codon. This redundancy is an important
factor that provides accuracy in production of protein.
These synonymous codons are not used randomly. There
are some codons that are used more often than other
codons. This phenomenon is referred to as codon usage
bias (Tao etal., 2009; Moratorio etal., 2013; Wang etal.,
2016; Van etal., 2016).
Studies on codon usage have determined several fac-
tors that could in uence codon usage pattern, including
mutational pressure, natural or translational selection,
secondary protein structure, replication and selective
transcription, hydrophobicity of the protein, and the
external environment. Among these, the major factors
responsible for codon usage variation among different
organism are considered to be compositional constraints
under mutation pressure and natural selection. Numbers
of previous studies on codon usage of different viruses
have highlighted mutation pressure as the major factor
in shaping codon usage patterns compared with natural
selection. But with increasing understanding of codon
usage it appears that although mutational pressure is
still a dominating force, it is certainly not the only one
when different viruses are considered (Sharp et al.,
1988; Cristina etal., 2015; Xiang etal.,2015, Butt etal.,
2016 ).
Analysis of codon usage patterns of Flaviviridae
would not only provide a base for better understanding
of biased usage of synonymous codons, the evolution
and pathogenesis of Flaviviridae, but also improve our
understanding of the regulation of viral genes expres-
sion and aid vaccine design, where the ef cient expres-
sion of viral protein may be required to generate immu-
nity. In order to gain insight into these matters, we have
analysed codon usage and base composition of the 114
species of Flaviviridae family. The patterns of preferred
codons for each individual amino acid in each species
were identi ed.
MATERIALS AND METHODS
Total 114 complete genome sequences of viruses of Fla-
viviridae family were downloaded from the National
Centre for Biotechnology (NCBI) database (http://www.
ncbi.nlm.nih.gov) in FASTA format. The accession
numbers and other detailed information of the selected
genomes were listed in [supplementary material Table 1].
Open reading frames (ORF) of all the genomic sequences
were identi ed by using NCBI ORF nder (https://www.
ncbi.nlm.nih.gov/orf nder/).
In order to understand the frequencies of occurrence
of each nucleotide in ORFs, composition analysis was
conducted. The overall frequency of occurrence of the
nucleotides (A %, C %, U %, and G %) was calculated
along with the frequency of each nucleotide at the third
site of the synonymous codons (A
3
, C
3
, U
3
and G
3
). Also
the overall GC, AU and GC
3
content were calculated to
investigate the compositional properties. The codons
AUG and UGG are the only codons for Met and Trp,
respectively, and the termination codon UAA, UAG, and
UGA do not encode any amino acids. Therefore, these
ve codons are excluded from the analysis.
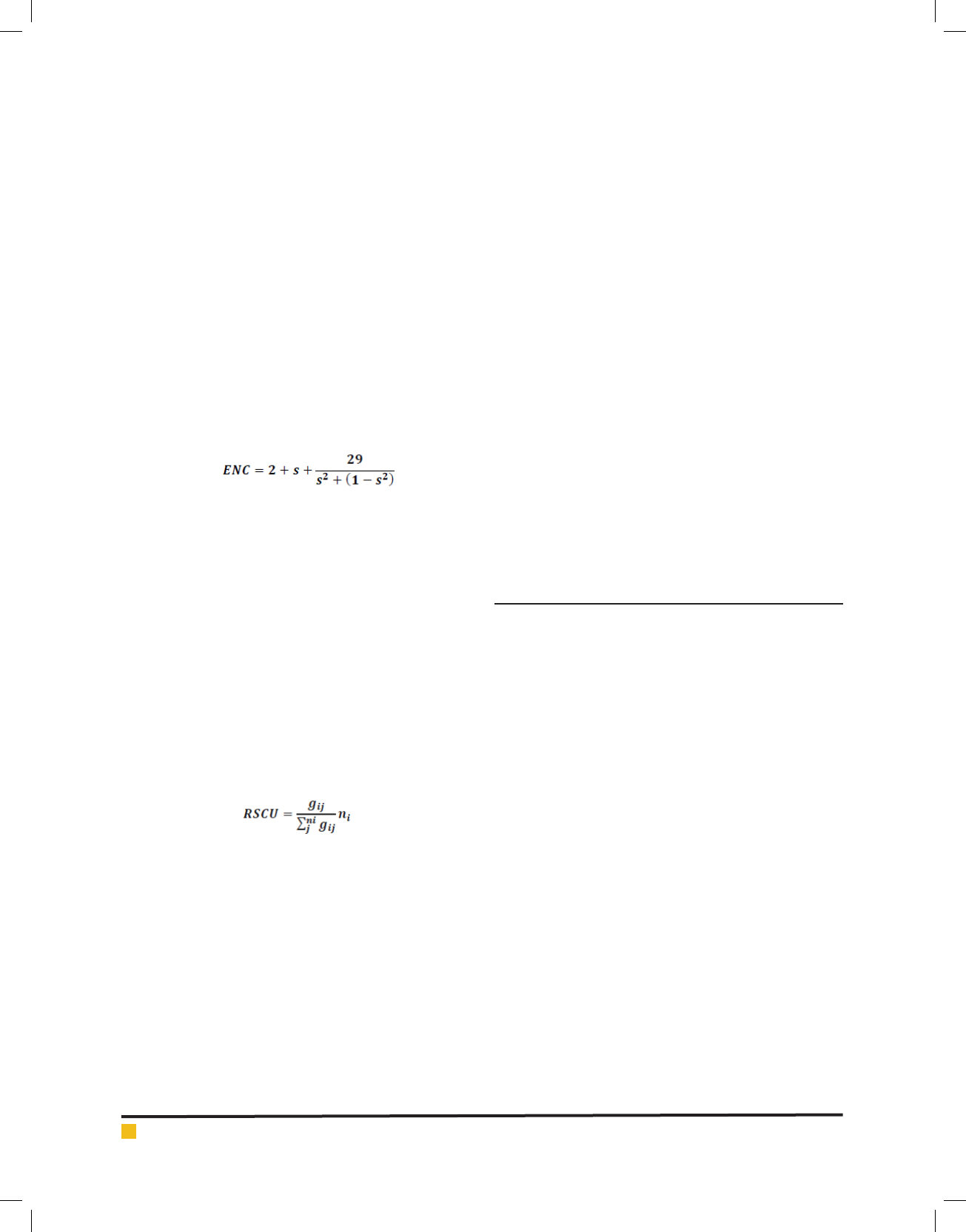
The ENC is a very effective estimator to measure the
magnitude of codon usage bias in the coding sequences
of members of Flaviviridaefamily. The ENC value ranges
from 20 (when only one synonymous codon is chosen
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS GENOME-WIDE COMPARATIVE ANALYSIS OF THE CODON USAGE PATTERN IN
FLAVIVIRIDAE
FAMILY 681
Anjusha Mune etal.
by the corresponding amino acid) to 61 (when all syn-
onymous codons are used equally) (Lu etal., 2005). In an
extremely biased gene where only one codon is used for
each amino acid, this value would be 20; if all codons
are used equally, it would be 61; and if the value of
ENC is greater than 40, the codon usage bias is regarded
as low (Wright F, 1990). We calculated ENC to meas-
ure the degree of departure from equal use of synony-
mous codons of ORF of members of Flaviviridaefamily.
The values of ENC were obtained by EMBOSS CHIPS
program. These ENC values were further analysed as
suggested by Wright’s ENC – plot (Zhang etal., 2011).
The ENC values are plotted against GC
3
as a method
to understand the pattern of codon usage. The viruses,
whose codon choice is constrained only by a mutation
bias, will lie on or just below the curve of the predicted
values. The predicted values of ENC were calculated as
Wheresrepresents the given (G+C)
3
% value.
Relative synonymous codon usage (RSCU) Analysis:
The RSCU values of codons in each ORF for all the mem-
ber of Flaviviridaefamily were calculated by the given
formula to determine the characteristics of synonymous
codon usage. The synonymous codons with RSCU values
> 1.0 have positive codon usage bias and were de ned
as preferred codons, while those with RSCU values <
1.0 have negative codon usage bias and were de ned as
less- preferred codons. When the RSCU value is 1.0, it
means there is no codon usage bias for that amino acid
and the codons are chosen equally or randomly. More-
over, the synonymous codons with RSCU values >1.6
and < 0.6 were treated as over-represented and under-
represented codons, respectively (Wong etal., 2010; Ma
etal., 2013).
Where g
ij
is the observed number of the ith codon for
the jth amino acid, which has n
i
types of synonymous
codons.
The correlation analysis was performed between each
general nucleotide composition (U%, A%, C%, and G
%) and each nucleotide composition in the third site of
codon (U
3
%, A
3
%, C
3
%, and G
3
%) and the value A%,
T%, C%, G% and A
3
%, T
3
%, C
3
%, G
3
% were compared
with GC%,, GC
3
% and ENC Using statistical software
SPSS 19 for windows. It is used to identify the relation-
ship between nucleotide composition and synonymous
codon usage pattern of viruses Flaviviridae family.
Principal component analysis (PCA): In this study
PCA was performed to analyse the major trend in codon
usage pattern among members of aviviridae family.
Principal component analysis is one of the most fre-
quently used multivariate statistical techniques (Su MW
et al., 2009; Yadav and Swati D, 2012; Kanaya et al.,
2001; Wang et al., 2011). PCA is an orthogonal linear
transformation that is used to transform the original
data set into a new coordinate system. It involves a
mathematical transformation procedure that transforms
some correlated variable (RSCU) into a smaller number
of uncorrelated variables called principal components.
The greatest variance represented by the data lies on
the rst coordinate, thus known as the rst principal
component (PC), the second greatest variance is on the
second PC, and so on. One can use top 2 or 3 PCs to rep-
resent the data instead of the large number of original
variables (in this case, 59 Variables). In this study PCA
was done by constructing a 114 59 RSCU data matrix.
In the matrix each row denotes the codon usage pattern
of a speci c virus, demonstrated by its RSCU value. Each
member of Flaviviridae family was represented as a 59
dimensional vector and each dimension corresponds to
the RSCU value of one sense codon, which only included
several synonymous codons for a particular amino acid,
excluding Met (AUG), Trp (UGG) and three stop codon.
RESULTS AND DISCUSSION
COMPOSITIONAL PROPERTIES OF ORFS OF 114
FLAVIVIRIDAE GENOMES
The nucleotide contents (A, U, C, G and AU, GC %) and
each nucleotide contents in the third site of codon (A
3
,
U
3
, C
3
, G
3
and GC
3
%) in the orf of members of Flaviviri-
dae family do not show similarity and are found to be
quite different from each other. [Table 1 supplementary
material].The genome of genus Flavivirus is enriched for
purines (A and G) compared to Pyrimidines (U and C)
with high frequency of base G ranging from 21.52-34.01.
The purine richness is maintained throughout the genus
without getting affected by the vector choice of that
virus. But the content of G is higher in tick born viruses
as compared to others. The effect of purine richness can
be observed on the selection of codons as the most pref-
erentially used codons are A - ended or G – ended codons
with higher preference to A – ended codons except AUC
for Ile in Flavivirus. Members of genus Pestivirus are also
enriched for purine bases, with high A content ranging
from 31.54-36.94 unlike Flavivirus .Most preferentially
used codons are A - ended or G – ended codons with
higher preference to A – ended codons except AGU for
Ser in Pestivirus. The genus Pegivirus and Hepacivirus
are rich in GC content with High content of G base in
Pegivirus ranging from 27.67-32.41 and content of base
C is almost similar in both the genus. The GC and GC
3
682 GENOME-WIDE COMPARATIVE ANALYSIS OF THE CODON USAGE PATTERN IN
FLAVIVIRIDAE
FAMILY BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Anjusha Mune etal.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS GENOME-WIDE COMPARATIVE ANALYSIS OF THE CODON USAGE PATTERN IN
FLAVIVIRIDAE
FAMILY 683
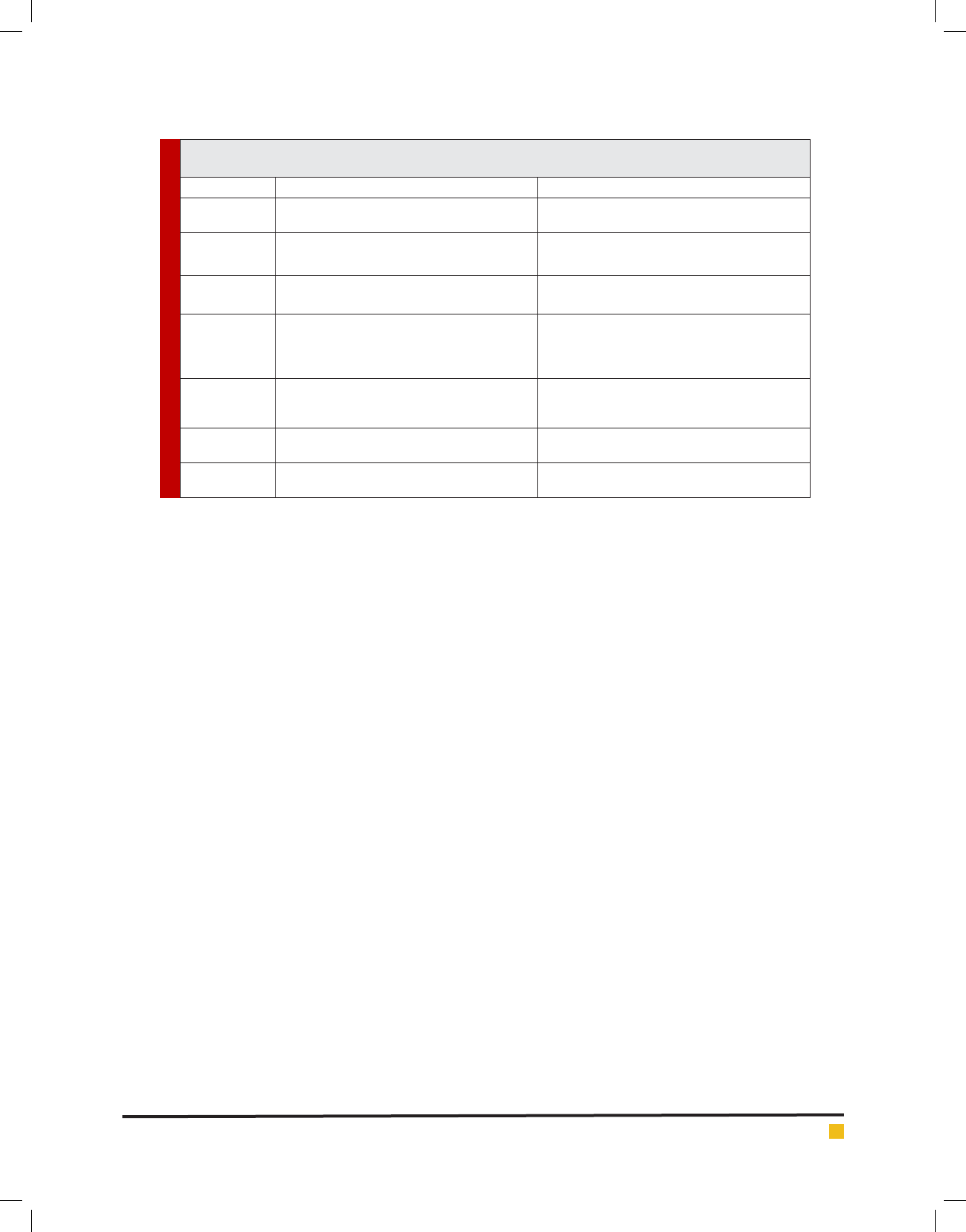
Table 1. List of Over-represented and Under-represented codons of four genus and unclassi ed members of
Flaviviridae family.
Genus Over-represented codons Under- represented codons
Flavivirus
(mosquito born)
UUG and CUG (Leu), GUG (Val), GGA (Gly), UCA
(Ser), CCA(Pro), ACA(Thr), AGA and AGG (Arg),
UUA (Leu), GUA (Val), CCG (Pro), GCG (Ala), GGU
(Gly)
Flavivirus
(tick born)
CUG (Leu), AUC ( Ile), GUG (Val), CCA (Pro), AGA
and AGG (Arg), GGA (Gly), AGU and AGC (Ser)
UUA and CUA (Leu), GUA (Val), UCG (Ser), CCG
(Pro), GCG (Ala), CGU and CGA (Arg)
Flavivirus
(NKV)
UUG (Leu), UCA (Ser), CCA (Pro), AGA & AGG
(Arg), GGA (Gly), ACA (Thr), GUG (Val)
GUA (Val), CGC & CGU (Arg)
Pestivirus CUA and CUG (Leu), AUA (Ile), GUG and GUA
(Val), UCA, AGC and AGU (Ser), CCA for Pro, GCA
for Ala, AGA and AGG for Arg , GGG for Gly,
ACA (Thr)
UCG (Ser), GCG (Ala), CGU, CGC, CGA and CGG
(Arg), UCG (Ser) and CCG (Pro).
Pegivirus UUG and CUG (Leu), AUC (Ile), GUG (Val), UCU
and UCC (Ser), ACU and ACC (Thr), CGC and CGG
(Arg)
GUA (Val), UUA and CUA (Leu), AUA (Ile), and AGA
(Arg) and GGA (Gly).
Hepacivirus AUC (Ile), CUC (Leu), GGC (Gly), AGG (Arg), UCC
(Ser)
UUA and CUA (Leu), GUA (Val), CGA (Arg)
Unclassi ed UUG (Leu), GUG (Val), CCA (Pro)
GGA (Gly)
GUA (Val), UUA (Leu)
compositions also highlight the richness of these nucleo-
tides in Pegivirus and Hepacivirus.
As a result of this they prefer using G - ended or
C - ended codons with higher preference to C – ended
codons. The unclassi ed group of virus show similarity
with genus Flavivirus, enriched for purines, hence most
preferentially used codons are A- ended or G with higher
preference to A – ended codons. Two out of the four
genus, Flavivirus and Pestivirus, are AG rich and show
similarity in preferred base. On the other hand, Pegivirus
and Hepacivirus are GC rich and show similarity in pre-
ferred base. We observed that the four possible nucleo-
tides are not used at equal frequencies. Flavivirus and
Pestivirus genus show low C content whereas Pegivirus
and Hepacivirus genus show low A content. The base U
is observed to be stable in the entire Flaviviridae family.
EFFECTIVE NUMBER OF CODON USAGE (ENC)
Different species have different tendencies to prefer spe-
ci c codons, symbolized by Effective number of codons
values. To investigate the overall codon usage pattern
of Flaviviridae family, the ENC values for each orf is
calculated and compared among the four genus [Supple-
mentary material Table 1]. The values were analysed and
compared within a genus and between different genus.
Overall, the observed ENC values range between 44.99
(Norway rat Pestivirus) to 58.97 (Aedes Flavivirus) with
the average being 53.74 across the Flaviviridae family.
We also observed the values across 4 Genus. The codon
bias of Flavivirus genus was on average 53.54 and
ranged from 58.97 (Aedes Flavivirus) to 47.30 (Tamana
bat virus) with standard deviation of 2.14. The overall
codon bias of Hepacivirus genus was on average 55.07
and ranged from 57.02 (Hepatitis GB virus B) to 51.27
(Norway rat Hepacivirus) with standard deviation of
1.85. The overall codon bias of Pegivirus genus had an
average value of 54.17 and ranged from 57.37 (Human
Pegivirus 2) to 50.59 (Rodent Pegivirus) with standard
deviation of 2.48.The overall codon bias of Pestivirus
had an average value of 50.71 and ranged from 54.08
(Atypical porcine Pestivirus 1) to 44.99 (Norway rat
Pestivirus) with standard deviation of 2.19. Finally, the
codon bias of unclassi ed members was represented
by an average value of 55.89 and ranged from 58.15
(Anopheles avivirus variant 1) to 52.30 (Bamaga virus)
with standard deviation of 1.75.
Among the entire genus Flavivirus showed highest
variation in ENC value and members of unclassi ed
group have shown the least variation. The codon varia-
tion of Flavivirus genus is higher than the variation of
other three genus, implying that the evolution speed of
these viruses is higher than the speed of the remain-
ing viruses of the family. Conceptual value is com-
prised between 21 (if only single codon is used for each
amino acid) and 61 (if all codons are used with equal
frequency). In general, the overall codon bias of the four
genus and unclassi ed members of Flaviviridae viruses
is considerably weak. This is in agreement with previ-
ous reports about some other RNA viruses, for example
BVDV (ENC=51.42), H5N1 (ENC=50.91) and SARS-covs
(ENC=48.99), NDV (ENC=56.15) (Wang etal., 2011; Zhou

Anjusha Mune etal.
684 GENOME-WIDE COMPARATIVE ANALYSIS OF THE CODON USAGE PATTERN IN
FLAVIVIRIDAE
FAMILY BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
et al., 2005; Gu et al., 2003; Wang et al., 2011). The
possible explanation of weak codon bias in RNA virus
is that a weak bias is helpful for ef cient replication of
virus in host cells. (Zhong etal., 2007)
MUTATION PRESSURE AFFECTS THE CODON
USAGE PATTERN
Mutational pressure and natural selection are considered
the two major factors that shape codon usage patterns
(Jenkins and Holmes, 2003). A general mutational pres-
sure, which affects the whole genome, would certainly
account for the majority of the codon usage among cer-
tain RNA viruses (Tatarinova et al., 2010). To identify
whether the evolution and variation pattern of codon
usage had been driven alone by mutation pressure or
also contributed by natural selection, we compared the
correlation between overall nucleotide composition (A,
U, C, G) and nucleotide composition at the third position
of codon (A
3
, U
3
, C
3
, G
3
) and correlation between overall
nucleotide composition (A, U, C, G, A
3
, U
3
, C
3
, G
3
) and
GC, GC
3
and ENC for individual genus using Pearson s
correlation [supplementary material ( Tables 2-3).
In genus Flavivirus GC and GC
3
show signi cant pos-
itive correlation with G(r=0.87, P<0.01) (r=0.80, P<0.01),
C (r=0.75, P<0.01) (r=0.80, P<0.01) and G
3
(r=0.86,
P<0.01) (r=0.85, P<0.01), C
3
(r=0.75, P<0.01) (r=0.82,
P<0.01).and negative correlation with A (r=-0.84,
P<0.01) (r= -0.85, P<0.01), U (r= -0.70, P<0.01) (r= -0.65,
P<0.01), and A
3
(r= -0.76, P<0.01), (r= -0.81, P<0.01),
U
3
(r= -0.69, P<0.01) (r= -0.68, P<0.01). ENC shows posi-
tive signi cant correlation with C (r= 0.73, P<0.01) and
C
3
(r= 0.70, P<0.01), and negative correlation with A (r=
-0.69, P<0.01) and A
3
(r= -0.65, P<0.01)
,
and non-sig-
ni cant correlation with U, G, and U
3.
A shows positive
correlation with A
3
, negative correlation with C
3
and G
3,
and non-signi cant correlation with U
3.
U shows signi -
cantly negative correlation with C
3
and G
3 ,
positive cor-
relation with U
3
, and non-signi cant correlation with
A
3.
G and C show signi cantly negative correlation with
A
3
and U
3
and signi cantly positive correlation with C
3
and G
3
. When we study correlation vector wise, tick born
and NKV viruses show signi cant correlation in com-
parison with mosquito borne viruses of the genus. In
genus Pestivirus an interesting and complex correlation
was observed.
To sum up, the GC, GC
3
and ENC have highly posi-
tive signi cant correlation with C (r=0.84, P<0.01)
(r=0.86, P<0.01) (ENC=0.87), C
3
(r=0.90, P<0.01) (r=0.94,
P<0.01) (ENC=0.89) and G (r=0.94, P<0.01) (r=0.91,
P<0.01) (ENC=0.74),G
3
(r=0.98, P<0.01) (r=0.97, P<0.01)
(ENC=0.82)
.
And signi cantly negative correlation with
A (r= -0.99, P<0.01) (r= -0.99, P<0.01) (ENC= -0.92),
A
3
(r= -0.97, P<0.01) (r= -0.99, P<0.01) (ENC= -0.90)
and U (r= -0.89, P<0.01) (r= -0.84, P<0.01), U
3
(r= -0.82,
P<0.01) (r= -0.80, P<0.01) (ENC= -0.66, P<0.05). A
3
and
U
3
show signi cantly positive correlation with A and
U, and signi cantly negative correlation with C and
G, whereas C
3
and G
3
show signi cantly negative cor-
relation with A and U and signi cantly positive cor-
relation with C and G. In genus Hepacivirus A
3
shows
positive correlation with A and has non-correlation with
U, C, G and GC and GC
3
. Similarly, A shows non-cor-
relation with U
3
, C
3
, G
3
and GC, GC
3
and ENC. GC and
GC
3
show signi cantly negative correlation with U (r=
-0.94, P<0.01) (r= -0.97, P<0.01) U
3
(r= -0.92, P<0.01)
(r= -0.96, P<0.01) and highly positive correlation with
G (r=0.95, P<0.01) (r=0.94, P<0.01), G
3
(r=0.96, P<0.01)
(r=0.97, P<0.01) and C (r=0.98, P<0.01) (r=0.95, P<0.01),
C
3
(r=0.96, P<0.01) (r=0.99, P<0.01). ENC of Hepacivirus
show non-correlation with A, U, C, G, U
3,
G
3.
In genus Pegivirus the GC and GC
3
show signi cantly
positive correlation with G (r=0.83, P<0.01) (r=0.83,
P<0.01), C (r=0.84, P<0.01) (r=0.71, P<0.05), C
3
(r=0.82,
P<0.01) (r=0.84, P<0.01) and signi cantly negative cor-
relation with U (r= -0.77, P<0.05) (r= -0.91, P<0.01)
and A
3
(r= -0.89, P<0.01) (r= -0.73, P<0.05). ENC have
highly signi cant correlation with A, U, C, G, A
3
, C
3
and
non-correlation with U
3
and G
3.
A shows signi cant cor-
relation with A
3
but does not show signi cant correla-
tion with U
3
, C
3 ,
G
3
. U shows signi cant correlation with
U
3
, C
3
and G
3
, and non-signi cant correlation with A
3.
C
show signi cant correlation with A
3
and C
3
and non-
signi cant correlation with U
3
and G
3
. G shows signi -
cant correlation with G
3
and non-signi cant correlation
with A
3,
U
3,
and C
3.
The members of unclassi ed group
do not show signi cant correlation with other nucleo-
tides, they show signi cant positive correlation with the
same type of nucleotide like A show positive correla-
tion with A
3
. The GC and GC
3
show positive correlation
with C (r=0.73, P<0.01) (r=0.62, P<0.05) and G (r=0.81,
P<0.01) (r=0.67, P<0.01) and negative correlation with
A (r=-0.75, P<0.01) (r=-0.69, P<0.01) and U (r=-0.78,
P<0.01) (r=-0.59, P<0.05). ENC does not show signi -
cant correlation with any nucleotide. This analysis col-
lectively indicates that mutational pressure is most likely
responsible for the patterns of nucleotide composition
and, therefore, codon usage patterns in all four genus of
Flaviviridae family.
VARIATION OF RELATIVE SYNONYMOUS
CODON USAGES IN FLAVIVIRIDAE FAMILY
In order to investigate the extent of codon usage bias in
aviviridae family, all RSCU values of different codons
in genus Flavivirus (69), Hepacivirus (14), Pegivirus (8),
Pestivirus (11) and unclassi ed members (12) were cal-
culated. The heat map [supplementary material Fig. 1]
Anjusha Mune etal.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS GENOME-WIDE COMPARATIVE ANALYSIS OF THE CODON USAGE PATTERN IN
FLAVIVIRIDAE
FAMILY 685
show the RSCU results of all codons in the 114 viruses of
Flaviviridae family. Green represent lower RSCU value,
black represent moderate RSCU, and red represents
greater RSCU values. The common over - represented
and common under - represented codons are listed for
each genus of Flaviviridae family [Table 1]. As we know
genus Flavivirus is classi ed into three groups on the
basis of vector. The over - represented and under - rep-
resented codons are identi ed vector wise for this genus.
Viruses in this genus show similarity in choice of
codon with their subtype or genotype like the four sero-
type of Dengue show similar choice of codons. Preferred
codons in four serotype are UUG and CUG for Leu, AUA
for Ile, GUG for Val, UCA for Ser, CCA for Pro, ACA for
Thr, GCC and GCA for Ala, AGA and AGG for Arg, GGA
for Gly. Less preferred codons are GUA for val, UCG for
Ser, CCG for Pro, ACG for Thr, GCG for Ala, CGU and
CGC, CGA and CGG for Arg, GGU and GGC for Gly.
Similarly, westnile 1 and westnile 2 virus show similar
choice of codon usage UUG, CUC and CUG for Leu, AUC
for Ile, GUG for Val, UCA for Ser, CCA for Pro, ACC and
ACA for Thr, GCU and GCC for Ala, AGU and AGC for
Ser, AGA and AGG for Arg, GGA for Gly. The less pre-
ferred codons are UUA for Leu, GUA for Val,UCG for Ser,
CCG for Pro,GCG for Ala, CGU and CGA for Arg, GGU
for Gly. In genus Hepacivirus Equine and Bovine show
similar choices for preference of codon in comparison to
the other members of the group. The unclassi ed mem-
bers of Flaviviridae family show similarity with genus
Flavivirus like, Lammi virus shows similarity with mos-
quito born Flaviviruses especially with West Nile virus
in choice of preferred and less preferred codon. Nhu-
mirim virus shows similarity with nkv group of Flavi-
viruses especially with Paraiso virus show similarity in
preferred codons CCC, CCA & CCG for Pro, GCG for Ala,
CGA & CGG for Arg, AGU for Ser and GGA & GGG for
Gly. IIomantsi virus and Donggang virus show similar-
ity in preferred codon CCC & CCG for Pro, GCG for Ala,
CGA & CGG for Arg, AGU for Ser and GGA & GGG
for Gly. IIomantsi ,Lammiand Nienokoue viruses show
higher degree of similarity in choice of preferred codon
with mosquito born Flavivirus. GUG for Gly is the only
common codon in the entire Flaviviridae.
In general, the amount of the over - represented
codon is more than the amount of under - represented
codon in the four genus of family and this feature is
consistent with all the 114 ORF`s, suggesting that the
evolution process of viral genome of all four genus is
similar to some degree and the codon usage bias is weak
which is supporting the results we observed from ENC
values. The nucleotide composition also plays an impor-
tant role in choosing preferred codons, therefore Flavi-
virus and Pestivirus shows preference to A and G ending
codons, as they are rich in purines. And Hepacivirus and
Pegivirus show preference to G and C ending codons as
they are rich in GC content.
In addition, the RSCU values of the eight codon con-
taining CpG (CCG, GCG, UCG, ACG, CGC, CGG, CGU,
and CGA) in four genus were analysed. All of these eight
codons were not preferential codons and were found
suppressed in genus Flavivirus and Pestivirus. In genus
Pegivirus and Hepacivirus six codons are under - repre-
sented except CGC and CGG. The explanation for CpG
scarcity in these viruses is attributed to their property to
escape the host immune response. A high CpG content
leads to increased unmethylated CpGs which has immu-
nostimultory property and therefore are easily recog-
nized by the host’s innate immune system as a pathogen
signature. This is injurious to the small DNA (or RNA)
viruses. Thus high mutational rates are observed in CpGs
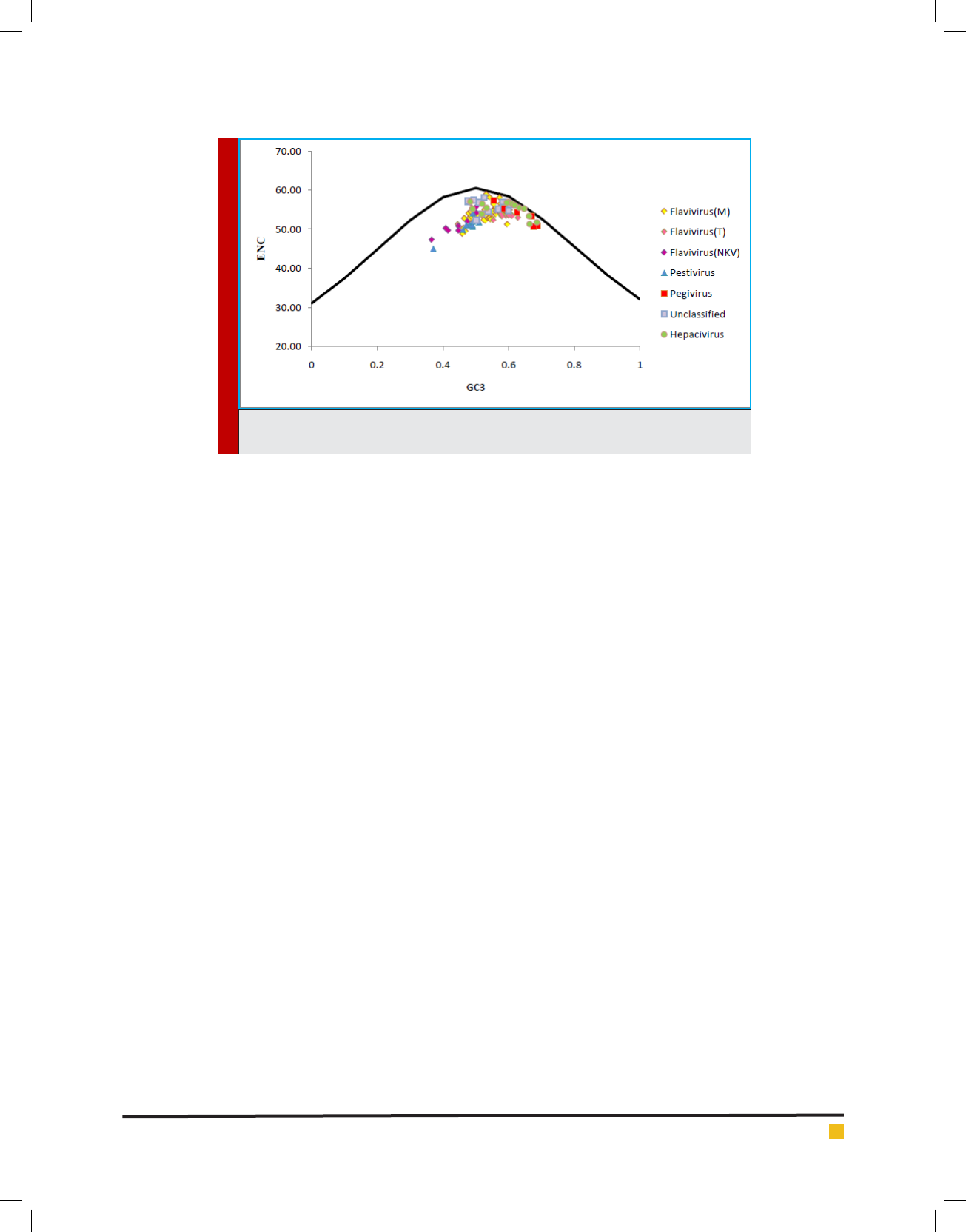
FIGURE 1. The ENC vs GC3 plots of four genus and unclassi ed members (Pestivirus,
Pegivirus, Hepacivirus, and Flavivirus) of Flaviviridae family.
Anjusha Mune etal.
686 GENOME-WIDE COMPARATIVE ANALYSIS OF THE CODON USAGE PATTERN IN
FLAVIVIRIDAE
FAMILY BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
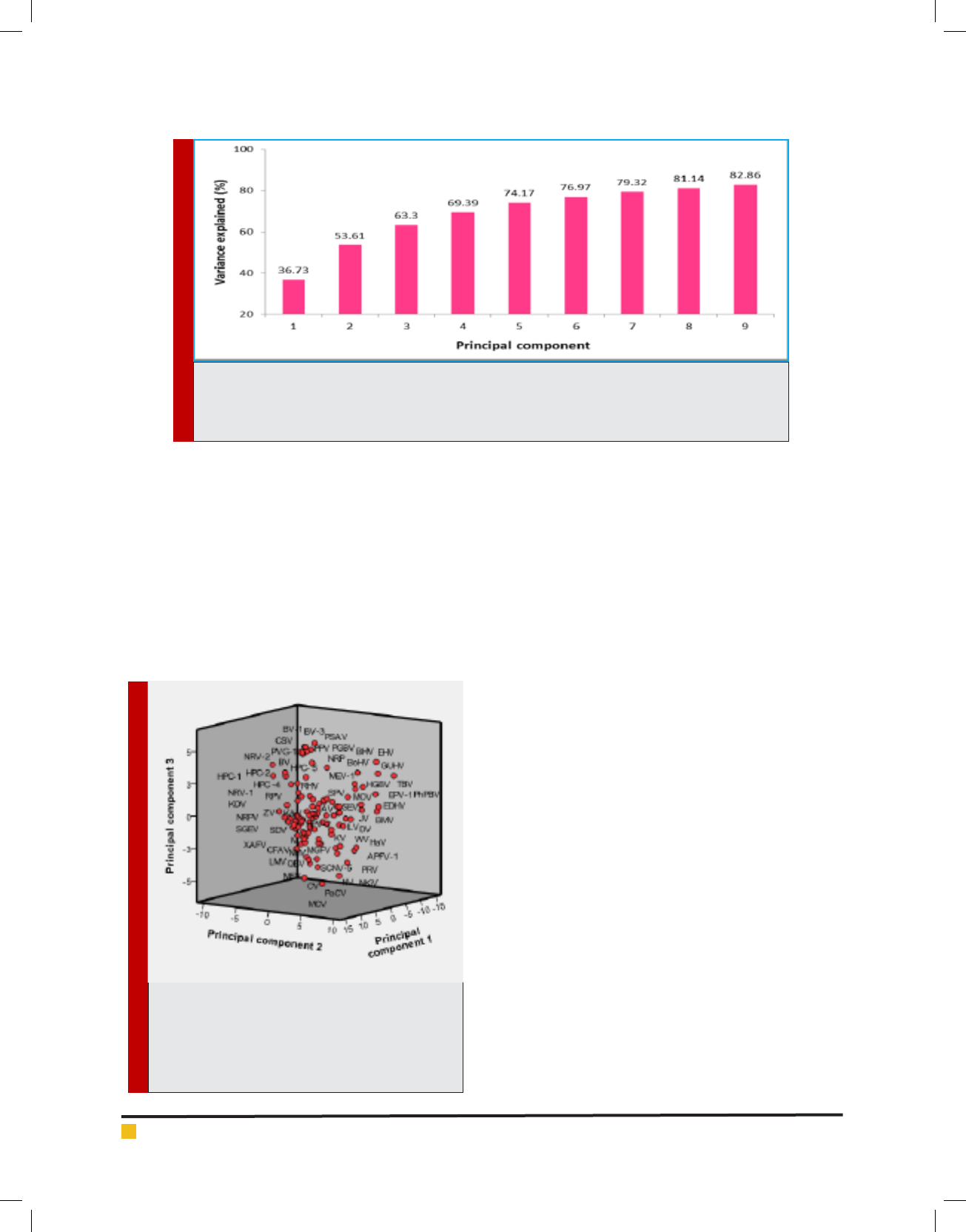
FIGURE 2. Principal components (PCs) and variances explained in the analysis of the 59 relative syn-
onymous codon usage (RSCU) indices. The first 9 PC vectors are listed on with accumulated variance
(%). The plot on the shows that the first ve PCs have explained more than 70% of the variance of the
original data.
FIGURE 3. Principal component analysis (PCA) plot
for analysis of the relative synonymous codon usage
(RSCU) indices of 114 RNA viruses. The PCA scores of
the 114 viruses were plotted in a three-dimensional
coordinate system using the first three principal com-
ponent vectors as axes.
since its de cit will enable virus to infect the host. (Dorn
and Kippenberger, 2008; Krieg, 2003)
CORRELATION ANALYSIS BETWEEN ENC AND
GC
3
VALUE
A plot of ENC versus GC
3
is widely used to study codon
usage variation among different organisms. It is the
most important part of investigation of codon usage
pattern. The ENC values of each member of Flaviviridae
family were plotted against its corresponding GC
3
values
and all values lie below the expected curve as shown in
[Fig. 1]. Therefore it can be hypothesized that the codon
usage bias, in all these 114 viruses is principally in u-
enced by the mutational pressure.
IDENTIFICATION OF SIMILARITIES AND DIFFERENCES IN
CODON USAGE PREFERENCES BY PCA
The identi cation of similarities and differences in
codon usage preferences is an involved process that can
be handled by using the Principal Component Analy-
sis (PCA) approach. The PCA is a classical data analysis
method that identi es patterns and focuses on similari-
ties and differences in a multivariate data set. The explo-
ration of codon usage pattern differences among these
RNA viruses involves processing of the 114 × 59 RSCU
matrix by PCA. This enables calculating the principal
components (PCs) which in turn are employed to high-
light the similarities and differences in codon usages.
[Fig.2] shows the trend of the rst 9 PCs. PCs with Eigen
value greater than or equal to 1 are usually considered
as being of statistical signi cance (the Kaiser criterion)
as indicated in [Supplementary material Table 4] .The
rst PC is associated with 36.73% of the variance among
the 59 RSCU indices. The rst two PCs taken together
account for 53.61% of the variance whereas the rst
three PCs combined together account for 63.30% of the
variance in codon usage.
The variances of a total of 114 PCs generated from
PCA are listed in [Supplementary Material Table 4].
Fig. 3 is the three-dimensional PCA plot using the rst
three PCs of these 114 viruses as axes [the correspond-
ing PCA coordinates are listed in Supplementary Mate-
rial Table 5]. The PCA score diagram shows that the all
viruses can be broadly classi ed into four categories.
This classi cation is essentially based on different hosts,

Anjusha Mune etal.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS GENOME-WIDE COMPARATIVE ANALYSIS OF THE CODON USAGE PATTERN IN
FLAVIVIRIDAE
FAMILY 687
vectors and ecological niche. Genus Flavivirus display
negative values on the second and third PC axes. Mem-
bers of genus Pegivirus displayed positive values on the
three PC axes. The genus Hepacivirus displays more pos-
itive values on rst and third PC axes. The genus Pes-
tivirus displays negative values on rst and second PC
axes whereas all positive values appear on the third axis.
The unclassi ed members manifest a heterogeneous dis-
tribution of values and, consequently, do not represent
a fth category but get merged into the four categories.
CONCLUSION
Our analysis reveals that the overall codon usage bias in
Flaviviridae family is slightly biased and mutation pres-
sure is the main factor that affects codon usage variation
in viruses. Other factors like Compositional constraint and
natural selection also signi cantly in uence codon usage
variation. Results show RNA viruses with same vector
choice share similar codon usage preferences. However,
more detailed analysis is needed to understand the rela-
tionship of codon choices between viruses and hosts.
REFERENCES
Blitvich BJ, Firth AE. (2015) Insect-speci c aviviruses:
a systematic review of their discovery, host range, mode of
transmission, superinfection exclusion potential and genomic
organization. Viruses, Vol.7, 1927-1959.
Brand C, Bisaillon M, Geiss, BJ. (2017) Organization of the
Flavivirus RNA replicase complex. Wiley Interdiscip Rev RNA,
Vol.8.
Butt AM, Nasrullah I, Tong Y. (2013) Genome-Wide Analysis of
Codon Usage and In uencing Factors in Chikungunya Viruses.
PLoS One 9(3): e90905, Vol.9.
Butt AM, Nasrullah I, Qamar R, Tong Y. (2016) Evolution of
codon usage in Zika virus genomes is host and vector speci c.
Emerging Microbes & Infections, Vol.5, 10.
Chen Y. (2013) A Comparison of Synonymous Codon Usage
Bias Patterns in DNA and RNA virus Genomes: Quantifying the
Relative Importance of Mutational Pressure and Natural Selec-
tion. BioMed Research international, Vol.2013.
Cristina J, Moreno P, Moratorio G, Musto H. (2015) Genome-
wide analysis of codon usage bias in Ebola virus. Virus
Research, Vol.196, 87-93.
Dorn A, Kippenberger S. (2008) Clinical application of CpG-,
non-CpG-, and antisense oligodeoxynucleotides as immu-
nomodulators. Current opinion in molecular therapeutics, Vol.
10, 10-20.
Gu W, Zhou T, Ma J, Sun X, Lu Z. (2003) Analysis of synony-
mous codon usage in SARS Coronavirus and other viruses in
the Nidovirales. Virus Research, Vol. 101, 155-161.
Huang YJ, Higgs S, Horne KM, Vanlandingham DL. (2014)
Flavivirus-mosquito interactions. Viruses, Vol.6, 4703-4730.
Jenkins GM, Holmes EC. (2003) the extent of codon usage bias
in human RNA viruses and its evolutionary origin. Virus Res,
Vol. 92, 1–7.
Kanaya S, Kinouchi M, Abe T, Kudo Y, Yamada Y, Nishi T,
Mori H, Ikemura T.(2001) Analysis of codon usage diversity of
bacterial genes with a self-organizing map (SOM): characteri-
zation of horizontally transferred genes with emphasis on the
E.coli O157 genome. Gene, Vol. 276, 89-99.
Krieg AM. (2003) CpG DNA: trigger of sepsis, mediator of pro-
tection, or both? .Scandinavian journal of infectious diseases,
Vol.35, 653-659.
Lobo FP, Mota BE, Pena SD, Azevedo V, Macedo AM, Tauch A,
Machado CR, Franco GR. (2009) Virus-host coevolution: com-
mon patterns of nucleotide motif usage in Flaviviridae and
their hosts. PLoS One, Vol.4, e6282.
Lu G, Huang J, Yang Q, Xu H, Wu P, Fu C, Li S. (2017) Identi -
cation and genetic characterization of Hepacivirus and Pegivi-
rus in commercial equine serum products in China. PLoS One,
Vol.7, 12.
Lu H, Zhao WM, Zheng Y, Wang H, Qi M, Yu XP. (2005) Analy-
sis of synonymous codon usage bias in Chlamydia. Acta Bio-
chim Biophys Sin (Shanghai), Vol. 37, 1-10.
Ma J-J, Zhao F, Zhang J ,Zhou J H, Ma L, Ding Y (2013). Anal-
ysis of Synonymous Codon Usage in Dengue Viruses. Journal
of animal and veterinary advances, Vol. 12, 88-98.
Moosavi.F, Mohabatkar.H, Mohsenzadeh.S. (2011) Analysis of
synonymous codon usage bias and nucleotide and amino acid
composition in 13 species of Flaviviridae. Journal of Cell and
Molecular Research, Vol. 3, 1-11.
Moratorio G, Iriarte A, Moreno P, Musto H, Cristina J. (2013)
A detailed comparative analysis on the overall codon usage
patterns in West Nile virus. Infection, genetics and evolu-
tion: journal of molecular epidemiology and evolution-
ary genetics in infectious diseases. Elsevier, vol. 14, 396-
400.
Sharp PM, Cowe E, Higgins DG, Shields DC, Wolfe KH, Wright
F. (1988). Codon usage patterns in Escherichia coli, Bacil-
lus subtilis, Saccharomyces cerevisiae, Schizosaccharomyces
pombe, Drosophila melanogaster and Homo sapiens; a review
of the considerable within-species diversity. Nucleic Acids
Research, Vol.16, 8207-8211.
Su MW, Lin HM, Yuan HS, Chu WC. (2009) Categorizing host-
dependent RNA viruses by principal component analysis of
their codon usage preferences. Journal of Computational Biol-
ogy, Vol. 16, 1539-47.
Tatarinova TV, Alexandrov NN, Bouck JB, Feldmann KA (2010)
GC3 biology in corn, rice, sorghum and other grasses. BMC
Genomics, Vol.11, 308.
Tao P, Dai L, Luo M, Tang F, Tien P, Pan Z. (2009) Analysis of
synonymous codon usage in classical swine fever virus. Virus
Genes, Vol. 38,104-12.
Tautz N, Tews BA, Meyers G. (2015) The Molecular Biology
of Pestiviruses. Advances in Virus Research, Vol. 93, 47-
160.

Anjusha Mune etal.
688 GENOME-WIDE COMPARATIVE ANALYSIS OF THE CODON USAGE PATTERN IN
FLAVIVIRIDAE
FAMILY BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Theze J, Lowes S, Parker J, Pybus OG. (2015) Evolutionary and
Phylogenetic Analysis of the Hepaciviruses and Pegiviruses.
Genome Biology and Evolution, Vol. 7, 2996-3008.
Van Hemert F, Berkhout B. (2016) Nucleotide composition of
the Zika virus RNA genome and its codon usage. Virology
Journal, Vol.13, 95.
Velazquez-Salinas L, Zarate S, Eschbaumer M, Pereira Lobo
F, Gladue DP, Arzt J, Novella IS, Rodriguez LL. (2016) Selec-
tive Factors Associated with the Evolution of Codon Usage
in Natural Populations of Arboviruses. PLoS One, Vol. 25,
11.
Wang M, Zhang J, Zhou JH, Chen HT, Ma LN, Ding YZ, Liu
WQ, Liu YS. (2011) Analysis of codon usage in bovine viral
diarrhoea virus. Archives of Virology, Vol. 156,153-60.
Wang M, Liu YS, Zhou JH, Chen HT, Ma LN, Ding YZ, Liu WQ,
Gu YX, Zhang J. (2011) Analysis of codon usage in Newcastle
disease virus. Virus Genes, Vol.42, 245-253.
Wang H, Liu S, Zhang B, Wei W.(2016) Analysis of Synony-
mous Codon Usage Bias of Zika Virus and Its Adaption to the
Hosts. PLoS One, Vol.11, 11.
Wong EH, Smith DK, Rabadan R, Peiris M, Poon LL (2010)
Codon usage bias and the evolution of in uenza A viruses.
Codon Usage Biases of In uenza Virus. BMC Evolutionary
Biology, Vol.10, 253.
Wright F. (1990) the ‘effective number of codons’ used in a
gene. Gene, Vol.87, 23-29.
Xiang H, Zhang R, Butler RR 3rd, Liu T, Zhang L, Pombert JF,
Zhou Z (2015) Comparative Analysis of Codon Usage Bias Pat-
terns in Microsporidian Genomes. PLoS One, Vol.10, 6.
Yadav MK, Swati D. (2012) Comparative genome analysis of
six malarial parasites using codon usage bias based tools. Bio-
information, Vol. 8, 1230-1239.
Zhang J, Wang M, Liu WQ, Zhou JH, Chen HT, Ma LN, Ding YZ,
Gu YX, Liu YS. (2011) Analysis of codon usage and nucleotide
composition bias in polioviruses .Virology Journal, Vol. 8, 146.
Zhang X, Jia R, Shen H, Wang M, Yin Z, Cheng A.(2017) Struc-
tures and Functions of the Envelope Glycoprotein in Flavivirus
Infections. Viruses, Vol.9, 338.
Zhong J, Li Y, Zhao S, Liu S, Zhang Z. (2007) Mutation pres-
sure shapes codon usage in the GC-Rich genome of foot-and-
mouth disease virus. Virus Genes, Vol.35, 767-776.
Zhou JH, Gao ZL, Zhang J, Chen HT, Pejsak Z, Ma LN, Ding
YZ, Liu YS.(2012) Comparative codon usage between the three
main viruses in Pestivirus genus and their natural susceptible
livestock. Virus Genes, Vol.44, 475-481
Zhou T, Gu W, Ma J, Sun X, Lu Z. (2005) Analysis of syn-
onymous codon usage in H5N1 virus and other in uenza A
viruses. Biosystems, Vol.81, 77-86.