Biotechnological
Communication
Biosci. Biotech. Res. Comm. 10(4): 676-679 (2017)
PCR-based detection of microsporidia in silkworms
using non-conventional RNA polymerase primers
Gourab Roy, Kalidas Mandal and G. Ravikumar*
Seri-Biotech Research Laboratory, Central Silk Board, Carmelaram Post, Kodathi, Bangalore, 560 035, India.
ABSTRACT
Microsporidia are obligate intracellular, spore-forming parasites that infect both invertebrates and vertebrates. They
infect silkworms causing the deadly pebrine disease leading to heavy crop loss in sericulture. Because of the horizon-
tal and vertical transmittance, outbreaks should be detected at an early stage and persistent infections should also
be identi ed to prevent further transmittance. So far, microscopic examination method remains the conventional
detection method for screening of microsporidia in sericulture. Molecular diagnosis tools have an advantage over
microscopic detection as they are more speci c, sensitive and aid in early detection. Microsporidia detection by PCR
method using primers designed from SSU-rRNA is widely used. In this study, we developed a PCR assay for the detec-
tion of microsporidia using primers designed from the conserved regions of RNA polymerase gene. Under optimized
PCR conditions, the assay yielded a ~650 bp DNA fragment from microsporidia infected silkworms, Bombyx mori
and Antheraea mylitta. Sequence analysis of the ampli ed products has shown homology to various microsporidia
including Nosema bombycis and N. antheraea. No non-speci c products were observed. This method could help in
early detection of microsporidia infection at any developmental stage of the silkworm and thereby reducing the crop
loss.
KEY WORDS: DETECTION, MICROSPORIDIA, PCR
676
ARTICLE INFORMATION:
*Corresponding Author: ravikumarpillai@gmail.com
Received 21
st
Oct, 2017
Accepted after revision 18
th
Dec, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF: 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at:
http//www.bbrc.in/
DOI: 10.21786/bbrc/10.4/10
INTRODUCTION
Silkworm, Bombyx mori is one of the most important
domesticated insects, which produces luxuriant silk
thread in the form of cocoon by consuming mulberry
leaves during larval period. In India the bulk of the
commercial silk produced is mulberry silk whereas, Eri,
Tasar & Muga silk contribute to a lesser extent. These
silkworms are susceptible to various diseases resulting
in substantial crop loss which is estimated to be 40%
in India (Singh et al., 2012). The common pathogens
infecting them are microsporidians including Nosema

Gourab Roy, Kalidas Mandal and G. Ravikumar
bombycis, nucleopolyhedrovirus (NPV) and densovirus
(mainly DNV1&2), infectious acherie virus (IFV), cyto-
plasmic polyhedrovirus (CPV) and bacteria. The micro-
sporidians cause pebrine disease; NPV causes grasserie;
and DNVs, IFV and bacterial pathogens cause ach-
erie diseases. Among all, the microsporidians disease
is responsible for the signi cant economic loss in the
sericulture industry. Microsporidiasis remained a threat
to silk industry since time immemorial, because of its
unique and recurrent occurrence and is the only dis-
ease transmitted both horizontally and vertically (Bhat
et al., 2009). Several species and strains of microsporidia
have been isolated from infected silkworms among
which pebrine caused by Nosema bombycis is the most
prevalent. Other microsporidian species (Vairimorpha,
Pleistophora, Thelohania etc.) which differ in their spore
morphology, sites of infection and virulence, have also
been isolated from silkworms (Kawarabata, 2003, Gupta
et al., 2017).
Since the control of disease is often met with lim-
ited success, early detection of pathogens is essential to
control of emerging, reemerging, and in preventing the
spread of infectious diseases. Microsporidian are easily
detected by light microscopy when infections are heavy
and spores are present. However, early infections without
spores, or light infections with low numbers of spores are
easily missed. This limitation has made it dif cult to con-
duct investigations into microsporidian prevalence and
transmission. To overcome these dif culties, PCR- based
techniques have been developed to detect the major path-
ogens of silkworms with great speci city and sensitivity
(Hatakeyama and Hayasaka 2003, Hamiduzzaman et al.,
2010, Ravikumar et al., 2011, Fu et al., 2016).
Due to the availability of sequence information and
the presence of conserved and variable sequence regions
within the SSU rRNA genes, PCR-based methods have
typically used primers of this gene for the detection of
microsporidians (Franzen and Muller, 1999). Herein, we
report that primers designed from the RNA polymerase
of microsporidians can also be used to detect micro-
sporidians from silkworms. To our knowledge, this is the
rst report on the detection of microspordians using its
RNA polymerase primers from silkworms.
MATERIALS AND METHODS
SILKWORM AND MICROSPORIDIAN INFECTION
The silkworm rearing and microsporidian infection were
essentially performed as reported by us (Ravikumar
et al, 2011). Silkworms, B. mori (Pure Mysore) were fed
on mulberry leaves. For Microsporidian infection, 3rd
instar day 1 larvae were orally fed with 2000 spores/
larva and periodical observations were taken. Control
larvae did not receive microsporidian infection. On 4
th
and 8
th
day post infection (p.i.), larval mid gut tissues
were dissected out and used for DNA extraction, fol-
lowed by PCR. DNA was also extracted from pupa, adult
and eggs of infected and normal silkworms.
DNA EXTRACTION
DNA extracted from the mid gut of infected and control
using Hi-Pure DNA extraction Kit (Himedia) according to
manufacturer’s protocol. DNA from mulberry leaves and
pebrine infected A. mylitta DNA were used as reported
earlier (Ravikumar et al., 2011). The DNA was analyzed
in 1% agarose gel electrophoresis and quanti ed using a
Nanodrop (Thermo Corporation) spectrophotometer.
PCR AND CLONING
A set of primers were designed from the conserved region of
available microsporidian RNA polymerase sequences from
NCBI database. The primers used were: Sense: 5’-CCICAY-
TTYCCIAARGARGAYTA-3’ and antisense: 5’-AARGAY-
ITIGARGGIACIAAYGA-3’. (I: deoxyinosine; R: A, G; Y: T,
C). PCR reactions were carried out using 1X Taq buffer,
2.5 mM dNTPs, 25 mM MgCl2, 0.5U Taq DNA polymerase
(Fermentas) and 100 ng of DNA. The DNA from control
silkworms and mulberry DNA were employed as negative
controls. PCR reactions were carried out (Eppendorf) using
the following cycles: 94°C for 2 min, 30 cycles of 94°C for
40 s, 48°C for 30 s, and 72°C for 30s and 1 cycle of 72° C
for 5 min. PCR products were analyzed in 1% agarose gel
electrophoresis, stained in Sybergreen (HiMedia) and visu-
alized under UV transillumination. The PCR products were
cloned in pJET blunt end cloning vector (Fermentas) and
positive clones were con rmed by colony PCR. Puri ed
plasmids were sequenced at Euro ns, Bangalore, followed
by BLAST analysis (NCBI).
RESULTS AND DISCUSSION
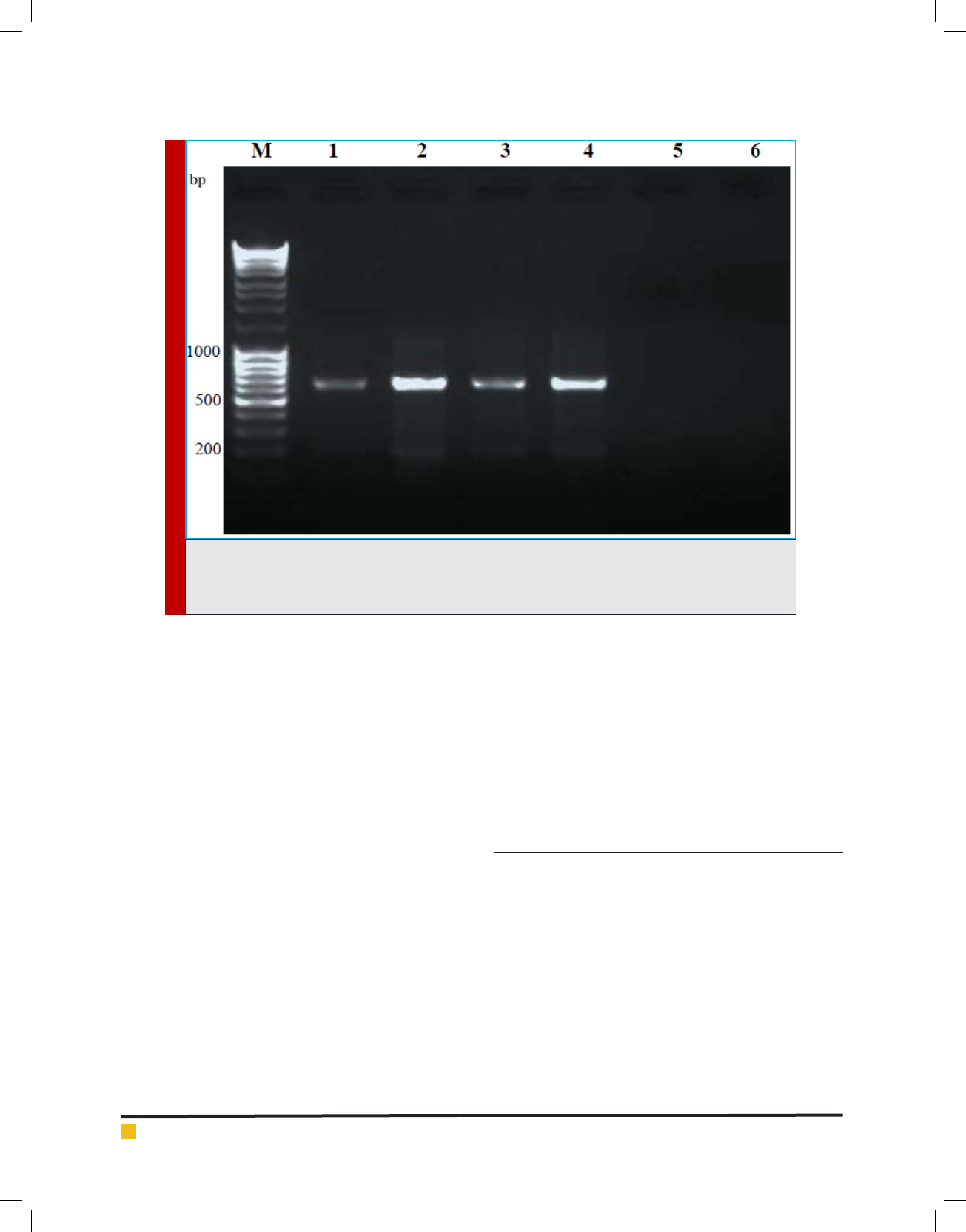
Results are presented in Figure 1. PCR ampli cations
have resulted in discrete and desired product. DNA
extracted from the microsporidian infected silkworm
yielded speci c ampli cation products of ~ 650 bp (Lane
1-4) using RNA polymerase primers. No non- speci c
products were observed. The negative controls; DNA
from normal silkworm and the plant DNA from mul-
berry showed no PCR products, indicating the speci c-
ity of the PCR. The banding intensity on day 8th was
higher to that of on 4th day showing the proliferation
of pathogen at an advanced stage of infection. Fur-
ther con rmation of the PCR products was done by
sequencing and BLAST analysis. BLAST showed 92- 99
% homology to RNA polymerases of various isolates of
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PCR-BASED DETECTION OF MICROSPORIDIA IN SILKWORMS 677
Gourab Roy, Kalidas Mandal and G. Ravikumar
FIGURE 1. PCR ampli cation of microsporidia DNA (~650bp). M: Molecular weight marker, Lane 1&2: DNA
from microsporidia infected B. mori larvae, day 4 and 8 p.i., respectively; Lane 3&4: DNA from micro-
sporidia infected A. mylitta larvae day 4 and 8 p.i. Lane 5&6: DNA from uninfected B. mori and mulberry
leaves, respectively, were used as controls.
N. bombycis, Nosema Sp, N. antheraea, N. ceranae, N.
pernyi and other microsporidia. The same results were
obtained from other developmental stages, pupa, adult
and eggs (data not shown) of the silkworm. In addi-
tion, the same primer sets could detect microsporidian
of tasar silkworm A. mylitta showing the ef cacy of the
RNA polymerase-based primers in detecting microspo-
ridian of other silkworm species than B. mori. The con-
served regions of RNA polymerase gene was effectively
utilized for the detection of microsporidia in the present
work and it can be used for detection of microsporidia
of other insects/organisms also. Highly conserved SSU-r
RNA gene primers were successfully used for the detec-
tion and classi cation of microsporidia across organ-
isms with high speci city and sensitivity (Weiss and
Vossbrinck, 1999; Jehle et al., 2006, Ravikumar et al.,
2011). PCR diagnosis of N. pernyi using SSU-rRNA prim-
ers provided increased speci city and sensitivity when
compared with light microscopy in Antheraea pernyi
(Jiang et al., 2011). For the effective control of pebrine
disease, outbreaks should be detected at an early stage
and persistent infections should also be identi ed to
prevent further transmittance of the disease.
In our study, microsporidia were detected by PCR at
4th day of p.i, whereas the spores were visible under
microscope only on day 8 and afterwards. Hence, this
method can be useful in the early detection of micro-
sporidia which is critical in reducing crop loss in seri-
culture. Further, real-time quantitative PCR assay can
be used with RNA polymerase primers for increased
sensitivity. The results of this study suggest that RNA
polymerase primers from microsporidia can be used
for pebrine detection in sericulture. To the best of our
knowledge, this is the rst study in which PCR was used
for the successful detection of microsporidia using RNA
polymerase primers in silkworms.
ACKNOWLEDGEMENTS
Authors are thankful to Messrs. S. N. Gundurao and N.
Pillapa for rearing silkworms. Financial support was
provided by Central Silk Board (CSB), Bangalore, India.
Mr. Gourab Roy is thankful to CSB for a Junior Research
Fellowship.
REFERENCES
Bhat S. A., Bashir I., Kamili A. S. (2009) Microsporidiosis of
silkworm, Bombyx mori (Lepidoptera- Bombycidae): A review,
African Journal of Agricultural Research, 4, 1519-1523.
Franzen C. and Müller A. (1999) Molecular techniques for
detection, species differentiation and phylogenetic analysis of
microsporidia, Clinical Microbiology Reviews, 12, 243-285.
678 PCR-BASED DETECTION OF MICROSPORIDIA IN SILKWORMS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Gourab Roy, Kalidas Mandal and G. Ravikumar
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PCR-BASED DETECTION OF MICROSPORIDIA IN SILKWORMS 679
Fu Z., He X., Cai S., Liu H., He X., Li M., Lu X. (2016) Quantita-
tive PCR for detection of Nosema bombycis in single silkworm
eggs and newly hatched larvae Journal of Microbiological
Methods, 120, 72-78.
Gupta S. K., Hossain Z., Nanu M. M., Mondal K. (2017) Impact
of microsporidian infection on growth and development of
silkworm Bombyx mori (Lepidoptera: Bombycidae), Agriculture
and Natural Resources, 50, 388-395.
Hamiduzzaman M., Novoa E. G., Goodwin P.H. (2010) A multi-
plex PCR assay to diagnose and quantify Nosema infections in
honey bees (Apis mellifera), Journal of Invertebrate Pathology,
105, 151-155.
Hatakeyama Y. and Hayasaka S. (2003) A new method of
pebrine inspection of silkworm egg using multiprimer PCR,
Journal of Invertebrate Pathology, 82, 148-151.
Jehle J.A., Lange M., Wang H., Hu Z., Wang Y., Hauschild R.
(2006) Molecular identi cation and phylogenetic analysis of
baculoviruses from Lepidoptera, Virology, 346, 180-193.
Jiang Y. R., Deng Z. H., Shi S.L., Yang R. S., Li Y.Z., Duan
Y. X., Qin L. (2011) Development of a PCR-based method for
detection of Nosema pernyi, African Journal of Microbiology
Research, 5, 4065-4070.
Kawarabata T. (2003) Biology of microsporidians infecting
silkworm, Bombyx mori, in Japan-Review, Journal of Insect
Biochemistry and Sericology, 72, 1-32.
Ravikumar G., Raje Urs S., Vijaya Prakash B., Rao C.G.P., Vard-
hana K.V. (2011) Development of a multiplex polymerase chain
reaction for the simultaneous detection of microsporidians,
nucleopolyhedrovirus, and densovirus infecting silkworms,
Journal of Invertebrate Pathology, 107, 193-197.
Singh T., Bhat M. M., Khan M. A. (2012) Microsporidiosis
in the silkworm, Bombyx mori (Lepidoptera: Bombycidae),
Pertanika Journal of Tropical Agriculture Science, 35, 387-
406.
Weiss L. M. and Vossbrinck C. R. (1999) Molecular biology,
molecular phylogeny, and molecular diagnostic approaches
to the microsporidia. In: Wittner, M., Weiss, L.M. (Eds.), The
Microsporidia and Microsporidiosis. American Society for
Microbiology, Washington, DC, pp. 129-171.