Microbiological
Communication
Biosci. Biotech. Res. Comm. 10(4): 612-622 (2017)
Production and partial characterization of extracellular
polysaccharide from endophytic
Bacillus cereus
RCR 08
Ananya Mukherjee
1
, Rituparna Das
1
, Anju Sharma
1
, Arundhati Pal
2
and A. K. Paul
1
*
1
Microbiology Laboratory, Department of Botany, University of Calcutta, 35, Ballygunge
Circular Road, Kolkata, India
2
Post Graduate Department of Botany, Serampore College, 9, William Carey Road, Serampore, Hooghly, India
ABSTRACT
The present study focuses attention on the production of extracellular polysaccharide (EPS) by bacterial endophytes
of Ricinus communis L. Among the 28 endophytic bacterial isolates screened for EPS production, a potent isolate
identi ed as Bacillus cereus RCR 08 (GenBank accession number MF159112) produced signi cant amount of EPS in
mineral salts medium under batch culture. In single factor system of analysis, glucose and ammonium chloride were
most suitable carbon and nitrogen sources respectively for EPS production. Maximum growth (7.1 g/L) and EPS yield
(10.24 g/L) was attained when glucose and ammonium chloride were used in the ratio of 25:1. The isolated polymer
contained carbohydrate (88.8%), protein (3.18%), RNA (6.0%) as well as DNA (3.2%) and showed characteristic FTIR
absorption spectrum with peaks at 3404, 2,933, 1,655, and 1,042 cm
−1
. The emulsifying activity of the EPS was more
or less comparable with Tween 80. Though the EPS failed to show any antibacterial activity, it exhibited moderate
DPPH radical scavenging activity and displayed a dose-dependent cytotoxic activity against hepato cellular car-
cinoma (Huh 7.5) cell line in MTT assay. A detailed physico-chemical analysis is, therefore, essential to assess the
signi cance and potential importance of this endophytic EPS in biotechnology.
KEY WORDS:
BACILLUS CEREUS
, ENDOPHYTIC BACTERIA, EXTRACELLULAR POLYSACCHARIDE, FTIR ANALYSIS,
RICINUS COMMUNIS
L.
612
ARTICLE INFORMATION:
*Corresponding Author: amalk_paul@yahoo.co.in
Received 11
th
Nov, 2017
Accepted after revision 19
th
Dec, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF: 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at:
http//www.bbrc.in/
DOI: 10.21786/bbrc/10.4/3
INTRODUCTION
Endophytes are microorganisms which colonize living
internal tissues of plants without causing any apparent
negative impact on the host plant. They occur ubiqui-
tously in almost all plants and are bene tted from the
host by deriving organic nutrients, shelter as well as
transmission to the next host generation. On the other
hand endophytes favour the infected host plants by
xation of atmospheric nitrogen, production of growth

Ananya Mukherjee et al.
promoting substances, imparting tolerance to stress and
toxicity to herbivores, nematodes and pathogens (Borges
et al., 2009). Recently, attention has been paid to the
endophytes for the production of biopolymers including
extracellular polysaccharides (EPS) and their utilization
for potential industrial applications (Donot et al., 2012;
Kusari et al., 2014).
Microbial extracellular polymeric substances, the
heterogenous matrix of polymers comprising of poly-
saccharides, proteins, nucleic acids, uronic acids, humic
substances, lipids etc. (Wingender et al., 1999) are bio-
synthesized by bacteria and fungi via intracellular or
extracellular processes (Freitas et al., 2011). In recent
years, a variety of structurally different EPSs with bio-
active potentials have been reported from endophytes
(Guo et al., 2014; Mahapatra and Banerjee, 2016; Liu
et al., 2017 ).
Production of such endophytic EPS in culture depends
on media components such as carbon and nitrogen
sources, minerals, surfactants and cultivation conditions
including incubation temperature, pH and aeration (Liu
et al., 2009). The EPS so produced in their natural habitat
play a key role in plant-endophyte interactions and are
essential for the survival in the host plant (Wingender et
al., 1999). Owing to their interesting physico-chemical
and biological activities, the endophytic EPS has been
considered as potential candidate for nutraceuticals,
bioleaching, bioremediation, waste water treatment and
pharmaceutical industries. Special attention has also
been paid for the use of EPS as a hydrophilic matrix for
controlled release of drugs (Gandhi et al., 1997), anti HIV
agent (Yamada et al., 1997), enhancement of nonspeci c
immunity (Sutherland, 1998), antimicrobial (Orsod et
al., 2012), antioxidant (Liu et al., 2009) and antitumour
activities (Chen et al., 2013). Furthermore, they have
been found to be extremely susceptible for biodegrada-
tion in nature and thus are environment friendly.
Ricinus communis L. (Euphorbiaceae), a perennial
owering shrub, is an indigenous oil-yielding plant of
India having medicinal as well as agrochemical impor-
tance. Its oil and seeds have been used in folk medicine
for disorders like severe constipation, worm infestation,
rheumatism, intestinal in ammation and also for birth
control. Castor oil is an effective motor lubricant and
also used as a component of avour and ingredient for
preparing protective coatings for tablets. Apart from
these, a range of biologically active compounds have
been isolated especially from rhizosphere and endo-
sphere associated fungi of R. communis L. with probable
industrial applications (Rajkumar and Freitas, 2008; Jain
and Sharma, 2015).
The increasing demand for natural polymers with
industrial applications has thus led to an interest in EPS
production by microorganisms which have high yield
and better quality than plant or animal derived poly-
saccharides (Moscovici, 2015). In this article we report
the screening of bacteria endophytic to R. communis L.
for production of EPS, determine the in uence of nutri-
tional and environmental conditions for EPS production
by a selected potent strain under batch culture and char-
acterization of the partially puri ed polymer.
MATERIAL AND METHODS
A total of 28 endophytic bacterial isolates of Ricinus
communis L. used in the present study were isolated in
the Microbiology Laboratory, Department of Botany,
University of Calcutta. Pure cultures of endophytic bac-
terial isolates were maintained on slopes of nutrient agar
by repeated sub-culturing at an interval of 30 days.
The selected potent EPS producing isolate was char-
acterized following standard morphological and physio-
biochemical tests (Gerhardt et al., 1994). Antibiotic sen-
sitivity of the bacterial isolate was detected following the
Kirby Bauer disc-diffusion assay (Bauer et al., 1966) using
antibiotic impregnated discs (Himedia, India, 6 mm dia).
The 16S rRNA gene sequence of the isolate was deter-
mined by direct sequencing of PCR ampli ed 16S rDNA.
The genomic DNA was isolated from the overnight
grown culture and puri ed according to the modi ed
method of Marmur (1961). The 16S rDNA was ampli ed
using the universal primers 27F (5’AGAGTTTGATCCTG-
GCTCAG3’) and 1492R (5’GGTTACCTTGTTACGACTT3’)
and the ampli ed product was puri ed using QIAquick
gel extraction kit (Qiagen, Netherlands). The sequencing
reaction was performed with ABI PRISM Dye Termina-
tor cycle-sequencing ready reaction kit (Applied Biosys-
tems) and products were puri ed and electrophoresed on
polyacrylamide sequencing gel using an ABI 377 auto-
mated DNA sequencer. Sequencing data were analyzed
by ABI version 3.0.1 b3 software and compared with ref-
erence sequences using the NCBI BLASTN programme.
Multiple sequence alignments were carried out by using
BLOSUM 62 matrix with the program package Clustal-
W employing the neighbour-joining algorithm method
(Saitou and Nei, 1987) with MEGA version 6.0.
Mineral salts medium was inoculated with freshly pre-
pared inoculum (2% v/v) of the endophytic bacteria and
incubated at 32 °C on rotary shaker (120 rpm). Growth
was determined by measuring dry weight of the washed
cell mass harvested by centrifugation (10,000×g, 20 °C
for 10 min). Cell pellet was transferred to pre-weighed
aluminium cup and dried to constant weight at 80 °C.
The EPS of the cell-free culture ltrate was precipitated
with double volume of chilled acetone, kept overnight
at 4 °C and recovered by centrifugation (12,000×g, 4 °C
for 20 min). Cell-bound EPS was extracted with EDTA
(0.05M), precipitated with chilled acetone and recovered
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE 613

Ananya Mukherjee et al.
by centrifugation. The soluble and cell-bound EPS frac-
tions were pooled and dissolved in known volume of
distilled water prior to quanti cation.
The EPS was quanti ed following the phenol sulphu-
ric acid method of Dubois et al. (1956). To 1 mL of EPS
solution, 1 mL of 5% (w/v) phenol solution was added
and mixed thoroughly. To the reaction mixture, 5 mL
of concentrated H
2
SO
4
was purged in and the optical
density was measured at 490 nm using Systronics col-
orimeter. The amount of EPS was determined from the
calibration curve using glucose as the standard.
For isolation and puri cation of the EPS, the selected
isolate RCR 08 was grown in mineral salts medium
under continuous shaking for 64 h. The cell-bound EPS
was extracted following washing of cell mass with 0.05
M EDTA and separated by centrifugation (12,000 × g
for 20 min). The EPS was recovered from the superna-
tant by precipitation with chilled acetone. The soluble
EPS from the cell-free culture ltrate was obtained by
the same acetone precipitation method. The soluble and
bound EPS were pooled, dissolved in distilled water and
subjected to dialysis in sterile water for 24 h at 4 °C with
regular change of dialysate. On completion of dialysis,
the EPS was further treated with chilled acetone at 4
°C and the precipitate was collected by centrifugation
(12,000×g, 4 °C, 20 min) as partially puri ed EPS. To
remove protein and nucleic acid, trichloroacetic acid
(20%) was added to the partially puri ed EPS solution
and incubated in ice for 30 minutes prior to centrifuga-
tion (15,000 × g, 4 °C, 30 min) (Bales et al., 2013). The
supernatant was treated with double volume of chilled
ethanol at 4 °C and the precipitate was collected by cen-
trifugation (12,000 × g, 4 °C, 20 min).
The partially puri ed EPS was analyzed for its car-
bohydrate, protein and nucleic acid contents. While
the carbohydrate content was estimated following the
phenol-sulphuric acid method of Dubois et al. (1956),
protein content was estimated by folin-phenol reagent
using bovine serum albumin as standard (Lowry et al.,
1951). DNA and RNA contents of the EPS were estimated
by diphenylamine (Soni et al., 2011) and orcinol meth-
ods (Almog and Shirley, 1978) respectively.
The absorbance of the crude and puri ed EPS in dis-
tilled water was recorded in the range of 200 to 300 nm
using UV-VIS spectrophotometer (Jenway, Model 6505).
The Fourier transform infrared (FTIR) spectra of the
puri ed EPS were recorded in a Perkin Elmer RX-1 FTIR
spectrometer. The dried sample was grinded with potas-
sium bromide (KBr) and pressed into pellet for spectro-
photometric scanning in the frequency of 400 to 4000
cm
−1
.
The emulsi cation assay was carried out following
the method as described by Cooper and Goldenberg
(1987). The puri ed EPS solution (2.5 mL, 0.5% w/v) was
mixed with 2.5 mL hydrocarbons, vortexed to homog-
enity and left to stand for 24 h at 4 °C. The emulsi-
fying activity was expressed as the percentage of the
total height occupied by the emulsion. The hydrocarbon
substrates used were benzene, palm oil, olive oil, soy-
bean oil, kerosene, petrol, octane, hexane, tetradecane
and hexadecane.
The aqueous solution of puri ed EPS was lter steri-
lized and screened for antibacterial activity following
agar-cup assay method using four test organisms like
Escherichia coli, Bacillus subtilis, Staphylococcus aureus
and Pseudomonas cepacia. Modi ed method of Liu et al.
(2010) was used for the DPPH radical scavenging activ-
ity of the EPS. The reaction mixture containing 0.5 mL
of puri ed EPS, 0.2 mL of DPPH solution (0.4 mM DPPH
in methanol) and 2.5 mL distilled water was shaken vig-
orously, incubated for 30 min at room temperature and
the optical density was measured at 517 nm. Vitamin
C (ascorbic acid) was used as the positive control. The
percentage of scavenging of free radical was calculated
according to the following formula:
% scavenging activity = {1- (A
1
-A
2
/A
0
)} × 100
Where,
A
1
= O.D. of reaction mixture
A
2
= O.D. of reaction mixture without DPPH
A
0
= O.D. of reaction mixture with DPPH but without
sample
Proliferation of Huh 7.5 cells in response to EPS
produced by B. cereus RCR 08 was measured by using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay as described by Slater et al. (1963).
Huh 7.5 cells in DMEM medium were incubated over-
night in 96 microtiter plate. The cells were treated with
lter sterilized EPS of different concentrations and
incubated for 48 h following an additional incubation
of 4 h with 20 μL of MTT (5 mg/mL). The MTT-trans-
formed crystals were dissolved in MTT solvent [4 mM
HCl, 0.1% Nondet P-40 (NP40) in isopropanol] and the
absorbance was measured at 595 nm with a reference
lter of 620 nm by using a microplate reader (Molecular
Devices, Sunnyvale, USA). The relative cell viability was
expressed as the mean percentage of viable cells relative
to the respective control.
All experiments were carried out in triplicates and
results represent mean ± standard deviation.
RESULTS AND DISCUSSIONS
Endophytic microorganisms, the bacteria in particular
have long been recognized as important bioresources
for production of structurally and functionally diverse
extracellular polymeric substances. All 28 endophytic
614 PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE 615
Ananya Mukherjee et al.
Table 1. Screening of bacterial endophytes of
Ricinus communis L. for production of extracellular
polysaccharide
Category of
producer
Production of
EPS, g/L
% EPS
producer*
Good >1.0 3.57
Moderate 0.31-1.0 85.71
Poor 0.14-0.3 10.71
*Expressed out of total 28 isolates
FIGURE 1. Colony morphology of potent EPS produc-
ing bacterial isolate Bacillus cereus RCR 08 endophytic
to root tissues of Ricinus communis L. in mineral salts
agar plate
bacteria isolated from Ricinus communis L. were screened
for EPS production during growth under batch cultiva-
tion in glucose containing mineral salts medium. The
EPS content (bound and free) of each isolate was quan-
ti ed in terms of their carbohydrate content (Dubois et
al., 1956) and almost all the endophytic isolates of R.
communis L. were capable of producing EPS (Table 1).
Majority of the isolates were poor to moderate producers
with the exception of isolate RCR 08, which produced
good amount of EPS (1.5 g/L) and was selected for fur-
ther studies. Liu et al. (2017) in a recent review have
summarized the EPS-producing endophytic bacteria and
their host plants which include rice, sorghum, sugar-
cane, Artimisia annua, Ophiopogon japonicas, etc.
Morphological and physiological analysis revealed
that the endophytic isolate RCR 08 endophytic to root
tissues of R. communis L. is a rod-shaped, Gram-pos-
itive, motile and endospore forming bacterium which
form white smooth colonies on mineral salts agar (Figure
1). The isolate could tolerate wide range of pH (3.5-8.0)
and temperature (30-40 °C) and produced a number of
hydrolytic enzymes such as catalase, amylase, protease,
pectinase, lipase, gelatinase and inulinase. It produced
acid from glucose, fructose, sucrose, maltose and galac-
tose and was resistant to antibiotics ampicillin, bacitra-
cin, penicillin and methicillin. Based on these charac-
teristics, the endophytic isolate RCR 08 was tentatively
identi ed as a member of the genus Bacillus. Sequence
analysis of 16S rDNA of the isolate Bacillus RCR 08
showed 99% similarity with Bacillus cereus strain ATCC
14579, reasonably high score and e-value being zero.
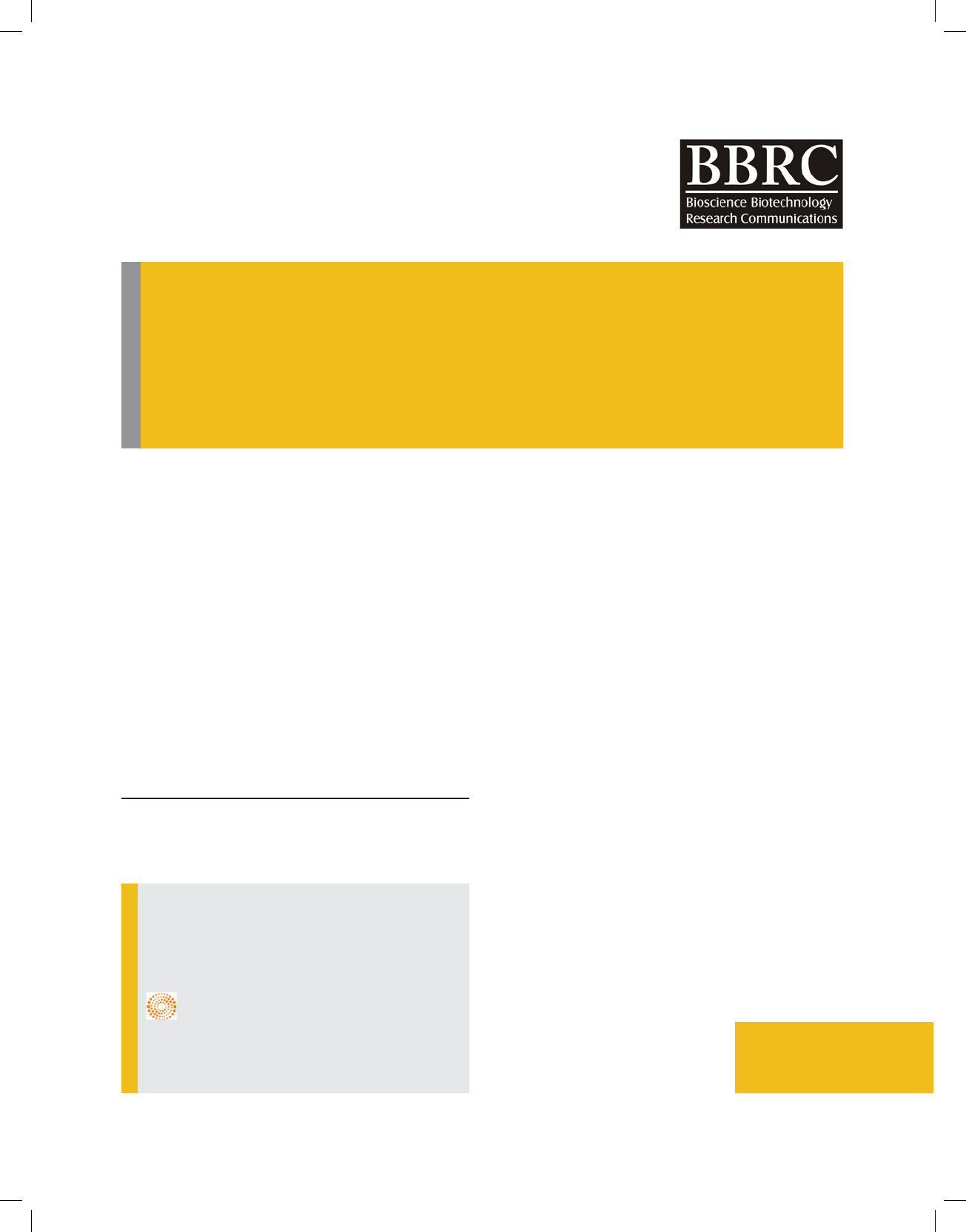
The evolutionary relationship of the endophytic isolate
RCR 08 as depicted from the dendrogram showed clear
rooted evolution (Figure 2). The 16S rDNA sequence of
the isolate RCR 08 has been deposited to the GenBank
under the accession number MF159112 and the isolate
has been designated as Bacillus cereus RCR 08. Similar
to B. cereus RCR 08, production of EPS by B. cereus
SZ1 endophytic to Artimisia annua L. is not uncommon
(Zheng et al., 2016). Likewise endophytic B. amylolique-
faciens (Chen et al., 2013) and B. licheniformis (Singh
et al., 2011) isolated from Ophiopogon japonicas and
Gracilaria dura respectively are well recognized as EPS
producers.
The production of EPS by bacteria in culture depends
on phases of growth, media components, nutritional
status and the environmental conditions. Media com-
ponents including carbon and nitrogen sources, min-
eral elements, etc. on EPS production have been tested
using the single factor method. Out of eight different
media tested, B. cereus RCR 08 showed maximum EPS
production (7.65 g/L) in ammonium chloride containing
mineral salts medium (Table 2). Yeast extract medium
supported signi cant biomass formation but not the EPS
production. Tryptic soy and Luria Bertani media failed
to support both biomass as well as EPS production by B.
cereus RCR 08.
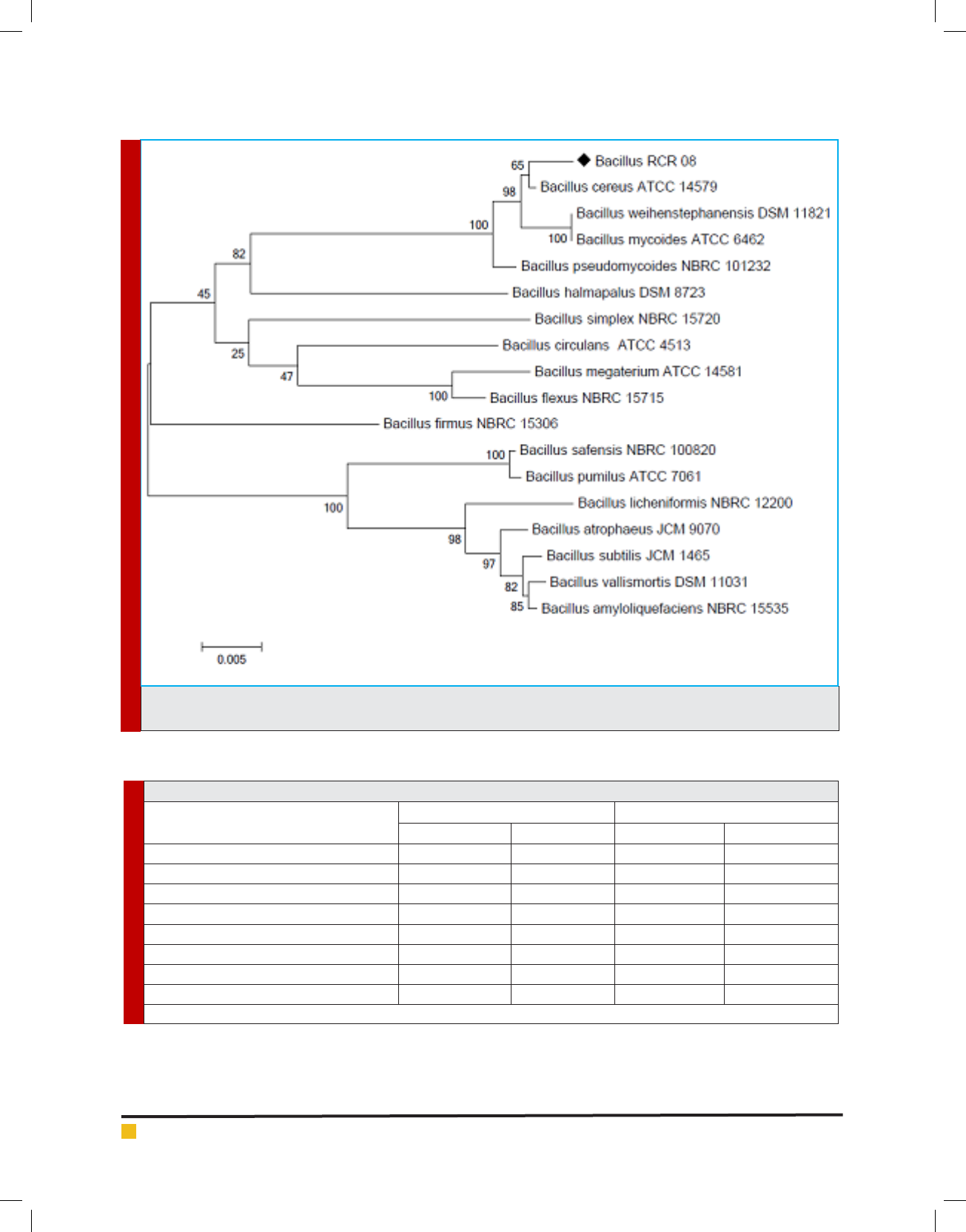
During growth under shake ask condition in min-
eral salts medium, the extracellular polymer accumu-
lation by the endophytic isolate B. cereus RCR 08 was
found to be more or less parallel with growth and con-
tinued to increase till late stationary phase of growth.
The highest EPS production (9.48 g/L) was obtained after
64 h of incubation (Figure 3). This supports the earlier
observations of Decho (1990) and Manca et al. (1996).
EPS synthesis was accompanied by increasing cell mass
formation until glucose, the sole source of carbon, was
consumed. In addition, production of EPS was accompa-
nied with decline of pH of the medium (data not shown).
Ability to utilize nine different carbon sources for
growth and EPS production by B. cereus RCR 08 was
tested and EPS production was highest (9.48 g/L) in glu-
cose followed by mannitol (6.93 g/L) and maltose (5.07
g/L) (Figure 4). Though, the isolate B. cereus RCR 08 pre-
ferred maltose for growth, it failed to utilize galactose.
Different carbon sources have been utilized for EPS pro-
duction by endophytes (Liu et al., 2009; Bragadeeswaran
et al., 2011) and glucose and sucrose are reported to be
the most suitable ones.
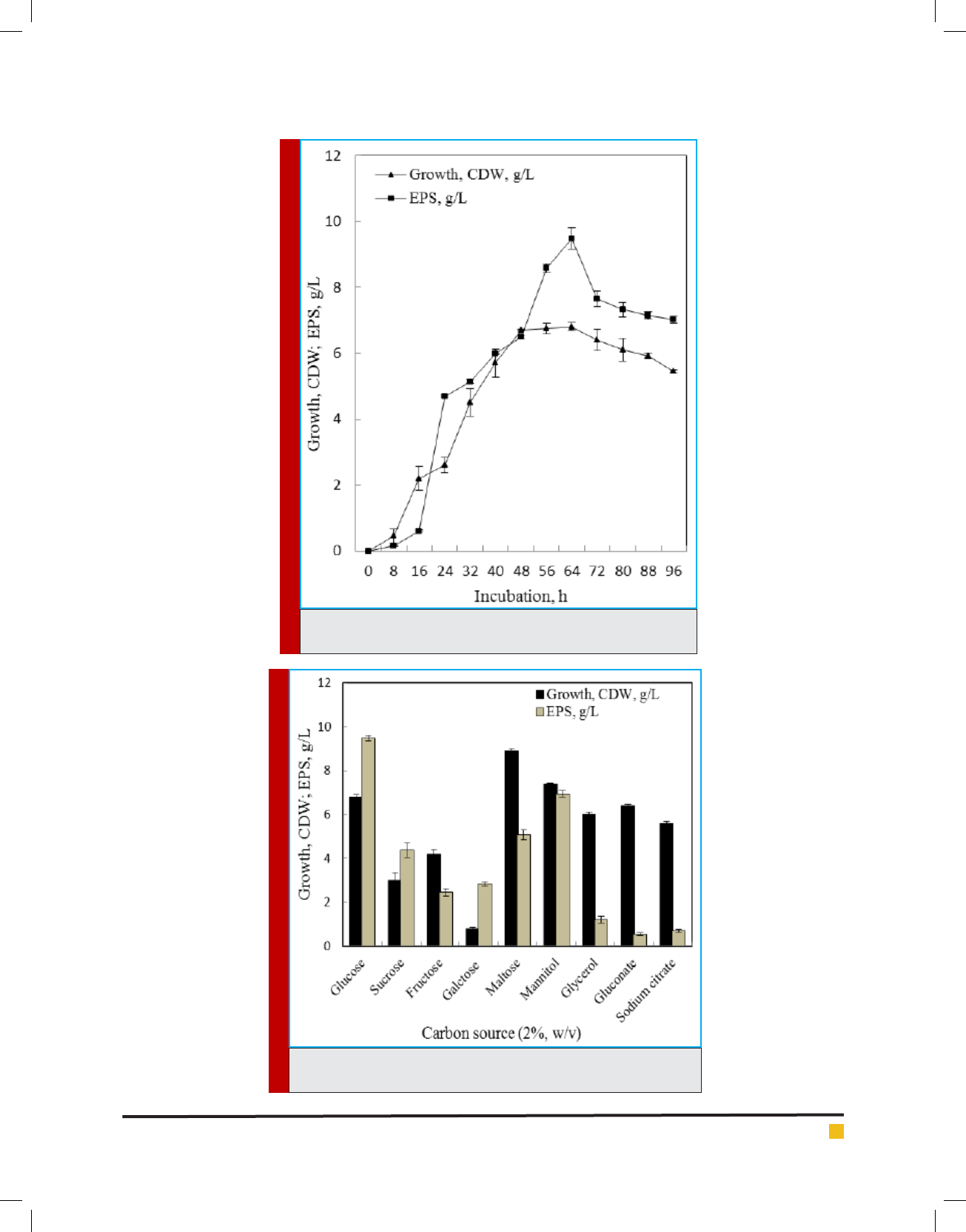
Recently, Li et al. (2016) and Mahapatra and Baner-
jee (2016) have showed that organic nitrogenous com-
pounds supported higher biomass and EPS yield than the
inorganic ones. Supplementation of both inorganic and
organic nitrogen in the growth medium at 0.1% (w/v)
616 PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Ananya Mukherjee et al.
FIGURE 2. Phylogenetic relationship of Bacillus RCR 08 endophytic to Ricinus communis L. with closely allied Bacillus spp.
based on 16S rDNA sequence analysis.
Table 2. Effect of different media on growth and EPS production by the endophytic bacterial isolate B. cereus RCR 08
Medium
Growth, CDW, g/L EPS, g/L
48 h 72 h 48 h 72 h
Davis and Mingioli’s medium 1.4 ± 0.02 2.4 ± 0.02 2.09 ± 0.04 1.81 ± 0.03
Mineral salts medium 1.6 ± 0.01 1.9 ± 0.04 1.05 ± 0.03 1.38 ± 0.04
Mineral salts medium with NH4Cl 6.7 ± 0.04 6.4 ± 0.03 6.07 ± 0.05 7.65 ± 0.07
Glutamate-mannitol medium 4.0 ± 0.02 4.0 ± 0.03 1.36 ± 0.04 2.13 ± 0.04
Tris-Glucose medium 1.8 ± 0.01 2.0 ± 0.02 1.65 ± 0.02 1.90 ± 0.02
Yeast extract medium 4.7 ± 0.03 4.5 ± 0.01 2.94 ± 0.03 3.75 ± 0.02
Tryptic soy medium 4.6 ± 0.03 4.5 ± 0.03 1.06 ± 0.03 1.41 ± 0.04
Luria Bertani medium 3.4 ± 0.01 2.8 ± 0.04 1.73 ± 0.02 1.19 ± 0.02
Values represent mean of triplicate readings ± S.D.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE 617
Ananya Mukherjee et al.
FIGURE 3. Time course of growth and EPS production by the endo-
phytic bacterial isolate B. cereus RCR 08 in batch culture
FIGURE 4. Effect of carbon source on growth and EPS production by
endophytic bacterial isolate B. cereus RCR 08
618 PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Ananya Mukherjee et al.
FIGURE 5. Effect of nitrogen source on growth and EPS produc-
tion by the endophytic bacterial isolate B. cereus RCR 08
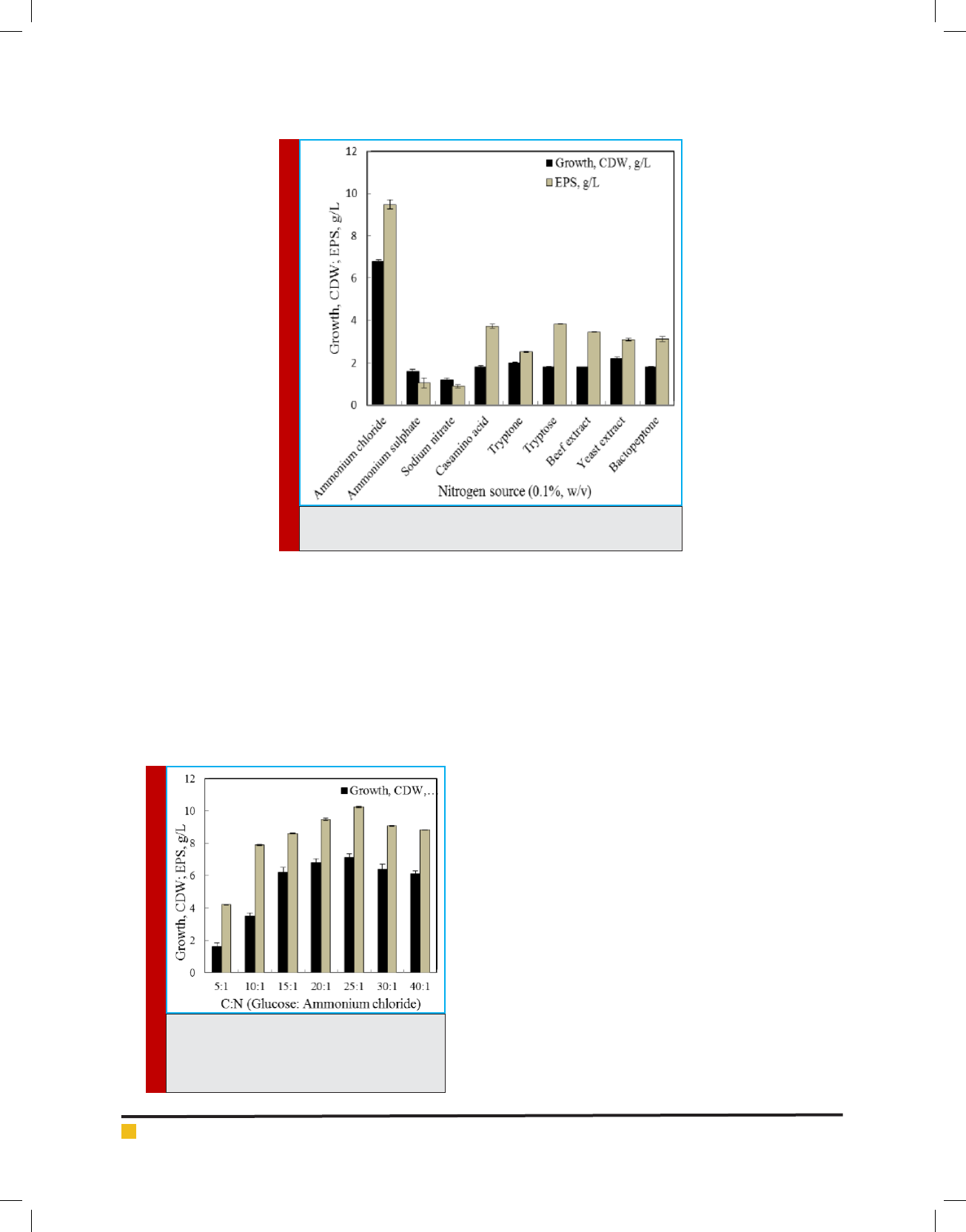
FIGURE 6. Effect of C:N (glucose: ammonium
chloride) ratio on growth and EPS production
by the endophytic bacterial isolate B. cereus
RCR 08
level showed discrete variation in the growth and poly-
mer production by the isolate B. cereus RCR 08, how-
ever maximum EPS production (9.48 g/L) was recorded
in presence of ammonium chloride and was followed
by organic nitrogenous compounds such as tryptone,
casamino acid and beef extract (Figure 5). When glu-
cose and ammonium chloride in the medium were main-
tained at a ratio of 25:1 maximum growth (7.1 g/L) and
EPS production (10.24 g/L) were recorded (Figure 6).
Miqueleto et al. (2010) studied the in uence of different
carbon sources and the C/N ratio on the production of
EPS by immobilized bacterial biomass and found that
high C/N ratio favored the biopolymer production.
Compositional analysis of the partially puri ed EPS
of B. cereus RCR 08 revealed that it was composed of
88.8% carbohydrate, 3.18% protein, 6.0% RNA as well
as 3.2% DNA. The partially puri ed EPS showed charac-
teristic peaks of protein and nucleic acids at 260 and 280
nm, respectively (Figure 7). Following TCA treatment,
the EPS, however showed characteristic spectrum similar
to those of authentic polysaccharides such as galactan
and dextrin (Figure 7).
FTIR spectrum of puri ed EPS showed characteristic
absorption peaks at 3404, 2,933, 1,655, and 1,042 cm
−1
(Figure 8). The strong band at 3404 cm
-1
was assigned to
the hydroxyl stretching vibration of the polysaccharide,
while the band at 2933 cm
-1
was due to C-H stretching
vibration. The bands in the region of 1500 and 1200
cm
-1
were assigned to C-H deformation vibration and
the bands between 1100 and 1075 cm
-1
corresponded to
C-O-C and C-O-H stretching vibration. A characteristic
absorption at 928 cm
-1
was possibly due to the stretch-
ing vibration of pyran ring (Liu et al., 2010). A similar
spectrum was also observed by Sonawdekar and Gupte
(2016) for EPS from B. cereus.
The emulsifying activity of extracellular polysacharides
as tested by the method of Cooper and Goldenberg (1987)
revealed that all the hydrocarbons (except petrol and hex-
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE 619
Ananya Mukherjee et al.
FIGURE 7. Comparision of UV absorption spectra of the puri-
ed exopolysaccharide obtained from the endophytic bacterial
isolate B. cereus RCR 08 with other authentic polysaccharides
FIGURE 8. FTIR spectra of the puri ed EPS produced by the endophytic bacterial isolate B. cereus RCR 08
adecane) showed effective emulsi cation (Table 3). The
highest emulsifying activity of the EPS was obtained with
benzene (76.37%) followed by tetradecane (70%) and hex-
ane (66.66%). However, tween 80 showed higher emulsify-
ing activity for kerosene (73.07%) than the EPS of B.cereus
RCR 08. Chowdhury et al. (2011) reported high emulsify-
ing activity of B. megaterium RB-05 EPS in coconut oil,
mustard oil and xylene while B. cereus isolated by Son-
awdekar and Gupte (2016) showed 53% emulsi cation.
Though there are several reports of EPS with antimi-
crobial activities (Orsod et al., 2012), the EPS produced
by the endophytic isolate RCR 08 failed to show any
antibacterial activity when tested against E. coli, B. sub-
tilis, S. aureus and P. cepacia by agar-cup assay. Simi-
larly, antioxidant properties of EPS (Liu et al., 2009) are
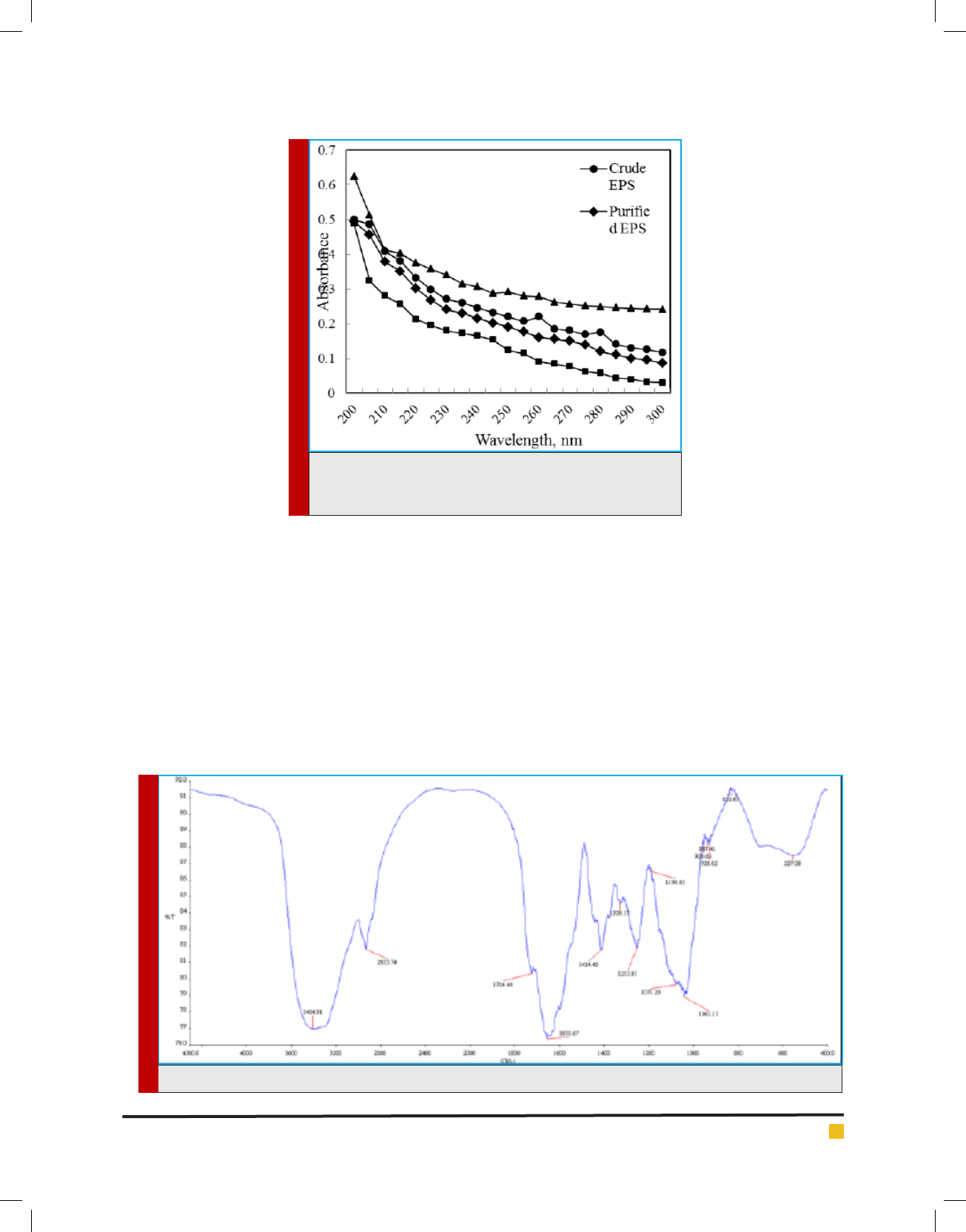
also not rare. The DPPH radical scavenging activity of
the EPS isolated from B. cereus RCR 08 increased with
increasing concentrations and a scavenging activity of
16% was recorded at a concentration of 10 mg/mL but
was much lower as compared to vitamin C (Figure 9).
The scavenging activity exhibited by the EPS might be
attributed due to their hydrogen donating abilities. The
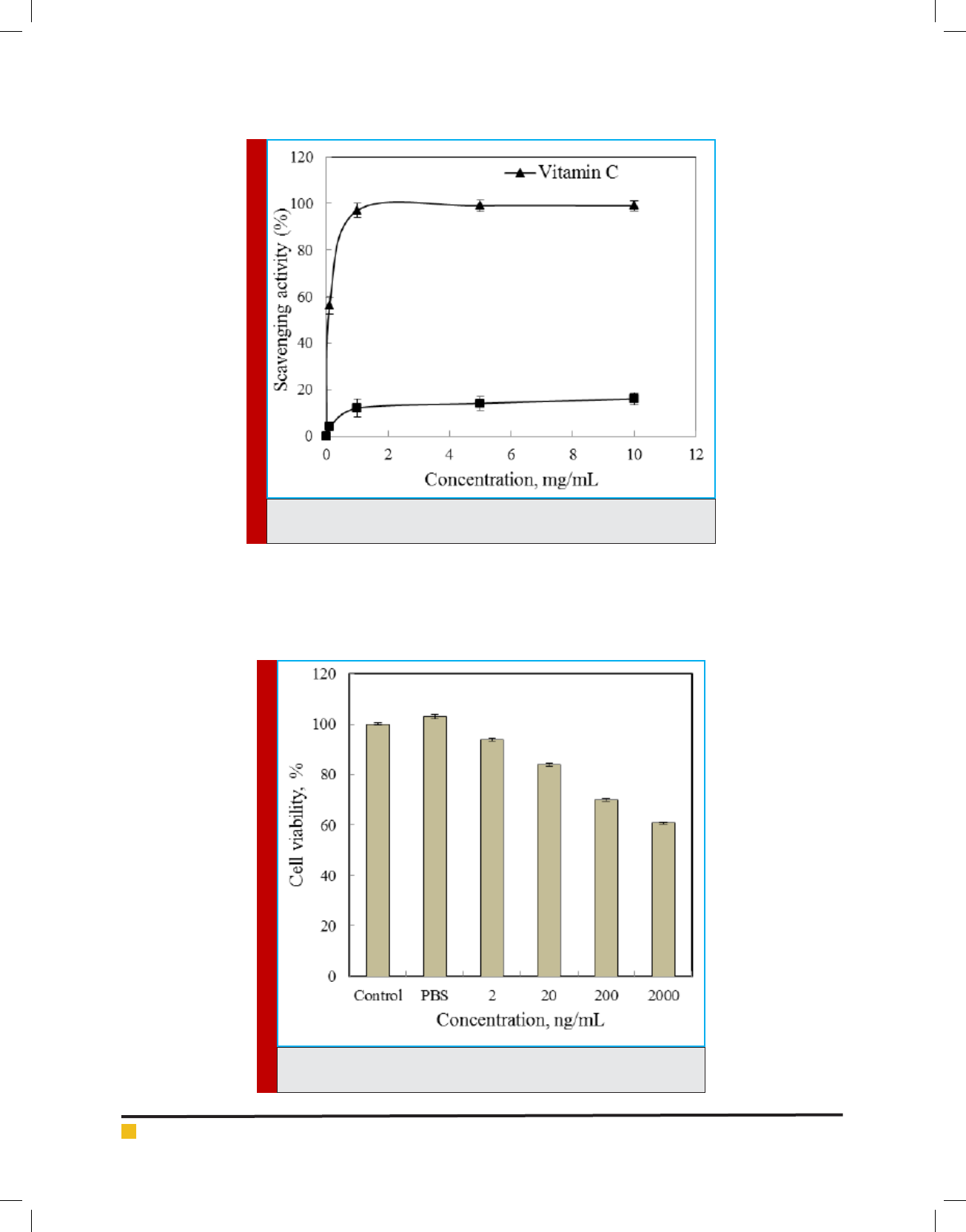
effect of EPS extracted from B. cereus RCR 08 on the
viability of Huh 7.5 cells was determined by MTT assay.
620 PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Ananya Mukherjee et al.
FIGURE 9. DPPH radical scavenging activity of the EPS produced by the endo-
phytic bacterial isolate B. cereus RCR 08
FIGURE 10. Effect of EPS produced by the endophytic bacterial isolate B.
cereus RCR 08 on the viability of Huh 7.5 cell line
The EPS displayed a dose-dependent cytotoxic activity
against Huh 7.5 cell line in culture. The antiproliferative
activity of the EPS gradually increased with increasing
concentration. The EPS exhibited 60.8% viability of the
Huh 7.5 cells at a concentration of 2000 ng/mL (Figure
10). Li and Shah (2016) also reported strong antiprolif-
erative activity of EPS isolated from Streptococcus ther-
mophilus ASCC 1275 on Caco-2 and HepG2 cells.

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE 621
Ananya Mukherjee et al.
CONCLUSION
Endophytes have been recognized as important sources
of structurally and functionally novel extracellular
polysaccharides which could nd applications in medi-
cal, pharmaceuticals, chemical and other industries. The
present study demonstrates that Bacillus cereus RCR 08,
endophytic to Ricinus communis L., is capable of pro-
ducing a substantial amount of extracellular polymeric
substance employing a suitable carbon and nitrogen
source in a de nite ratio. Results so obtained appear to
be bene cial for further assessment of enhancing the
production of B. cereus RCR 08 EPS in large scale. The
signi cant oil emulsifying activity along with antioxi-
dant and antiproliferative activity against Huh 7.5 cell
line deserve special attention. Thorough chemical analy-
sis of these carbohydrate polymers is required to exploit
them in pharmacy in adjunction to cancer trials.
ACKNOWLEDGEMENTS
This work was partially supported from the grant received
by one of us (RD) from the Department of Science and
Technology, New Delhi (Sanction No. DST-INSPIRE Fel-
lowship/REL3/2013/2).
CONFLICT OF INTEREST
All authors have declared no con icts of interest in this
communication.
REFERENCES
Almog, R. and Shirley, T. L. (1978): A modi ed orcinol test for
the speci c determination of RNA. Ann Biochem. 91, 130-137.
Bales, P. M., Renke, E. M., May, S. L., Shen, Y. and Nelson, D. C.
(2013): Puri cation and characterization of bio lm-associated
EPS exopolysaccharides from ESKAPE organisms and other
pathogens. PloS One. 8, e67950.
Bauer, A. W., Kirby, W. M. M., Sherris, J. C. and Turck, M.
(1966): Antibiotic susceptibility testing by a standardized sin-
gle disk method. Am J Clin Pathol. 36, 493-496.
Borges, W. D. S., Borges, K. B., Bonato, P. S., Said, S. and Pupo,
M. T. (2009): Endophytic fungi: Natural products, enzymes and
biotransformation reactions. Curr Org Chem. 13, 1137-1163.
Bragadeeswaran, S., Jeevapriya, R., Prabhu, K., Sophia Rani,
S., Priyadharsini, S. and Balasubramanian, T. (2011): Exopoly-
saccharide production by Bacillus cereus GU812900, a fouling
marine bacterium. Afr J Microbiol Res. 5, 4124-4132.
Chen, Y. T., Yuan, Q., Shan, L. T., Lin, M. A., Cheng, D. Q. and
Li, C. Y. (2013): Antitumour activity of bacterial exopolysac-
charides from the endophyte Bacillus amyloliquefaciens sp.
isolated from Ophiopogon japonicas. Oncol Lett. 5, 1787-1792.
Chowdhury, S. R., Manna, S., Saha, P., Basak, R. K., Sen, R.,
Roy, D. and Adhikari, B. (2011): Composition analysis and
material characterization of an emulsifying extracellular poly-
saccharide (EPS) produced by Bacillus megaterium RB-05: a
hydrodynamic sediment-attached isolate of freshwater origin.
J Appl Microbiol. 111, 1381-1393.
Cooper, D. G. and Goldenberg, B. G. (1987): Surface active
agents from two Bacillus species. Appl Environ Microbiol. 53,
224-229.
Donot, F., Fontana, A., Baccou, J. C. and Schorr-Galindo, S.
(2012): Microbial exopolysaccharides: Main examples of syn-
thesis, excretion, genetics and extraction. Carbohydr Polym.
87, 951-962.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. and
Smith, F. (1956): Calorimetric method for determination of
sugars and related substances. Ann Chem. 28: 350-356.
Freitas, F., Alves, V. D. and Reis, M. A. (2011): Advances in
bacterial exopolysaccharides: From production to biotechno-
logical applications. Trends Biotechnol. 29, 388-398.
Gandhi, H. P., Ray, R. M. and Patel, R. M. (1997): Exopolymer
production by Bacillus species. Carbohydr Polym. 34, 323-327.
Gerhardt, P., Murray, R. G. E., Wood, W. A. and Krieg, N. R.
(1994): Methods for general and molecular bacteriology.
Washington DC: American Soceity for Microbiology.
Guo, S., Mao, W., Yan, M., Zhao, C., Li, N., Shah, J., Lin, C., Liu,
X., Guo, T. and Wang, S. (2014): Galactomannan with novel
structure produced by the coral endophytic fungus Aspergillus
ochraceus. Carbohydr Polym. 105, 325-333.
Jain, P. and Sharma, P. (2015): Isolation and preliminary
screening of endophytic fungi of Ricinus communis for their
antimicrobial potential. J Microbiol Biotech Food Sci. 5, 230-
233.
Table 3. Emulsifying activity of EPS produced by B.
cereus RCR 08 and commercial emulsi er Tween 80
Hydrocarbons
Emulsi cation, %
EPS of B. cereus
RCR 08
Tween 80*
Benzene 76.37 ± 2.50 68.00 ± 1.20
Palm oil 47.61 ± 1.22 48.00 ± 1.22
Olive oil 60.00 ± 1.25 68.18 ± 0.09
Soybean oil 63.15 ± 1.23 61.53 ± 0.12
Kerosene 62.50 ± 1.26 73.07 ± 0.08
Petrol - 62.50 ± 0.01
Octane 60.00 ± 1.23 68.00 ± 0.00
Hexane 66.66 ± 1.27 64.00 ± 0.15
Tetradecane 70.00 ± 1.26 61.23 ± 0.12
Hexadecane - 29.62 ± 1.25
*Expressed as the percentage of the total height occupied by the oil
water emulsion after 24 h; each value represents the average of
three measurements ± S.D.

622 PRODUCTION AND PARTIAL CHARACTERIZATION OF EXTRACELLULAR POLYSACCHARIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Ananya Mukherjee et al.
Kusari, S., Singh, S. and Jayabaskaran, C. (2014): Biotechno-
logical potential of plant-associated endophytic fungi: Hope
versus hype. Trends Biotechnol. 32, 297-303.
Li, S. and Shah, N. P. (2016): Characterization, anti-
In ammatory and antiproliferative activities of natural and
sulfonated exo-polysaccharides from Streptococcus thermo-
philus ASCC 1275. J Food Sci. 81, 1167-1176.
Li, Y., Guo, S. and Zhu, H. (2016): Statistical optimization of
culture medium for production of exopolysaccharide from
endophytic fungus Bionectria ochroleuca and its antitumour
effect in vitro. EXCLI J. 15, 211-220.
Liu, J., Luo, J., Ye, H., Sun, Y., Lu, Z. and Zeng, X. (2009): Pro-
duction, characterization and antioxidant activities in vitro of
exopolysaccharides from endophytic bacterium Paenibacillus
polymyxa EJS-3. Carbohydr Polym. 78, 275-281.
Liu, J., Luo, J., Ye, H., Sun, Y., Lu, Z. and Zeng, X. (2010):
Medium optimization and structural characterization of
exopolysaccharides from endophytic bacterium Paenibacillus
polymyxa EJS-3. Carbohydr Polym. 79, 206-213.
Liu, J., Wang, X., Pu, H., Liu, S., Kan, J. and Jin, C. (2017):
Recent advances in endophytic exopolysaccharides: Produc-
tion, structural characterization, physiological role and bio-
logical activity. Carbohydr Polym. 157, 1113-1124.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J.
(1951): Protein Measurement with the Folin Phenol Reagent. J
Biol Chem. 193, 265-275.
Mahapatra, S. and Banerjee, D. (2016): Production and struc-
tural elucidation of exopolysaccharide from endophytic
Pestalotiopsis sp. BC55. Int J Biol Macromolec. 82, 182-191.
Manca, M. C., Lama, L., Improta, R., Esposito, A., Gamba-
corta, A. and Nicolaus, B. (1996): Chemical composition of
two exopolysaccharides from Bacillus thermoantarcticus. Appl
Environ Microbiol. 62, 3265-3269.
Miqueleto, A. P., Dolosic, C. C., Pozzi, E., Foresti, E. and Zaiat,
M. (2010): In uence of carbon sources and C/N ratio on EPS
production in anaerobic sequencing batch bio lm reactors for
wastewater treatment. Bioresour Technol. 101, 1324-1330.
Moscovici, M. (2015): Present and future medical applications
of microbial exopolyssaccharides. Front Microbiol. 6, 1012.
Orsod, M., Joseph, M. and Huyop, F. (2012): Characterization of
exopolysaccharides produced by Bacillus cereus and Brachy-
bacterium sp. Isolated from Asian Sea Bass (Lates calcarifer).
Malaysian J Microbiol. 8, 170-174.
Rajkumar, M. and Freitas, H. (2008): In uence of metal resist-
ant-plant growth-promoting bacteria on the growth of Ricinus
communis in soil contaminated with heavy metals. Chemos-
phere. 71, 834-842.
Saitou, N. and Nei, M. (1987): The neighbor-joining method:
A new method for reconstructing phylogenetic trees. Mol Biol
Evol. 4, 406-425.
Singh, R. P., Shukla, M. K., Mishra, A., Kumari, P., Reddy,
C. R. K. and Jha, B. (2011): Isolation and characterization of
exopolysaccharides from seaweed associated bacteria Bacillus
licheniformis. Carbohydr Polym. 84, 1019-1026.
Slater, T. F., Sawyer, B. and Strauh, U. (1963): Studies on suc-
cinatetetrazolium reductase system. Ill. Points of coupling of
four different tetrazolium salts. Biochem Biophys Acta. 77, 383-
393.
Sonawdekar, S. and Gupte, A. (2016): Production and charac-
terization of exopolysaccharide produced by oil emulsifying
bacteria. Int J Curr Microbiol App Sci. 5, 254-262.
Soni, H., Singhai, A. K., Swarnkar, P., Sharma, S. and Kumar, V.
(2011): Spectrophotometric method for quantitative estimation
of DNA isolated from various parts of Catharanthus roseus. Int
J Pharm Pharm Sci. 3, 529-532.
Sutherland, I. W. (1998): Novel and established applications of
microbial polysaccharides. Trends Biotechnol. 16, 41-46.
Wingender, J., Neu, T. R. and Flemming, H. C. (1999): What
are bacterial extracellular polymer substances. In: Microbial
Extracellular Polymer Substances. Springer, Berlin, pp. 1-19.
Yamada, T., Ogamo, A., Saito, T., Watanabe, J., Uchiyama, H.
and Nakagawa, Y. (1997): Preparation and anti-HIV activity of
low-molecular-weight carrageenans and their sulfated deriva-
tives. Carbohydr Polym. 32, 51-55.
Zheng, L. P., Zou, T., Ma, Y. J., Wang, J. W. and Zhang, Y. Q.
(2016): Antioxidant and DNA damage protecting activity of
exopolysaccharides from the endophytic bacterium Bacillus
cereus SZ1. Molecules. 21, 174.