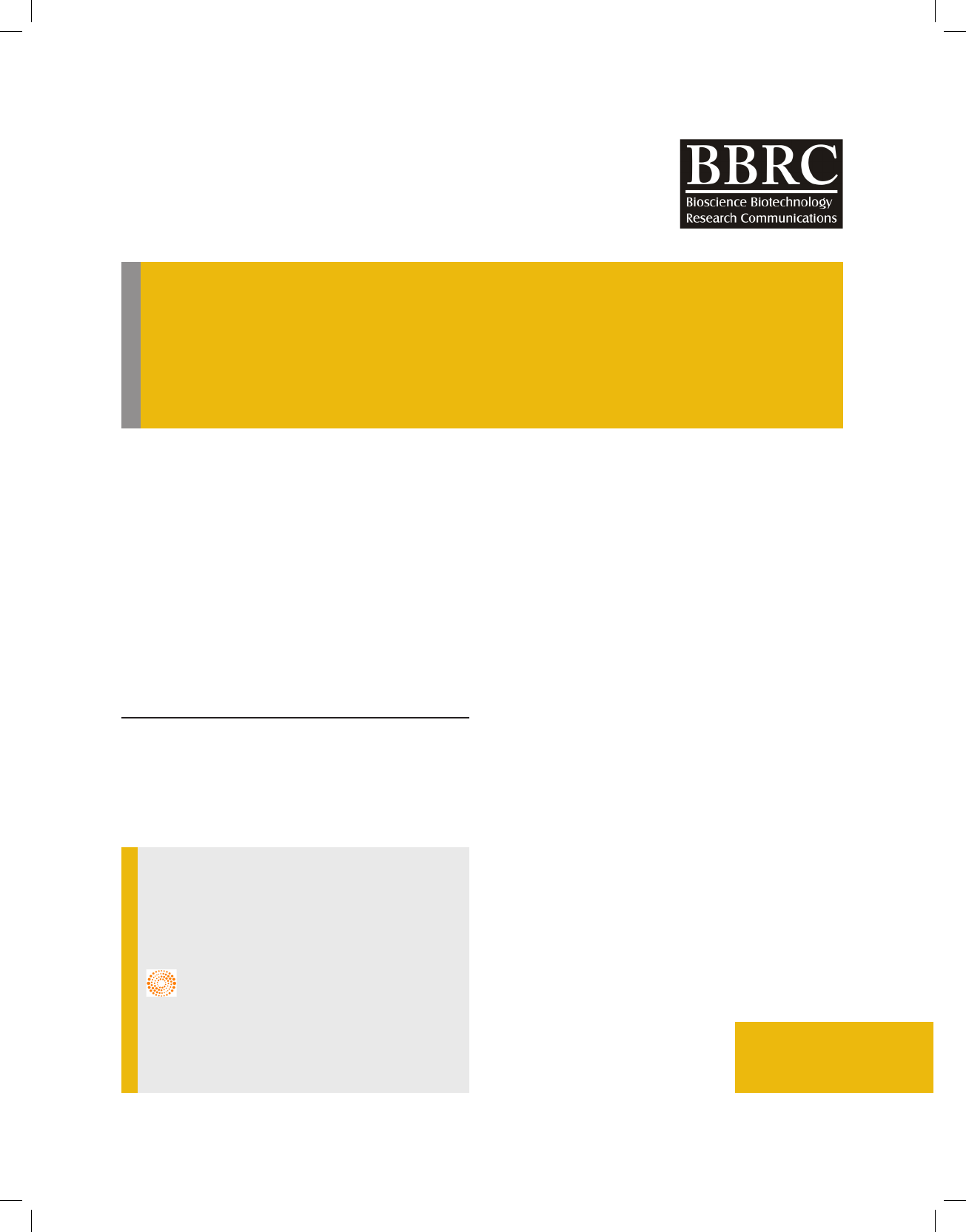
Diversity analysis and characterization of antagonistic
endophytic population from
Stevia rebaudiana
Ankita Verma
1
*, Sandeep Saini
2
, B. N. Johri
1
and Anil Prakash
1
1
Department of Biotechnology, Barkatullah University, Bhopal-462026
2
Department of Biotechnology, Faculty of Science, RKDF University, Bhopal-462036
ABSTRACT
Antagonistic endophytic fungal community resident in medicinal plant Stevia rebaudiana Bertoni was studied at two
sites within Bhopal, M.P. Among 339 recovered endophytic isolates from foliar tissues, 40 fungal isolates were found
antagonistic against Sclerotinia sclerotiorum, casual agent of disease stem rot in stevia and soybean (Glycine max.).
Antagonistic fungal population (40 isolates) consisted of 52.5% Hyphomycetes and 2.5% each of Coleomycetes, Basidi-
omycetes, Ascomycetes and Sterile mycelia. The percent colonization frequency of antagonistic endophytic community
in foliar tissues ranged from 0.3%-5.3% whereas percent dominance was of the order, ranged from 2.31%-40.8%. Diver-
sity analysis of the antagonistic endophytic population was determined in terms of Shanon index, Simpson index, Spe-
cies eveness, Menhinick and Margalef richmess index .Antagonistic endophytic population was also evaluated for IAA
production, siderophore and phosphorus solubilisation, considered as plant growth promotory attributes. Identi cation
of the antagonistic endophytes was carried out by rDNAsequencing of the ITS region.
KEY WORDS: DIVERSITY INDEX,
S. SCLEROTIORUM
, ITS REGION, ANTAGONISTIC ENDOPHYTES, SEQUENCE PHYLOGENY
754
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(4): 754-765 (2018)
INTRODUCTION
Fungal endophytes possess huge diversity morpho-
logically and biochemically (Strobel and Daisy, 2003).
Endophytic fungi are known to reside in the tissues of
plants above ground as well as below ground, parts of
the plant (Zhang et al. 2006; Kusari et al. 2012). Endo-
phytic fungi are an assemblage of microorganisms that
chie y belong to class Ascomycetes of kingdom fungi.
A signi cant literature is available so far to show that
these microorganisms, under laboratory culture condi-
tions, produce numerous structurally diverse biologi-
cally active secondary metabolites that include antimi-
crobial substances. Different ecological factors such as
seasonality, nearby vegetation and humidity in uence
the distribution of endophytic fungi in the host (Taylor
ARTICLE INFORMATION:
Corresponding Authors: ankita.verma1234@gmail.com
Received 19
th
Sep, 2018
Accepted after revision 23
rd
Dec, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
Mono of Clarivate Analytics and Crossref Indexed
Journal Mono of CR
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.4/28

Ankita Verma et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION 755
et al; 1999; Toofanee and Dulymamode; 2002 Lumyong
et al 2009, Dissanayake et al., 2016; Ratnaweera et al.,
2017, Ratnaweera et al., 2018).
Stevia rebaudiana Bertoniis an herbaceous polyan-
nual plant of the sun ower family (Fam. Asteraceae),
generally known as candy leaf,
sweet leaf, sweet leaf,
orsugar leaf. The Stevia leaves also contain variety of
glycosides compounds viz., avonoid glycosides, Stevi-
oside, Rebaudioside A, Rebaudioside C, coumarins, cin-
namic acids, phenylpropanoids and some essential oils,
(Midmore and Rank, 2002 Lavini et al., 2008).
Stevia rebaudiana Bert. Is a good source of sweeten-
ers and is about 300 times sweeter than sucrose owing
to presence of steviosides in its leaves. Previous stud-
ies have reported clearly the diversity of these fungal
fungal endpphytes from Stevia rebaudiana which also
indicates the presence of Alternaria,, Aspergillus, Mono-
dictys, and Curvularia fungal genus from leaf of Ste-
via rebaudiana (Bert.). (Prakash et al., 2008; Madhumita
and Chandra, 2013) Furthermore, these fungal isolates
have been reported from almost all climatic regions of
the globe viz., tropical, temperate and alpine (Arnold,
2007; Halmschlager et al 1993; Higgins et al 2007). The
application of biocontrol agents has become one of the
most promising tools for reducing the use of chemical
pesticides in agriculture. The antagonism of biocontrol
agent is based on different mechanisms i.e. nutrients,
mycoparasitism, plant growth promotion and induction
of the defense responses in plants (Howell, 2003, Sen
et al, 2012; Hamzah et al, 2018).
In the present investigation the endophytes recov-
ered from Stevia rebaudiana leaves have been tested for
antagonistic abilities against Sclerotinia sclerotiorum,
which is the major phytopathogen affecting varieties of
crop plants in Central India (Prakash et al., 2008, Verma
et al 2004).
Therefore, present investigation was carried out to
understand the generic diversity of endophytic fungi in
leaves of Stevia rebaudiana Bertoni and to compare the
antagonistic endophytic assemblages in samples collected
from two different sites in the same region. Thus a speci c
rationale for the selection of stevia plant for endophyte
isolation and natural product discovery is used.
MATERIALS AND METHODS
Stevia rebaudiana Bertoni: Stevia rebaudiana Bertoni
was selected as the target plant for isolation of fungal
endophytes. Sampling was carried out from two sites.
The rst site was Misrod (23º16’N; 76º36’E), a village
situated nearly 22 km away from the capital city Bhopal
in the state of Madhya Pradesh. The second site of sam-
pling was green house grown plants in the campus of
(23º20’N; 77º45’E) Barkatullah University, Bhopal.
Samples Collection and Surface sterilization: Healthy
and mature plants were carefully chosen for sampling
and leaves were collected randomly. Plant material was
brought to the laboratory in sterile bags. Surface steri-
lization of foliar tissues were done using reagents like
70% ethanol, 4% sodium hypochlorite and sterile dis-
tilled water for different time period for effective surface
sterilization process. Leaves were thoroughly washed
several times in sterile distilled water (SDW) for 5-10 sec
then sterilized by exposing them to 70% ethanol for 2
min followed by treatment with 4% sodium hypochlorite
for 2 min. The leaves were now immersed in sterile water
(SDW) for 2-5 sec and allowed to dry on blotting paper.
Isolation of endophytic fungi from foliar tissues of
plants: The surface sterilized leaf segments of 5mm size
were placed on Potato dextrose Agar (Howksworth et al
1995), supplemented with chloramphenicol (0.2gl-1) to
avoid bacterial contamination. Plates were incubated at
28±2 ºC in for 3-5 days and were observed regularly for
fungal growth.
Analysis of data: The colonization frequency (% CF)
of endophytic fungi was calculated according to Hata
and Futai (1995) and dominance as per Kumaresan and
Suryanarayanan (2002). Utilizing the data of percent-
age colonization of fungal endophytes in leaves, for two
sites. Simpson’s Diversity indices and Shanon-Wiener
indices were calculated. Species evenness and species
richness was calculated according to Simpson (1949),
Shanon and Weaver (1949), Ludwig & Reynolds (1988)
and Margelef and Menhinick (1964)
In vitro antagonistic activity of fungal endophyte
Fungal isolates were screened for antagonism against
Sclerotinia sclerotiorum by a dual culture technique on
Potato Dextrose Medium (Szekeres et al, 2005).
Characterization of endophytes: The endophytic
fungi were identi ed by their macroscopic & micro-
scopic characteristics such as the morphology of the
fruiting bodies and spore morphology. Morphological
characterization was done on the basis of color, margin,
reverse pigmentation & texture. (Rifai 1969).Antago-
nistic endophytic fungi are characterized functionally
employing plate assays for amylase cellulase, Protease,
pectinase, lipase and xylanase (Paterson & Bridge. 1994;
Teather & Wood. 1982; Shakeri et al. 2007; Pointing.
1999; Sierra. 1957, Mishra et al, 2013, Aneja, 2003).
Plant growth promoting attributes: Plant growth
promoting attributes of antagonistic endophyte were
also studied. This included IAA (Brick et al. 1991) and
siderophore production (Schwyn & Neilands. 1987) and
phosphate solubilisation ef ciency (Pikovskaya. 1948).
Both qualitative and quantitative estimation were made.
Molecular identi cation of antagonistic endophyte:
- Morphological identi cation of the organism was car-
Ankita Verma et al.
756 DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
ried out at National Fungal Culture Collection of India
(NFCCI), Agharkhar Research Institute, Pune. For molec-
ular identi cation, total genomic DNA of the endophytic
fungus was isolated directely from actively growing
mycelium growing in Potato dextrose broth (PDB), using
CTAB method (Sambrook and Russel, 2001). DNA ampli-
cation was performed by PCR using primer pair ITS1:
TCCGTAGGTGAACCTGCGG and ITS4: TCCTCCGCTTGA-
TATGC (White et al. 1990).PCR was carried out according
to the following protocol: initial denaturation at 95 ºC
for 5 min; denaturation at 95 ºC for 1 min; annealing at
55ºC for 45 sec; extension at 72 ºC for 10 min; steps 2-4
were repeated 35 times. Sequencing of PCR product was
carried at Xcelris Labs Ltd, Ahmedabad. The sequenced
data was subjected to BLAST algorithm and submitted to
Genebank for accession number. The potential antago-
nistic endophytes were submitted at National Agricul-
turally Important Culture Collection (NAIMCC), culture
collection facility at ICAR-NBAIM, Maunath Bhanjhan
(U.P).
Phylogenetic analysis:To know the phylogenetic
relationship among the isolates and also to confirm
their taxonom ical status, certain ITS rDNA sequences
were chosen from GenBank databases via BLAST search
analysis. The sequences were chosen from the top 20
database hits obtained in the blast search by querying
the obtained sequences individually. These sequences
were aligned using CLUSTAL W 1.83 (Thompson et al.,
1994). Phylogenetic trees were generated by neighbour-
hood joining method with 100 bootstrapping replicates
using MEGA version 5.
RESULTS
Antagonistic Action and Diversity Analysis: A total of
339 recovered endophytic fungal isolates were screened
for antagonistic ability against Sclerotinia sclerotiorum
(culture obtained from Directorate of Soybean Research,
Indore, M.P.) by using dual culture technique. The inhi-
bition zone in dual plate assay averaged between 5 to
17 mm. Misrod eld site harboured greater number of
antagonists compared to the endophytes recovered from
green house raised plants. Percent growth reduction of
the pathogenic culture was recorded after 24 hrs. Iso-
late Aspergillus avipes (NAIMCC-F-03153) strain 63
showed highest value of growth reduction i.e. 19% after
24 hrs followed by Alternaria alternata strain 99 and
Aspergillus niger strain 89 (NAIMCC-F-03157) showed
17% and 18% growth reduction respectively (Fig 1);
Least reduction of pathogen was recorded for isolate
Alternaria brassicae strain 17 i.e. 6.9%. The percent
growth reduction values ranged from 6.9% to 19%.
A total of 40 endophytic fungal isolates which showed
antagonistic activity against Sclerotinia sclerotiorum
consisted of 52.5% Hyphomycetes followed by 2.5%
Coleomycetes, Basidiomycetes, Ascomycetes and Sterile
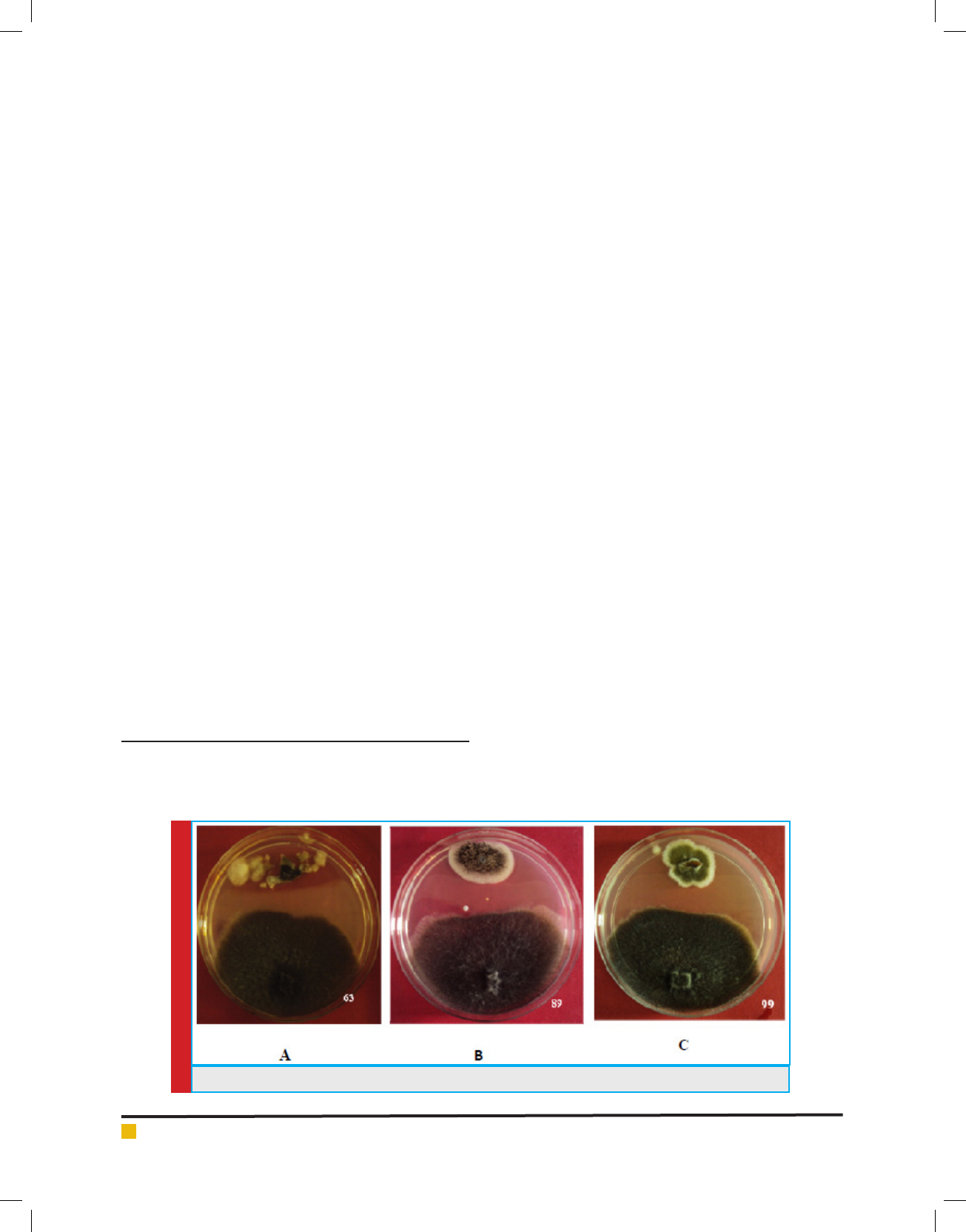
mycelia each. The percent colonization in tissues sam-
ples ranged from 1% - 36.6% (site 1) whereas 1%-10.6%
(site 2) (Fig 2) and percentage dominance of antagonistic
endophytes ranged from 1.1%-40.6% (site 1) and 4.3%-
45.3% at site2 (Fig 3). Diversity analysis of the antago-
nistic population was carried out which showed signi -
cant diversity index values at site 1 as compared to site
2, whereas Margalef & Menhinick’s richness index value
was maximum at site 2 as compared to site 1 (Table 1)
Characterization of antagonistic fungal endophytes
Morphological characterization of antagonistic fungal
endophytes: Based on the morphology different antago-
nistic endophytic fungal isolates were recovered on PDA
plate. Among 40 antagonistic endophytic isolates 62.5%
showed reverse pigmentation, 15% showed velvety
appearance on PDA plate while others appeared spory
and cottony texture. Isolates Aspergillus niger strain 89
FIGURE 1. Dual culture assay shown by isolate A) Strain 63 B) Strain 89 C) Strain 99
Ankita Verma et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION 757
FIGURE 2. % Colonization frequency of the antagonistic endophytic population from both sites.
FIGURE 3. % Dominance of the antagonistic endophytic population from both sites.
Table 1. Diversity indices of two sites (Site
1 & Site 2)
Diversity indices Site I Site II
Simpson index (1-D) 0.821 0.7982
Shanon index (H’) 1.856 1.781
Eveness (EH/s) 0.8001 0.8478
Menhinick S/√N 0.6305 1.121
Margalef S-1/ln(n) 1.378 1.638
(NAIMCC-F-03157), Aspergillus niger strain 88, Asper-
gillus sp. strain 37 (NAIMCC-F-03147), Aspergillus a-
vipes strain 63 (NAIMCC-F-03153) and Aspergillus niger
strain 50 (NAIMCC-F-03151), showed dispersed growth
on PDA medium.
Functional characterization of antagonistic fun-
gal endophytes: The antagonistic fungal endophytes
recovered from Stevia, were checked for their hydro-
lytic potential. They were screened for multiple enzyme
activity on starch, pectin, lipid, carboxy methyl cellu-

Ankita Verma et al.
758 DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Table 2. Plant growth promoting attributes of antagonistic endophytes
S.no Isolates Identity IAA (μg/ml)
Siderophore
(mg/ml)
% solubilising ef ciency
of Phosphate
1 SR/II/2 Alternaria porri - 8.42±0.20 75
2 SR/II/4 Alternaria alternata 6.2±0.03 1.6±0.20 52
3 SR/II/5 Alternaria brassicae - 3.79±0.05 71.4
4 SR/II/6 Alternaria porri 3±0.04 6.2±0.06 60
5 SR/II/42 Alternaria sp. 8±0.03 3.72±0.08 -
6 SR/II/45 Penicillium mallochii 6.3±0.05 4.2±0.04 32
7 SR/II/50 Aspergillus niger 4.8±0.01 - 6.25
8 SR/II/52 Phoma sp. - 6.6±0.03 10
9 SR/II/54 Phoma sp. 4.6±0.02 8.7±0.03 20
10 SR/I/76 Alternaria alternata - 3.2±0.02 5.4
11 SR/I/77 Alternaria sp. 5.5±0.03 6.9±0.02 22
12 SR/I/78 Alternaria tenuissima 6.5±0.02 8.02±0.03 11
13 SR/I/94 Alternaria alternata 3.8±0.01 2.8±0.03 8
14 SR/I/99 Alternaria alternata 5.3±0.3 - 36
15 SR/II/63 Aspergillus avipes 4.6±0.04 11.12±0.16 22.2
16 SR/II/1 Alternaria alternata - 17.09±0.1 -
17 SR/II/9 Alternaria alternata 9.8±0.6 7.45±0.12 17.3
18 SR/II/20 Alternaria sp. 6.3±0.06 8.03±0.11 70
19 SR/II/21 Alternaria alternata - 8.03±0.11 50
20 SR/II/23 Chaetomiumglobosum 6.7±0.05 15.51±0.12 14
21 SR/II/24 Alternaria alternata - 14.63±0.14 12
22 SR/II/26 Alternaria alternata 1.9±0.04 3.15±.10 10
23 SR/II/33 Phoma sp. 4.5±0.01 1.66±0.031 -
24 SR/II/36 Alternaria brassicae 8.2±0.7 27±20.22 65
25 SR/II/55 Alternaria alternata - 4.42±0.14 14
26 SR/II/60 Ustilago tritici 5.5±0.04 9.51±0.17 -
27 SR/I/72 Alternaria brassicae - 23±0.08 34
28 SR/II/81 Alternaria alternata - 22±0.06 -
29 SR/I/85 Alternaria alternata 5.7±0.05 6.07±0.03 37
30 SR/I/95 Alternaria alternata - 4.5±0.04 -
31 SR/II/100 Alternaria alternata 5.8±0.07 5.8±0.07 19
32 SR/I/83 Alternaria alternata 4.5±0.05 6.18±0.19 -
33 SR/II/18 Phoma sp. - 1.3±0.02 85.7
34 SR/II/37
Aspergillus sp. 11±0.01 13.3±1.12 -
35 SR/II/46 Aspergillus sp. 6.8±0.06 8.06±0.07 -
36 SR/I/88 Aspergillus niger 8.1±0.2 - -
37 SR/I/89 Aspergillus niger 11.2±0.4 6.72±1.4 -
38 SR/I/90 Alternaria alternata - - 35
39 SR/II/3 Sterile mycelia - 8.42±0.20 85.7
40 SR/II/17 Alternaria brassicae 6.25±0.05 24.33±0.12 -
lose, xylan and skim milk. Endophytes exhibited good
amylolytic, cellulolytic, proteolytic, pectinolytic activi-
ties while xylanolytic and lipolytic activities were pos-
sesed by only few isolates. The con rmation of enzy-
matic activity was recorded by the presence of zone
of clearance around the culture. Alternaria alternata
strain 76 showed maximum zone of about 15 mm on
starch agar plate while 11 mm zone was recorded for

Ankita Verma et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION 759
cellulolytic activity by isolate Alternaria alternata strain
26.Maximum pectinolytic activity was recorded by iso-
late Alternaria alternata strain 99, whereas isolate Alter-
naria alternata strain 4 (NAIMCC-F-03138), showed
maximum zone (11 mm) of clearance on CMC plate. The
isolates Aspergillus sp. strain 46, Phoma sp. strain18,
Phoma sp. strain 33 and Alternaria alternata strain 94,
Aspergillus niger strain 50, Alternaria porri strain 6
(NAIMCC-F-03139), and Alternaria brassicae strain 5
(NAIMCC-F-03140), showed minimum inhibition (2mm)
on same substrate.
Plant growth promotiong attributes of antagonistic
endophytes:The antagonistic endophytes were studied
for plant growth promoting traits such as siderophore
production, phosphate solubilisation, Indole acetic acid
production. A comprehensive overview of the PGP
traits of antagonistic endophytes is given in the Table
2. About 40% of the antagonistic endophytes were
positive for siderophore production and the amount of
siderophore produced ranged between 1.3-27 mgml
-1
.
On Pikovyaskya’s agar, 31% of the antagonistic endo-
phytes showed phosphate solubilisation; ef ciency of P
solubilisation ranged between 5.4%-85.7percent. Based
on the results isolates Phoma sp. strain 18 and Sterile
mycelia strain 3 were found to be most ef cient P solu-
bilizer. A total of 29% of the antagonistic endophytes
were positive for IAA production which ranged between
1.9-11μgml
-1
(Table 2).
Sequence analysis of ITS region of rDNA gene fragments
All the selected isolates produced a single PCR prod-
uct with approximately 600 bp. Puri cation of the PCR
product was performed using Banglore Genei puri-
cation kit and sequencing was performed by Xcelris
Genomics, Ahemdabad using the same set of primers as
mentioned earlier. The full length sequences of the iso-
lates were compared with the related fungal sequences
in the GenBank databases and sequence similarities were
determined using BLAST sequence similarity search tool
(Altschul et al., 1990). The sequences of ITS region of
rDNA gene of the fungal endophytes were deposited in
the GenBank and given accession numbers (Table 3).
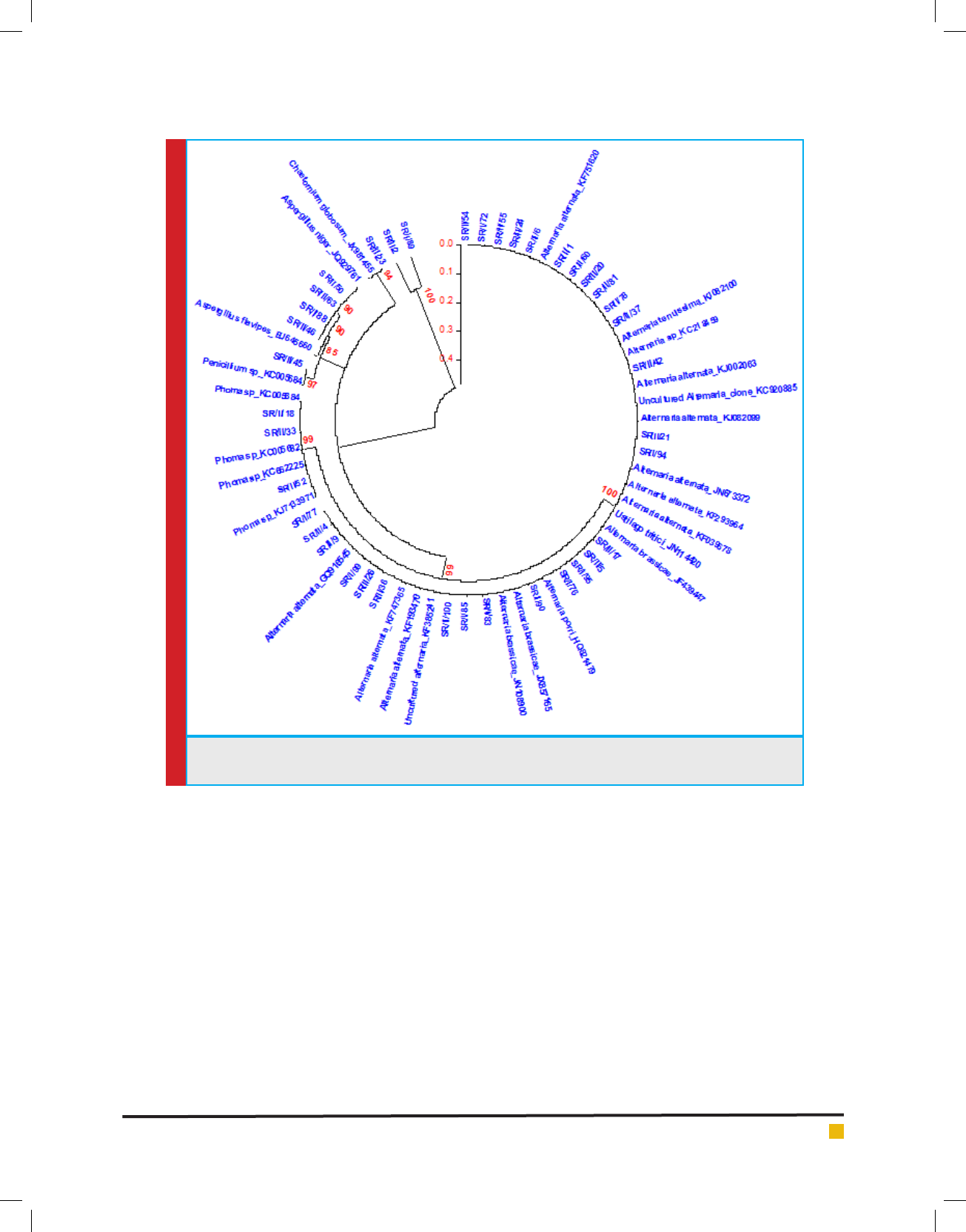
Phylogenetic analysis
To know the phylogenetic relationship among the iso-
lates and also to confirm their taxonom ical status, cer-
tain ITS rDNA sequences were chosen from GenBank
databases via BLAST search analysis. The sequences
were chosen from the top 20 database hits obtained in
the blast search by querying the obtained sequences indi-
vidually. These sequences were aligned using CLUSTAL
W 1.83 (Thompson et al., 1994). Phylogenetic trees were
generated by neighbourhood joining method with 100
bootstrapping replicates using MEGA version 5.( Fig:4)
DISCUSSIONS
Characterization of fungal endophytes from Stevia
rebaudiana Bertoni was considered important because
only few attempts have been made earlier to character-
ize fungal endophytes from this useful plant, (Begum
et al 2008; Kumari and Chandra 2013). Various fungal
diseases have been reported to pose serious problems
to S.rebaudiana Bertoni commonly known as Stevia, a
popular non calori c sweetener. These include Verticil-
lium dahlia on leaves (Farrar et al., 2000), S. sclerotio-
rum reported in Canada (Chang et al., 1997), S. rolfsii in
India (Kamalakannan et al., 2007) and Botryis cinerea in
Itlay (Garibaldi et al., 2009). Sclerotinia stem rot (white
mold) of soybean was rst reported in Hungary in 1924
and since has been reported in Argentina, Brazil, Canada,
India, Nepal, South Africa and United States. A great
economic loss to crop plants by different phytopatho-
gens results in low yields. The species composition of
the endophytic assemblage and frequency of infection
varies according to host species and site characteristic
such as elevation, exposure, associated vegetation, tis-
sue type (Fisher et al., 1994) and tissue age (Fisher et al.,
1986; Rodrigues, 1994).
In the present study, foliar endophytic microorgan-
isms were studied using various diversity indices viz
Simpson index (Simpson, 1949), Shannon index (Shan-
non and Weaver, 1949), evenness index (Ludwig and
Reynolds, 1988) and richness index (Margalef, 1958;
Menhinick, 1964). Higher value of Shannon index and
evenness index with lower values of Simpson index
indicated greater diversity. Bills et al. (2002) described
a signi cant difference between tropical and temperate
endophytes, in terms of their ability to produce num-
ber of bioactive natural compounds isolated from endo-
phytes. This observation suggests the importance of
the host plant in in uencing the general metabolism of
endophytic microbes.
Among 339 recovered endophytic fungal isolates,
40 isolates were screened out as potential antagonistic
endophytes against broad spectrum plant pathogen S.
sclerotiorum following using dual culture technique. It
was found that maximum numbers of antagonistic endo-
phytes were recovered from site1 as compared to site 2.
This may be explained as site 1 is open agricultural eld
which was exposed to wide variety of phytopathogens
so in order to overcome these pathogens several bioac-
tive compounds are produced by them whereas site 2
is closed area which was exposed to limited number of
phytopathogens. Sadrati et al. (2013) screened 20 endo-
phytic fungi from wheat which showed antimicrobial
activities against 12 pathogenic bacteria, yeast and two
phytopathogenic fungi. Percentage growth inhibition
ranged between 6.9%-19% after 24 hr of incubation.

Ankita Verma et al.
760 DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Table 3. Genetic relatedness of GenBank to antagonistic fungal endophytes recovered from
Stevia rebaudiana Bertoni using ITS rDNA gene sequence analysis
Isolate No. Site Accession no. % Similarity Organism
SR/II/2 1 KJ592050 99% Alternaria porri
SR/II/4 1 KJ592051 100% Alternaria alternata
SR/II/5 1 KJ592052 99% Alternaria brassicae
SR/II/6 1 KJ603463 98% Alternaria porri
SR/II/42 1 KJ713969 100% Alternaria sp.
SR/II/45 1 KJ713970 99% Penicillium mallochii
SR/II/50 1 KJ648618 100% Aspergillus niger
SR/II/52 1 KJ713971 99% Phoma sp.
SR/II/54 1 KJ648619 99% Phoma sp.
SR/I/76 1 KJ713972 99% Alternaria alternate
SR/I/77 2 KJ713973 100% Alternaria sp.
SR/I/78 1 KJ728832 100% Alternaria tenuissima
SR/I/94 1 KJ728833 100% Alternaria alternate
SR/I/99 1 KJ728834 99% Alternaria alternate
SR/II/63 1 KF671231 94% Aspergillus avipes
SR/II/1 1 KJ728835 100% Alternaria alternate
SR/II/9 1 KJ735925 99% Alternaria alternate
SR/II/20 1 KJ728836 98% Alternaria sp.
SR/II/21 1 KJ728837 99% Alternaria alternata
SR/II/23 1 KJ728838 100% Chaetomiumglobosum
SR/II/24 1 KJ728839 99% Alternaria alternate
SR/II/26 1 KJ728840 100% Alternaria alternate
SR/II/33 1 KJ728841 100% Phoma sp.
SR/II/36 1 KJ728842 99% Alternaria brassicae
SR/II/55 1 KJ728843 99% Alternaria alternata
SR/II/60 1 KJ735919 100% Ustilago tritici
SR/I/72 1 KJ735920 99% Alternaria brassicae
SR/II/81 1 KJ735921 100% Alternaria alternata
SR/I/85 1 KJ735922 100% Alternaria alternata
SR/I/95 1 KJ735923 100% Alternaria alternata
SR/II/100 1 KJ735924 100% Alternaria alternata
SR/I/83 2 KJ748009 100% Alternaria alternata
SR/II/18 1 KJ748010 100% Phoma sp.
SR/II/37 1 KJ767528 100% Aspergillus sp.
SR/II/46 1 KJ767529 100% Aspergillus sp.
SR/I/88 1 KJ767530 100% Aspergillus niger
SR/I/89 1 KJ767531 100% Aspergillus niger
SR/I/90 1 KJ767532 100% Alternaria alternata
SR/II/3 1 NS NS Sterile mycelia
SR/II/17 1 KJ767533 100% Alternaria brassicae
1= Misrod Agriculture Field 2= Green House NS= Not Sequenced
Ankita Verma et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION 761
FIGURE 4. Phylogenetic tree generated on the basis of ITS rDNAsequences of antagonistic endophytic fungal
isolates aligned with sequences in GenBank databases
Diversity analysis of the antagonistic endophytic popu-
lation showed signi cant diversity index values. Maxi-
mum antifungal activity against S. sclerotiorum was
recorded by Aspergillus avipes strain 63 followed by
strain Aspergillus niger strain 89 and Alternaria alter-
nata strain 99 after 24 hrs of incubation.
For instance, site1 is open agricultural eld with sur-
rounding vegetation like wheat and soybean, which
favours the establishment of endophytic colonization
whereas site 2 is devoid of natural open conditions of
environment which seems to be the major factor for tis-
sue speci c uctuations in the recovery of endophytes
The increased species richness in foliar tissues may be a
result of super infection of the leaves overtime by air-
borne inocula (Carroll et al., 1977; Suryanarayanan and
Vijaykrishna, 2001).
The 40 antagonistic endophytic fungal isolates were
studied for morphological, functional and genotypic
characterization. In the present study, signi cant func-
tional diversity was observed among the antagonistic
endophytes with respect to their hydrolytic potential
viz. amylolytic, cellulolytic, lipolytic, proteolytic, pec-
tinolytic and xylanolytic activities. 19% of the antago-
nistic endophytic fungal population showed amylase,
cellulase, pectinase and protease production whereas
17% were xylanase producing and rest 7% were lipase
producers. Fifty fungal strains isolated from medici-
nal plants (Alpinia calcarata, Bixa orellana, Calophyl-
lum inophyllum and Catharanthus roseus) showed 64%
were lipase producers, 62% were amylase and pectinase
whereas 32% were cellulase and 30% were laccase pro-
ducers (Sunitha et al 2013). Begum et al. (2008) reported

Ankita Verma et al.
762 DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
that majority of the endophytes from Stevia leaves were
cellulolytic in nature. Suganthi et al. (2011) isolated and
characterized Aspergillus niger (BAN 3E) out of ve fun-
gal isolates as the most potent -amylase producer. Sid-
key (2011) found endophytic strain F2Mbb to produce
extracellular amylase.
These potential antagonistic endophytes were screened
for plant growth promoting attributes like siderophore
production, Indole acetic acid production and phos-
phate solubilisation. In present study, about 40% of the
antagonistic endophytes were positive for siderophore
production and amount of siderophore produced ranged
between 1.3-27 mg ml
-1
, 31% of the antagonistic endo-
phytes showed positive results for phosphate solubilisa-
tion. Phosphate solubilisation ef ciency ranged between
5.4-85.7% whereas 29% of the antagonistic endophytes
were recorded positive for IAA production between the
range 1.9-11 μg ml
-1
.Certain endophytes were observed
to improve the ecological adaptability of host enhanc-
ing their tolerance to environmental stress and resist-
ance to phytopathogens (Kimmon et al., 1990; Struz et
al., 1999). In a study performed on Absidia corymbifera,
fungi isolated from rhizospheric soil, were found to pro-
duce siderophore in the range of 4-4.55 μg ml
-1
(Holz-
berg and Artis, 1983). Maliha (2004) found Aspergillus
avus, Aspergillus niger and P.canescens as the most
potent phosphate solubilizers, (Bilal et al, 2018).
Sequence analysis revealed that majority of fungal
endophytes belonged to Alternaria alternata followed
by Aspergillus niger, Phoma, Chaetomium globosum,
and Ustilago tritici. Mandyam et al. (2010) employed
sequencing of ITS region for studying Dark septate
endophytes (DSE) in annually burned tallgrass prairie. In
a nut shell, present investigation has shown that Stevia
rebaudiana Bertoni harbours a good deal of antagonistic
fungal endophytic community. These endophytes have
exhibited various characteristics features which may
pose better tness to Stevia plant and reveal ecological
signi cance of endophyte- host relationship.
ACKNOWLEDGEMENTS
First author is supported as Junior Research Fellowship
from M.P.Biotechnology Council, Bhopal. Authors are
grateful to Dr S.K. Singh, Coordinator, National Facility
(NFCCI), Pune for identi cation of fungi. Financial sup-
port from NASI for sequencing of the samples is highly
acknowledged. First author is extremely thankful to Dr.
S.K. Sharma, Incharge NAIMCC at ICAR-NBAIM, Mau-
nath Bhanjan (U.P) for culture deposition at his facil-
ity and providing accessions numbers. Authors are also
thankful to Dr. Nidhi Gujar for their help in conduct of
this research.
REFERENCES
Altschul SF, Gish W, Miller W, Myers EW and Lipman DJ (1990).
Basic local alignment search tool. J Mol Biol 215(3):403-410.
Aneja K R, Experiments in Microbiology (2003). Plant Pathol-
ogy and Biotechnology, 4th edition, New Age International.
Ankita Verma, B.N.Johri and Anil Prakash (2014). Antagonistic
Evaluation Of Bioactive Metabolite From Endophytic Fungus,
Aspergillus avipes KF671231. Journal of Mycology, Article ID
371218, 5 pages.
Arnold AE, Henk DA, Eels RL, Lutzoni F and Vilgalys R (2007).
Diversity and phylogenetic af nities of foliar fungal endo-
phytes in loblolly pine inferred by culturing and environmen-
tal PCR. Mycologia 99(2) 185-206.
Begum R (2010). Bioprospecting of endophytes from Stevia
rebaudiana Bertoni. PhD Thesis. Barkatullah University, Bho-
pal.
Bernstein ME, Carroll GC (1977). Internal fungi in old growth
Douglas r foliage. Can J of Bot 55: 644-653
Bhagopaty RK, Joshi SR (2011) Multilocus Molecular Char-
acterization of endophytic fungi isolated from ve medicinal
plants of Meghalaya, India. Microbiology 39 ( 2): 71-78.
Bilal L & Asaf S & Hamayun M, Gul H, Iqbal A, Ullah I, Lee IJ,
Hussain A (2018). Plant growth promoting endophytic fungi
Asprgillus fumigatus TS1 and Fusarium proliferatum BRL1
produce gibberellins and regulates plant endogenous hor-
mones. Symbiosis 2: 117-127.
Bills GA, Dombrowski F, Polishhook PJ (2002). Recent and
future discoveries of pharmacologically active metabolite from
tropical fungi. In: R Watling, J.C.Frenkland, A.M. Aineworth,
S.Issac and C.H. Robinson (ed), Tropical mycology: Micromy-
cetes vol 2 CABI Publishing , New York, pp. 165-194
Brick JH, Bostock RM, Silverstone SE (1991). Rapid in situ
assay for indole acetic acid production by bacteria immobi-
lized on nitrocellulose membrane. Appl Environ Microbiol
57:535-538.
Chang KF, Howard RJ, Guadiel RG (1997). First report of stevia
as a host for Sclerotinia sclerotiorum. Plant Dis 81(3): 311.
Choedon T , Mathan G, Arya S, Kumar VL (2006). Anticancer
and cytotoxic properties of the latex of Calotropis procera in
a transgenic mouse model of hepatocellular carcinoma. World
J Gastroenterol.
Choi YW, Hodgkiss IJ, Hyde KD (2005). Enzyme production by
endophytes of Brucea javanica . J Agricult Technol pp 55-66.
Clay K, Schardl C (2002). Evolutionary Origins and Ecological
Consequences of Endophyte Symbiosis with Grasses. Am Nat
160: 99-127.
Costa JM, Loper JE (1994). Characterization of siderophore
production by the biological control agent Enterobacter cloa-
cae. Mol Plant-Microbe Interact7:440–448.
De Bach PH (1964). Biological control of Insects Pests and
weeds. New York: Reinhold.
Dissanayake RK, Ratnaweera PB, Williams DE, Wijayarathne
CD, Wijesundera RLC, Andersen RJ, de Silva ED (2016). Anti-

Ankita Verma et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION 763
microbial activities of mycoleptodiscin B isolated from endo-
phytic fungus Mycoleptodiscus sp. of Calamus thwaitesii Becc.
J Appl Pharm Sci. 6: 1-6.
Farrar, JJ, Davis RM (2000). First report of Verticillium dahlia
On Stevia rebaudiana) in North America. Plant Dis 84: 922.
Fisher PJ and Petrini O (1987). Tissue speci city by fungi
endophytic in Ulex europaeus. Syddowia 40: 46-50.
Fisher PJ Anson AE and Petrini O (1986) Fungal endophytes in
Ilex gallii. T Brit Mycol Sco 86 (1): 153- 193.
Fisher PJ, Petrini O, Petrini LE, Sutton BC (1994). Fungal endo-
phytes from the leaves and twigs of Quercus ilex L. from Eng-
land, Majorca an Switzerland. New Phytologists 127, 133-137.
Garibaldi A, Bertetti D, Gullino ML (2009). First report of Bot-
rytis blight caused by Botrytis cinerea on owering Dogwood
(Cornus orida) in Italy. J Plant Dis 93(5): 549.
Gond S, Verma V, Kumar A and Kharwar R (2007). Study of
endophytic fungal community from different parts of Aegle
marmelos correae (Rutaceae) from Varanasi ( India). World J
Microbiol Biotechnol 23, 1371-1375.
Halmschlager E, Butin H, Donaubauer E. (1993) Endophytic
fungi in leaves and twigs of Quercus petraea. European Journal
of Forest Pathology 23: 51–63.
Hamzah TNT, Lee SH, Hidayat A, Terhem R, Hanum IF,
Mohamed R (2018). Diversity and characterization of endo-
phytic fungi isolated from the tropical Mangroove species,
Rhizophora mucronata, and identi cation of potential antago-
nists against the soil borne fungus, Fusarium solani 9: 1-17.
Hata K, Futai K (1995). Endophytic fungi associated with
healthy pine needles and needles infested by the pine needle
gall midge Thecodiplosis japonensis. Can J Bot 73: 384-390.
Hawksworth DL, Kirk PM, Sutton BC, Pegler DM (1995). Ains-
worth & Bisby’s dictionary of the fungi. 8
th
edition. CAB Inter-
national, Wallingford.
Higgins KL, Arnold AE, Miadlikowska J, Sarvate SD, Lutzoni
F. (2007) Phylogenetic relationships, host af nity, and geo-
graphic structure of boreal and arctic endophytes from three
major plant lineages. Molecular Phylogenetics and Evolution
42: 543–555.
Holzberg M, Artis WM (1983). Hyroxamate siderophore pro-
duction by opportunistic and systemic fungal pathogens.
Infect Immun 40(3): 1134-1139.
Howell CR (2003). Mechanisms employed by Trichoderma spe-
cies in the biological control of plant diseases: the history and
evolution of current concepts. Plant Dis. 87, 4-10.
Jeewon R, Ittoo J, Mahadeb D, Jaufeerally Y, Wang HK, Liu
Pong Ai ( 2013). DNA based Identi cation and Phyloge-
netic characterization of endophytic and saprobic fungi from
Antidesna madagascariense, a Medicinal Plant in Mauritius. J
Mycol 1-10.
Kamalakannan A,Valluvaparidasan V, Chitra K, Rajeswari E,
Salaheddin K, Ladhalakshmi D and Chandrasekaran ( 2007).
First report of root rot of Stevia caused by S.rolfsii in India. J
of Plant Pathol 56 (2): 350.
Kimmons CA Gwinn KD and Bernard EC (1990). Nematode
reproduction on endophyte infected and endo free tall fescue .
Plant Dis 74, 757-761.
Kumaresan V, Suryanarayanan TS (2002). Endophyte assem-
blages in young, mature and senescent leaves of Rhizophora
apiculata: evidence for the role of endophytes in mangrove
litter degradation. Fungal Diversity 9: 81-91.
Lavini A, Riccardi M, Pulvento C, Luca SD, Scamosci M,
Andria RD (2008). Yield, Quality and Water Consumption of
Stevia rebaudiana Bertoni Grown under Different Irrigation
Regimes in Southern Italy. Italian Journal of Agronomy 2:135-
143.
Lee Sunhu, Encarnacion FM, Zentella CZ, Flores Gracia L,
Escamilla JE and Kennedy C (2004). Indole acetic acid biosyn-
thesis is de cient in Glucanacetobacter diazotrophicus strains
with mutation in cytochrome C Biogenesis Genes. 186 (16):
5384-5391.
Lodge DJ, Fisher PJ and Sutton BC (1996). Endophytic fungi
of Manilkara bidentata leaves in Puerto Rico Mycologia 88:
733-738.
Ludwig JA, Reynolds JF (1988). Statistical Ecology. A primer
on methods and computing. John Wiley and Sons, New York,
p. 337
Lumyong, S, Techa W, Lumyong P et al (2009). Endophytic
fungi from Calamus kerrianus and Wallichia caryotoides (Are-
caceae) at Doi Suthep- Pui National Park, Thailand. Chiang
Mai Journal of Science, 36(2), 158-167.
Madhumita K and Chandra S. (2013). Localisation and isola-
tion of fungal endophytes from healthy tissue of Stevia rebau-
diana (Bert.). Internal journal of phytomedicine 5:435-440.
Maliha R, Samina K, Najma A, Sadia A, Farooq L (2004).
Organic acids production and phosphate solubilisation by
phosphate solubilising microorganism under in vitro condi-
tions. Pakistan. J Biol Sci 7: 187-196.
Mandyam K, Loughin T and Jumpponen A (2010). Isolation
and morphological and metabolic characterization of common
of endophytes in annually burned tallgrass prairie. Mycolo-
gia, The Mycological Society of America, Lawrence 102 (4) KS
66044- 8897.
Margalef R (1958). Temporal succession and spatial hetero-
geneity. In: Perspectives in Marine biology, Buzzati-Traverso
(ed). Univ California Press, Berkeley, pp- 323-347.
Menhinick EF (1964) A comparison of some species- individ-
ual diversity indices applied to samples of eld insects. Ecol-
ogy 45: 859-861.
Midmore D.J., Rank A.H. (2002). A new rural industry – Ste-
via – to replace imported chemical sweeteners. Report for
the Rural Industries Research and Development Corporation
02/022, 55 p.
Mishra KM, Anuradha P, Rao DS (2013). Screening a Pecti-
nolytic fungal strain; Aspergillus foetidus MTCC 10367 for
the production of multiple enzymes of industrial importance.
International Journal of Pharm and Bio Sciences. 2:1205 -
1209.

Ankita Verma et al.
764 DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Newman JD, Cragg Gorden M, Snader KM (2003) Natural
Products as sources of new drugs over the period 1981-2002. J
Natural Prod1022-1037.
Paterson RRM, Bridge PD (1994).Biochemical Methods for Fil-
amentous Fungi. IMI Technical Handbooks No. 1. Wallingford,
UK: CAB International
Pikovskaya RI (1948). Mobilization of phosphorus in soil in
connection with the vital activity of some microbial species.
Microbiology 17:62-370
Pirttila A, Joensu P Pospiech H Jalonen J, Hohtola A (2004).
Bud endophytes of Scots pine products adenine derivatives
and other compounds that affect morphology and mitigate
browning of callus cultures. Physiol Plant 121: 305-312.
Pointing SB, Buswell JA, Vrijmoed LLP, Jones EBG (1999).
Extracellular cellulytic enzyme pro les of ligninolous man-
grove fungi. Mycol Res 103(6): 696-700.
Prakash A, Begum R, Johri BN. (2008). Diversity of endophytic
fungi in leaves of Stevia rebaudiana Bertoni. In Proceedings of
the 12
th
International symposium on microbial ecology: 17-22
August, Crains Australia.
Ratnaweera PB, de Silva ED. Endophytic fungi: A remarkable
source of biologically active secondary metabolites (2017). In:
Endophytes: Crop productivity and protection. Sustainable
development and biodiversity. Maheshwari D, Annapurna K.
(eds). Vol 16. Springer, Cham, 2017, pp 191-212.
Ratnaweera PB, Walgama RC, Jayasundera KU, Herath SD,
Abira S, Williams DE, Andersen RJ and de Silva ED (2018).
Antibacterial activities of endophytic fungi isolated from six
Sri Lankan plants of the family Cyperaceae. Bangladesh Jour-
nal of Pharmacology 13:264-272.
Rifai MA (1969) A revision of the genus Trichoderma. Myco-
logical Papers.116: 1-56.
Rodriguez KF (1994). The foliar fungal endophytes of the Ama-
zonian plant Euterpe Xylariaceae. Boletim do Museu Paraense
Em lio Goeldi Botanica 2 (7): 429-439.
Rodriguez KF (1994). The foliar fungal endophytes of the Ama-
zonian plant Euterpe Xylariaceae. Boletim do Museu Paraense
Em lio Goeldi Botanica 2 (7): 429-439.
Rollinger JL and Langenhein (1993). Geographic survey of fun-
gal endophytes community composition in leaves of coastal
redwood. Mycologia 85 (2):149-153.
Sadrati N, Daoud H, Zerroug A, Dahamna S, Bounarati S
(2013). Screening of antimicrobial and antioxidant secondary
metabolites from endophytic fungi isolated from wheat ( T.
Durum) J Plant Protection Research. 53, 128-136.
Sambrook and Russell (2001). Molecular Cloning : A labora-
tory Manual. Cold Spring Harbor Laboratory. 3 edition.
Schippers B, Bakker AW, Bakker PAHM (1987) Interactions of
deleterious and bene cial rhizosphere microorganisms and the
effect of cropping practices. Annu Rev Phytopathol. 25: 339-358.
Schulz B, Boyle C, Draenger S, Rommert AK and Krohn (2002)
Endophytic fungi : a source of biologically active metabolites.
Mycological Res 106: 996-11004
Schwyn B, Neilands JB (1987) Universal chemical assay for
the detection and determination of siderophores. J Analytical
Biochem 160: 47-56.
Sen S, Biswas G, Basu SK, Acharya K (2012). Management of
leaf spot disease of Stevia rebaudiana Bertoni with antago-
nistic bacteria. Australian Journal of Crop Science 2:350-
356.
Shakeri J, Howard A (2007) Proteolytic activity and antibi-
otic production by Trichoderma harzianum in relation to
pathogenicity to insects. Foster Enz Microb Tech 40:961-
968.
Shannon CE, Weaver W (1949) The Mathematical Theory of
Communication. University of Illions Press, Urbana, USA.
Sidkey NM, MA Abo-Shadi, Balahmar R, Sabry R Badrany
(2011) Puri cation and characterization of amylase from
newly isolated Aspergillus avus F2Mbb.Int J of Microbiol-
ogy.2,96-103.
Sierra G (1957). A simple method for the detection of lipol-
ytic activity of microorganisms and some observations on the
in uence of the contact between cells and fatty substrates. Ant
Van Leewenhoek 23(1): 15-22.
Simpson EH (1949). The Measurement of diversity. Nature 163-
688.
Strobel G, Daisy B (2003). Bioprospecting for microbial endo-
phytes and their natural products. Microbiology and Molecular
Biology Reviews. 67:491-502.
Struz AV Christie BR, Matheson BG, Arsenault WJ, Buchanan
N (1999). Endophytic bacterial community in the periderm of
potato tubers and their potential to improve resistance to soil
borne plant pathogens. Plant Pathol 48 : 360-370.
Suganthi R, Benazir JF, Santhi R, Ramesh Kumar V, Hari A,
Meenakshi N, Nidhiya KA, Kavitha G, Lakshmi R ( 2011).
Amylase production by Aspergillus niger under solid state fer-
mentation using agro industries wastes. Inter J Eng Science
and Tech.3, 1756-1763.
Sullivan O, Gara FO (1992). Traits of uorescence Pseudomons
spp. involved in suppression of plants root pathogens. Micro-
biol Rev 56 ( 4): 662-676.
Sunitha VH, Nirmala Devi D, Srinivas C (2013). Extracellular
enzymatic activity of endophytic fungal strains isolated from
medicinal plants. World J of Agicultural Sci 9(1): 01-09.
Suryanaranan TS, Thirunavukkarasu N, Govindarajulu MB ,
Sasse F, Jansen R, Murali TS (2009). Fungal endophytes and
biosprospecting. Fungal Biol Rev 1, 11.
Suryanarayanan TS, Murali TS and Venkatesan G (2002).
Occurrence and distribution of fungal endophytes in tropical
forests across a rainfall gradient. Can J Bot 80: 818-826.
Suryanarayanan TS, Murali TS and Venkatesan G (2002).
Occurence and distribution of fungal endophytes in tropical
forests across a rainfall gradient. Can J Bot 80: 818-826.
Suryanarayanan TS, Venkatesan G, Murali TS (2003). Endo-
phytic fungal diversity and distribution patterns. Curr Sci, 85:
489-493.

Ankita Verma et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIVERSITY ANALYSIS AND CHARACTERIZATION OF ANTAGONISTIC ENDOPHYTIC POPULATION 765
Suryanarayanan, TS and Vijaykrishna D (2001). Fungal endo-
phytes of aerial roots of Ficus benghalensis. Fungal Diversity,
8; 155-161.
Szekeres A, Leitgeb B, Kredics L, Antal Z, Hatvani L, Mancz-
inger L, Vagvolgyi C (2005) Peptaibols and related peptaibiot-
ics ofTrichoderma—a review. Acta Microbiol Immunol Hung
52:137–168.
Taylor, 1.E., Hyde, K.D. and Jones, E.B.G. (1999). Endophytic
fungi associated with the temperate palm, Trachycarpus for-
tunei, within and outside its natural geographic range. New
Phytologist 142: 335-346.
Teather RM, Wood PJ (1982). Use of Congo red polysaccharide
interactions in enumeration and characterization of cellulo-
lytic bacteria from bovine rumen. Appl Environ Microbiol 43
(4):777-780.
Thompson JD, Higgins DG, Gibson TJ (1994). Clustal W:
Improving the sensitivity of progressive multiple sequence
alignment through sequence alignment through sequence
weighting position- speci c gap penalties and weight matrix
choice. Nucleic Acids Res 22(22): 4673-4680.
Toofanee, SB and Dulymamode, R. (2002). Fungal endophytes
associated with Cordemoya integrifolia. Fungal Diversity 11:
169-175.
Wakelin SA, Rosemary A, Warren Paul R, Ryder MH (2004).
Phosphate solubilisation by Penicillium spp. closely associated
with wheat roots. Biol Fertility Soils. 40:36-43.
Waller F, Achatz B, Baltruscha J, Fodor, Becker, K (2005) The
endophytic fungus Piriformospora indica reprograms barley to
salt stress tolerance, disease resistance and higher yield. Proc.
Nat.Acad.Sci.USA,102:13386-13391.
White TJ, Bruns T, Lee S, Taylor JW (1990). Ampli ca-
tion and direct sequencing of fungal ribosomal RNA genes
for phylogenetics. In: PCR Protocols: A Guide to Meth-
ods and Applications, (eds. Innis MA, Gelfand DH, Snin-
sky JJ, White TJ) Academic Press, Inc., New York. pp. 315-
322.
Wilson D, Carroll (1994). Infection studies of Discula quercina,
an endophyte of Quercus garryona. Mycologia 86 (5): 633-
647.