A review on the pharmagnostic evaluation of Meswak,
Salvadora persica
Deepak Kumar Sharma,
1
* K. R. Shah
2
and R. S. Dave
3
1
Department of Chemistry HVHP Institute of Post Graduate Studies and Research, Kadi, Gujarat, India
2
Department of Biotechnology, Pramukh Swami Science and H.D Patel Arts College, Kadi, Gujarat, India
3
Department of Chemistry, Arts, Science & Commerce College, Pilvai, Gujarat, India
ABSTRACT
Due to broad spectrum in physiologic diversity and their wide range of pharmacological activities, plants are playing
an important factor for the pharmaceutical industry. Meswak tree is shrub and botanically known as Salvadora persica
L. It has been used since ancient times as a chewing stick for oral hygiene. Many unique phytochemicals are naturally
present in Miswak, which are described by traditional medicine as a remedy for various disease symptoms with bene -
cial properties. The biological active compounds that are present in plants are referred as phytochemicals. These phyto-
chemicals are derived from different parts of plants such as leaves, barks, seed, seed coat, owers, roots and pulps and
thereby used as sources of direct medicinal agents. Phytochemistry describes the large number of secondary metabolic
compounds present in the plants. The plants are the reservoirs of naturally occurring chemical compounds and of struc-
turally diverse bioactive molecules. The extraction of bioactive compounds from the plants and their quantitative and
qualitative estimation is important for exploration of new biomolecules to be used by pharmaceutical and agrochemical
industry directly or can be used as a lead molecule to synthesize more potent molecules. This review includes the ana-
lytical methodologies in which extraction methods and the process of analysis for bioactive compounds present in the
plant extracts through the different Aaalytical techniques like HPLC, GC, GC, OPLC etc. and the detection of compound
by mean of FTIR, NMR, and MS.
KEY WORDS:
SALVADORA
, PHYTOCHEMISTRY, ANALYTICAL TECHNIQUES, MESWAK
734
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(4): 734-742 (2018)
ARTICLE INFORMATION:
Corresponding Authors: dbsikhwal@gmail.com
Received 15
th
Sep, 2018
Accepted after revision 21
st
Dec, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
Mono of Clarivate Analytics and Crossref Indexed
Journal Mono of CR
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.4/26

Sharma, Shah and Dave
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS A REVIEW ON THE PHARMAGNOSTIC EVALUATION OF MESWAK,
SALVADORA PERSICA
735
INTRODUCTION
Medicinal plants have been the keystone of traditional
herbal medicine amongst occupant of rural area world-
wide since old time. The therapeutic use of plants cer-
tainly goes back to the Sumerian and the Akkadian
civilizations in about the third millennium BC. Hip-
pocrates (ca. 460–377 BC), one of the ancient authors
who described medicinal natural products of plant and
animal origins, listed approximately 400 different plant
species for medicinal purposes. According to the World
Health Organization, a medicinal plant is the plant in
which one or more of its organs, contains substances
that can be used for therapeutic purposes, or which
are precursors for chemo-pharmaceutical compounds.
Medicinal plant will have chemical components that
are medically active in its parts including leaves, roots,
rhizomes, stems, barks, owers, fruits, grains or seeds,
which are employed in the control or treatment of a
disease condition. These chemical compounds or bioac-
tive components (non-nutritional) are often referred to
as phytochemicals (‘phyto-‘from Greek - phyto meaning
‘plant’) or phytoconstituents and are responsible for pro-
tecting the plant against microbial infections or infesta-
tions by pests (Doughari, et al. 2009) In present medical
world oral hygiene is one of the most important daily
routine practices for keeping the mouth and teeth clean
and prevents many health problems, (Halawany et al.
2012).
Recently, there have been considerable interest in
exploring the medicinal properties of S. persica. Metha-
nol, ethyl acetate, and diluted acetone extracts ofS. per-
sicawere screened for in vitro activity against someCan-
dida species with the extract of J. regia L. (Naumi et
al. 2009) The S. persica plant contain different ingredi-
ent which are helpful in the treatment of osteoporosis,
(Fouda et al. 2017). The aqueous extract of S. persica
leaves possesses analgesic activity and decreases car-
rageenan-induced inflammation in rat paw, (Ramadan
et al.2016). Another study has revealed that there are
5-O-caffeoylquinic acid and 4,5-O-Dcaffeoylquinic acid
present as the major phenolic compounds in the root of
S. persica while the stem is rich in 5-O-caffeoylquinic
acid, 3,5-O-Dcaffeoylquinic acid, catechin, and epicat-
echin, (Aumeeruddya et al. 2017). A high content of
5-O-caffeoylquinic acid, naringenine, and some alka-
loids, including caffeine, theobromine, and trigonelline
was also reported from the bark. A new sulphur-con-
taining imidazoline alkaloid, persicaline, along with ve
known compounds was identi ed in S. persica which
have different phytochemical activities, (Mohamed
Farag et al. 2018).
Several studies have probed into the biological pro le
of this plant and a wealth of literature has emerged and
published. In this direction we aimed to explore the up
to date data review regarding S. persica. On the basis of
this background, therefore, the purpose of this piece of
study is to provide baseline information of the effective-
ness ofS.persicastick extract in different aspects. The
phytochemical bio-application of S. persica in various
elds have also been systematically reviewed. Lastly,
possible future directions of research and priority are
also discussed.
HISTORY
According to ancient Greek and Roman literatures from
the 3500 BC, the evolution of the toothbrush may be
traced from chewing sticks that were used by Babyloni-
ans and to toothpicks that were chewed to help clean the
teeth and mouth, (Wu et al. 2001). During the old days,
the laws of Manu of ancient Vedic India stipulated that
the teeth be cleaned as part of the daily hygienic ritu-
als, (Hyson et al. 2003). This review includes the history
and the use of “Meswak” as an oral tool, as well as the
biological effects of S. persica extracts. Chewing sticks
are considered the most popular among all of the dental
care tools for their simplicity, availability, low cost and
their traditional and/or religious value (Halawany et al.
2012, Riggs el at. 2012).
Medical books of ancient India, Susruta Samhita and
Charaka Samhita, have also stressed on oral hygiene
using herbal sticks, (Dahiya et al. 2012). There are vari-
ous biological properties, including signi cant antibac-
terial, (Al-sieni et al. 2013, Rasouli et al. 2014) antifun-
gal, (Almas et al 1999) and anti-plasmodial effects in the
extract of miswak. During the 2nd century BC, the Greek
sophist, Alciphron, recommended a toothpick to clean
the ‘‘ brous residue’’ that remained between the teeth
after meals. The Greek word, karphos, Alciphron used
to describe the toothpick, is roughly translated to ‘blade
of straw’. The Romans had also used toothpicks from the
mastic tree (Pistacia lentiscus). The Gospel of Buddhism
mentions Buddha receiving a ‘‘tooth stick from the god,
Sakka’’. The Talmud mentions ‘‘quesem’’, a splinter or
wooden chip that was ‘‘divided at one end by chewing
and biting’’ and used like a toothbrush.
COMMON NAME
Salvadora persica is commonly known as tooth brush
tree but it does also have various names in different
languages, in Arabic it is used to be called “Miswak”
whereas in Hindi it is known a “Meswak” or “Pillu”. The
name of any plant varies as per the geographic areas. S.
persica is well known plant in India and has many local
names which include “Gudphala” in Sanskrit, “Uka” or
“Ukaay” or “Oamai” in Tamil, “Gonimara”, “Kankhira”

Sharma, Shah and Dave
736 A REVIEW ON THE PHARMAGNOSTIC EVALUATION OF MESWAK,
SALVADORA PERSICA
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
or “Genumar” in Kanada and “Gunnangi” in Telegu.
In the western-northern area of India S. persica grows
in large number. In Gujarat people know it as “Pilludi”
or Pilu” or “Kharijal” whereas Rajasthani language has
a name “Jaal” for it. In other languages it has vener-
able names, “Khabbar” in Sindhi, “Arak” in Assamese,
“Peelu” in Punjabi, “Khakan” in Marathi. In Dutch it is
known as “Zahnbürstenbaum”, “Misvak a
g
˘acı” in Turk-
ish, “Kerriebos” in Namibia, “Asawaki, kighir” in Nigeria
and “Chigombo” or “Iremito” or “Mkayo” in Tanzania.
DESCRIPTION OF THE PLANT
Traditionally rheumatism, leprosy, gonorrhoea, ulcers,
scurvy, tumours and dental diseases can be treated from
S. persica (Miswak) (Almas et al. 1995, Jindal et al.
1996). Dr. Laurent Garcin proposed the term Salvadora,
(Juan Salvadory Bosca, 1598–1681) while as persica
was oriented from Persia and L. is used to indicate Carl
Linnaeus (1707–1778), the father of modern taxonomy.
As a shrub the miswak pant has long branches, often
pendulous or semiscandent, glabrous or pubescent and
the leaves are sub succulent; blades coriaceous, landeo-
late to elliptic, occasionally orbicular, 1–3–10 cm long,
1–2–3 cm wide, rounded to acute at apex, cuneate to
subcordate at base. Flowers are small, greenish–white
with lateral and terminal panicles up to 10 cm long and
petals up-to 3 mm long. Drupes red or dark red purple
when ripe. (Malik S et al. 1987) Besides its medicinal
potentialities, it is also suitable in agroforestry systems
as a wind break and helps in land reclamation (Gururaja
GR et al. 2004, Bhatia B et al. 2000). The ripe fruits of
this tree are sweet and edible (locally called as Piloo)
and consumed by rural/tribal population. The seeds of
Salvadora yield a pale-yellow solid fat, rich in lauric and
myristic acid content which is used in making soaps,
illuminants, varnishes, paints as well as in food industry.
It is recognized as nonconventional oil seed tree crop.
TAXONOMIC POSITION
The genus Salvadora belongs to family ‘salvadoraeceae’.
It comprises three genera (i.e. Azima, Dobera and Salva-
dora) and 10 species distributed mainly in the tropical
and subtropical region of Africa and Asia. It belongs to
‘Magnoliophyta’ division which is further classi ed in
different classes in which Salvadora belong to ‘Magnoli-
opsida’. The order of plant is ‘Brassicales’.
USE AND PHYTOCHEMICALS
In Middle Eastern, some Asian and African cultures
chewing sticks are prepared from the roots and twigs
of S. persica. To prepare this type of sticks the stings or
roots are cut into pieces of 10-to 25-cm long. The sticks
of Miswak can usually be used 3–10 times daily con-
sidered as an inexpensive and an ef cient oral hygiene.
(AI-Bagieh NH et al. 1988) The Primary Health Care
Approach (PHCA) principles entirely consider the use
of Miswak. (Hyson JM el at. 2003) The use of chewing
sticks as an oral hygiene tool like Miswak, where it is
traditionally grown is encouraged and recommended by
the World Health Organization (WHO). (WHO et al. 1987)
In addition Miswak is also recommended for the teeth
whitening, the memory improving tool, the breath fresh-
ener, calming the bile, drying up the phlegm, the gums
strengthening, sharpening the vision and increasing the
appetite, (Almas et al. 2001). Antimicrobial substances
such as sulfur can be extracted from its roots and stems,
moreover Trimethylamine, benzyl isothiocyanate, Sal-
vadorine, beta cholesterol, tannins, saponines, sodium
chloride, potassium chloride, vitamin C, avonoids and
sterols are associated with anti-bacterial effects. Besides
this, the signi cant amounts of added silica can help
to remove plaque mechanically, (Almas et al.1995). In
this plant uoride is also found in measurable quanti-
ties, (Darout et al. 2000) which is easily dissolved and
released in water.
MATERIALS AND METHODS
PROCEDURE FOR SEARCHING INFORMATION
Relevant literature survey was done by scienti c web-
sites and tabs i.e Google Scholar, Scienti c journal.
Information was also obtained from books and e-arti-
cles. The scienti c name of the plant was validated using
The Plant List. Published review papers on S. persica
were used as guidelines to design the present study
and also to add missing data to ensure a more compre-
hensive and up-to-date review is obtained. The refer-
ence lists of review and research papers were searched
for further relevant information. Regarding the search
methodology, the following keywords were searched:
“Salvadora persica plant extraction”, (Google = 68,300
search results, Articles = 4,640).
PLANNING, DESIGN AND DESCRIPTION
OF SECTIONS
This review consists of different nine Sections cover-
ing the traditional uses, phytochemistry, pharmacologi-
cal properties, and bio-applications of S. persica. The
third subsection of seventh section highlights about the
extraction and separation of bioactive compounds of
Miswak plant. Section 8 and 9 include the separation
of phytochemicals in S. persica plant through different
extraction methods. The sections reviewed about the
phytochemicals and bioactivity of different compounds

Sharma, Shah and Dave
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS A REVIEW ON THE PHARMAGNOSTIC EVALUATION OF MESWAK,
SALVADORA PERSICA
737
from the plant extract. The detailed antimicrobial activ-
ity of S. persica has been displayed in the section 10,
lastly, section 11 provides an overview of the potential
applications of S. persica in various elds.
PLANT MATERIAL
First the fresh plant/plant parts can be collected ran-
domly from the semi-arid or xerophytic region. The
sticks of plant are dried at 55
0
C by use of an oven for
three to four days and then cut into slices then ground
into a ne powder using a mixture grinder. The extract
of plant Miswak can be prepared by adding 40 g of the
Miswak powder to 200 ml of solvent in which water,
ethanol and hexane are preferred, in a closed container
and stored at room temperature for 48 h. The solvents
are then ltrated through a Whatman No. 1 lter paper
and allowed to evaporate at 40
0
C in an oven for 72 h.
The dried extracts are considered 100% pure and used
to prepare different concentrations by adding the same
solvents in an amount of 100, 250 and 500 g/ml. differ-
ent commercial toothpaste brands can be used to control
for all of the antimicrobial tests by making the concen-
tration of 100 g/ml by allowing to dry and ground. 250
mg of dried extract is dissolved to prepare the Miswak
mouth wash by using of 1 L of distilled water. (Moham-
mad Abhary et al. 2016)
As scrutinizing the aimed review article, it is observed
that after the collection of plant extraction is car-
ried out by different methods according to the nature
of phytochemical which are present in plant. As the
review on Miswak, some common method of extrac-
tion includes cold extraction and solvent extraction
using Soxhlet apparatus. At present a common Univer-
sal Extraction System (Buchi) is used for the purpose of
extraction.
PLANT EXTRACTION
Cold extraction method
It is reviewed that several of extraction is done by this
method because of low costing and high productivity
with ef ciency. During the process of cold extraction,
Measurable weight of dried powder is taken and respec-
tive solvents is added into conical ask then allow at
room temperature for thirty-minute then after it is kept
for seven days and during this period shaking is allowed
after each twenty-four hours for seven days. Finally
lter the extract through Whatman lter paper under
vacuum and dry it at room temperature in watch glass
dish. Note down the weight of each dish prior to drying
of the extracts and after drying too. The difference was
calculated by the weight of the extract. (Harborne et al.
1973).
Solvent extraction method
Recently the Universal Extraction System (Buchi) is used
for solvent extraction. First the dried plant powder taken
various parts placed in glass thimble for extraction pur-
pose with the use of various solvents. For each extract
the procedures are carried out for 10 cycles, and the tem-
perature is adjusted just below the boiling point of the
respective solvents. The resulting solvent extract is l-
tered, concentrated in vacuum concentrator and used to
determine the presence of phyto constituents (
Harborne
et al. 1973)
Supercritical uid extraction (SFE)
Supercritical uid chromatography (SFC) provides a
useful alternative to gas chromatography and liquid
chromatography for some plant samples which involves
use of gases as mobile phase at a temperature and pres-
sure exceeding its critical point, usually CO
2
is used and
compressing them into a dense liquid under these condi-
tions the mobile phase is neither a gas nor a liquid. The
turbid liquid is then pumped through a cylinder contain-
ing the material which to be extracted. From there, the
extract- hampered liquid is pumped into a separation
chamber where the extract is separated from the gas and
the gas is recovered for re-use. CO
2
is commonly used
because its low critical temperature, 31 °C, and critical
pressure, 72.9 atm, are relatively easy to achieve and
maintain. Solvent properties of CO
2
can be manipulated
and adjusted by varying the pressure and temperature.
The advantages of SFE are, no solvent residues left in it
as CO
2
evaporates completely, (Patil et al. 2010).
Microwave-assisted extraction (MAE)
The combination of microwave and traditional solvent
extraction is simply termed as microwave extraction.
Microwave-assisted extraction is considered as the heat-
ing of solvents and plant tissue using microwave which
increases the kinetic of extraction (
Delazar et al 2012).
To remove the minute microscopic traces of moisture
present in plant cell, the extraction is heated in dried
plant material. As a result of the heating up of this mois-
ture inside the plant cell due the evaporation of moisture
occurs and generates tremendous pressure on the cell
wall. Due to the pressure the cell wall is pushed from
inside and the cell wall ruptures. Thus, from the ruptured
cells the exudation of active constituents occurs, hence
increasing the yield of phytoconstituents, (Gordy et al.
1953 and Goldman et al. 1963).
IDENTIFICATION OF PHYTOCHEMICALS
The separation of bioactive compound which are present
in the plant extract with different polarities is a chal-
lenging task for the process of identi cation and charac-
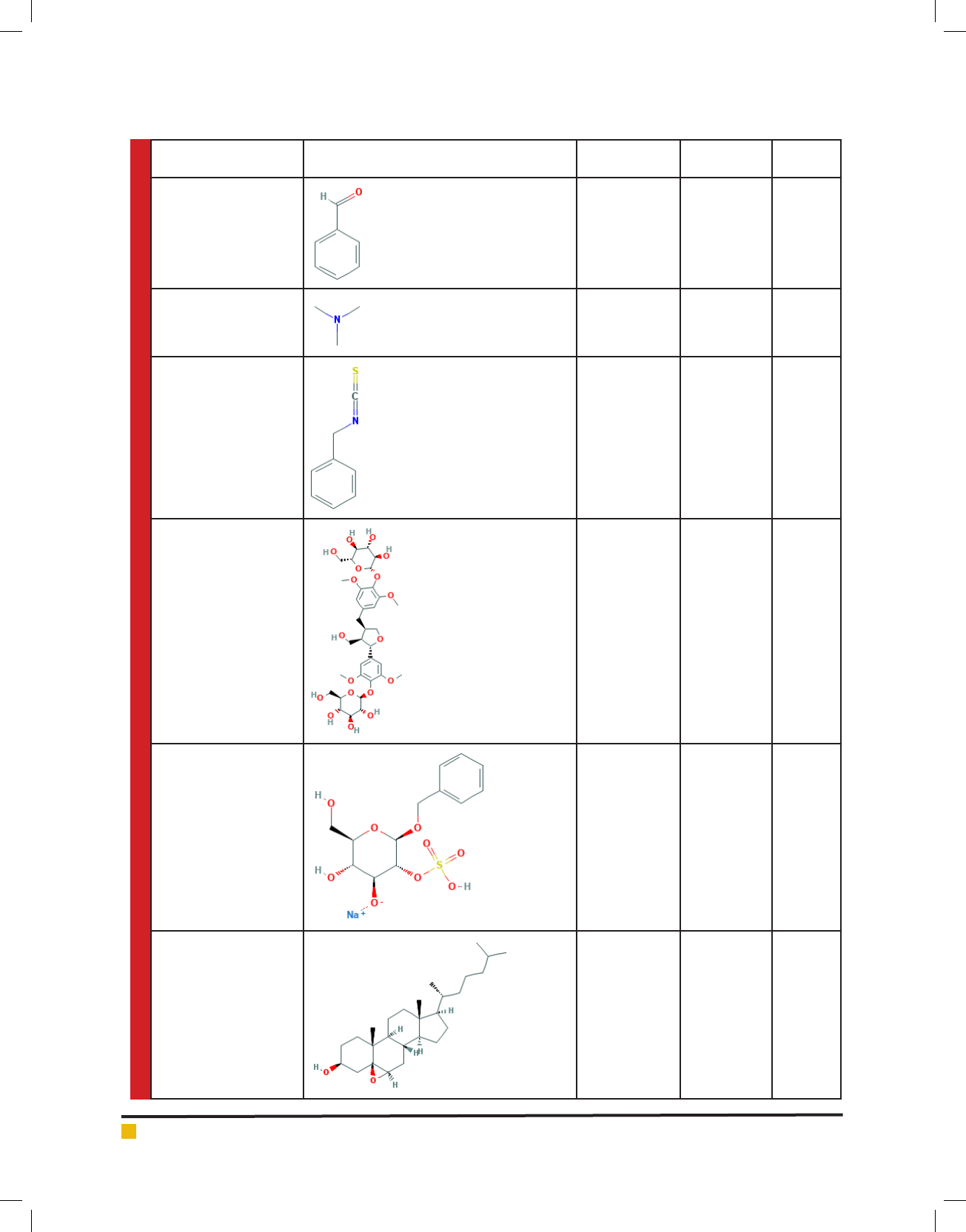
Sharma, Shah and Dave
738 A REVIEW ON THE PHARMAGNOSTIC EVALUATION OF MESWAK,
SALVADORA PERSICA
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Name of Compound Chemical Structure Molecular
Weight g/mol
Molecular
Formula
PubChem
CID
Benzaldehyde 106.124 C
7
H
6
O 240
Trimethyl amine 59.112 C
3
H
9
N 1146
Benzyl isothiocyanate 149.211 C
8
H
7
NS 2346
Salvadoraside 744.74 C
34
H
48
O
18
101630443
Salvadoside 372.32 C
13
H
17
NaO
9
S 23664985
Cholesterolbeta-epoxide 402.663 C
27
H
46
O
2
108109
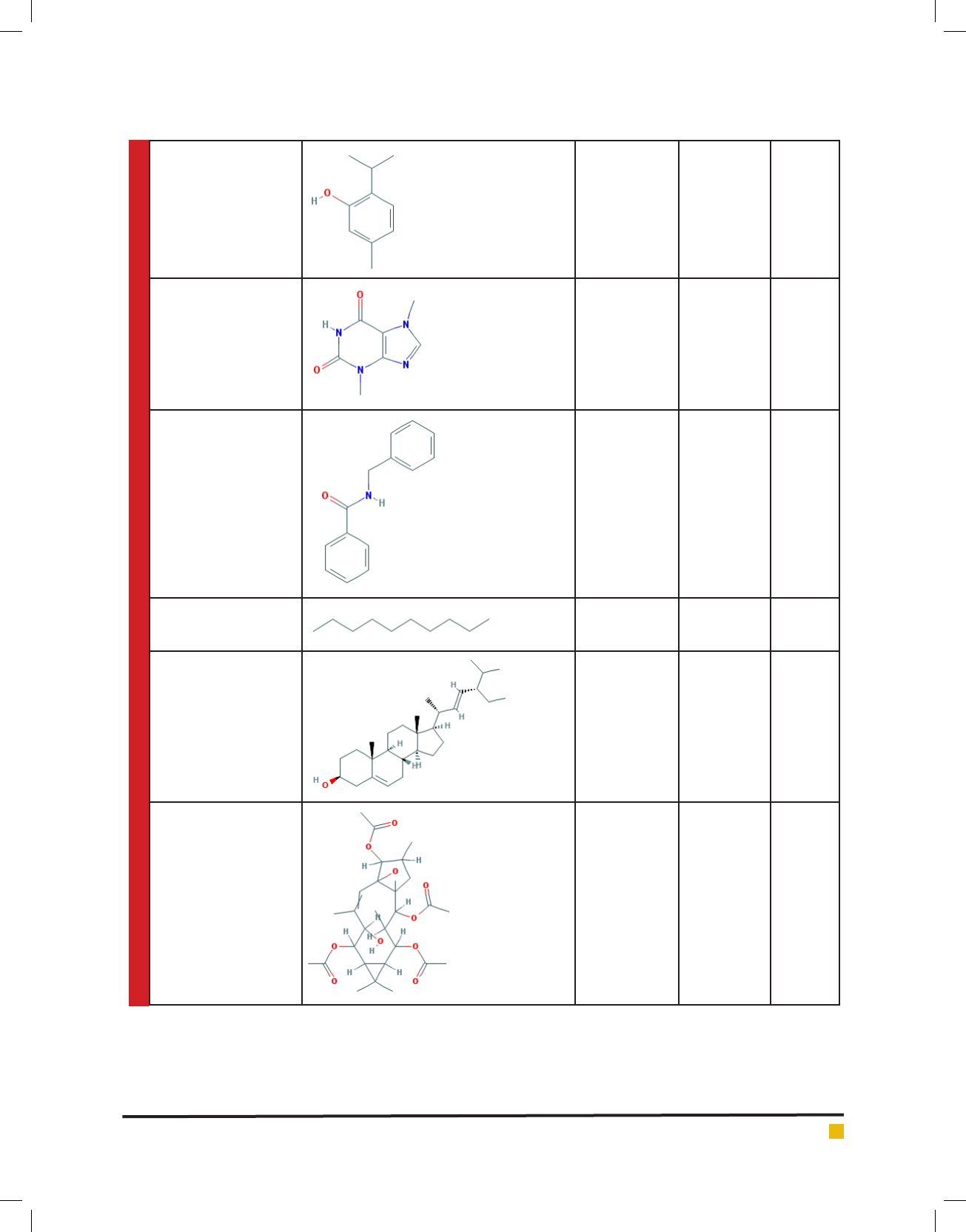
Sharma, Shah and Dave
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS A REVIEW ON THE PHARMAGNOSTIC EVALUATION OF MESWAK,
SALVADORA PERSICA
739
Thymol 150.221 C
10
H
14
O 6989
Theobromine 180.167 C
7
H
8
N
4
O
2
5429
N-Benzyl benzamide 211.264 C
14
H
13
NO 73878
Decane 142.2 C
10
H
22
15600
Stigmasterol 412.702 C
29
H
48
O 5280794
9-Desoxo-9-x-acetoxy-
3,8,12-tri-O-acetylingol
536.618 C
28
H
40
O
10
537583
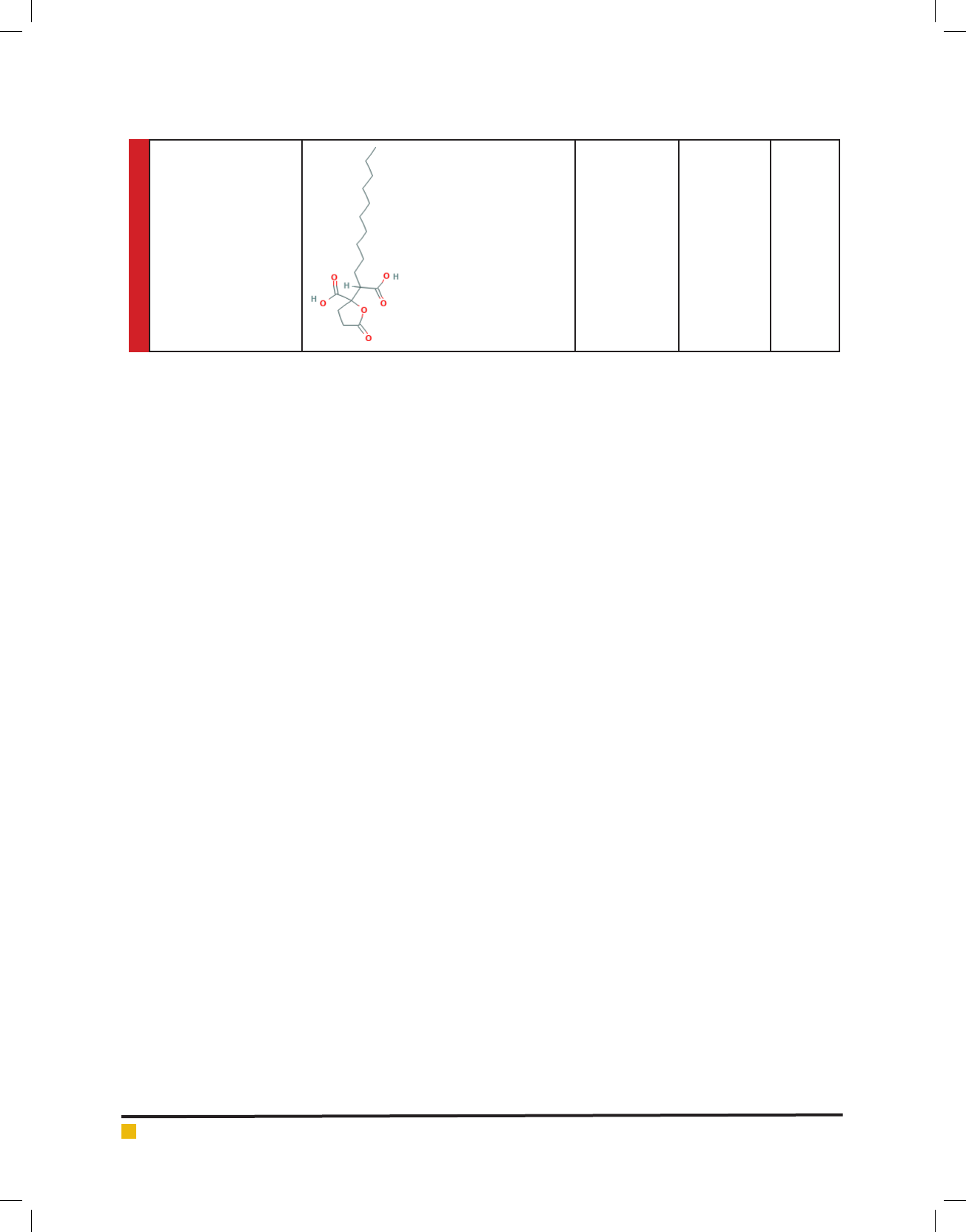
Sharma, Shah and Dave
740 A REVIEW ON THE PHARMAGNOSTIC EVALUATION OF MESWAK,
SALVADORA PERSICA
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
SpiculisporicAcid 328.405 C
17
H
28
O
6
316426
terization of bioactive compounds. It is a common prac-
tice to use TLC, HPTLC, paper chromatography, column
chromatography, Gas chromatography, OPLC and HPLC,
should be used to obtain pure compounds in isolation
of bioactive compounds. The pure compounds are then
used for the determination of structure and biological
activity, (Sasidharan et al. 2011).
Physicochemical and Phytochemical Studies
Phytochemical studies include extractive values, total
ash, acid insoluble ash, total sugar, starch, tannin, and
phenols can be calculated from the shade-dried and
powdered (60 mesh) plant material. (Peach K et al. 1955,
Ayurvedic Pharmacopeoeia of India. Part I et al. 2001,
2004). The Antioxidant Activity of the plant extracts
and standard was assessed on the basis of the radical
scavenging effect of the stable DPPH (2,2‐diphenyl‐1‐
picryl‐hydrazyl‐hydrate) free radical. (Verma et al 2012,
Gupta et al. 2015).
Methanol was used to make working solutions of
the test extracts. Ascorbic acid was used as the stand-
ard in solutions ranging from 1 to 50 μg/ml. In metha-
nol 0.002% DPPH solution is prepared. Then 2 ml of
this solution was mixed with 2 ml of sample solutions
(ranging from 25 μg/ml to 500 μg/ml) and the standard
solution to be tested separately. These solution mixtures
were kept in the dark for 30 min and optical density was
measured at 517 nm using a Shimadzu spectrophotom-
eter against methanol. The blank used was 2 ml of meth-
anol with 2 ml of DPPH solution (0.002%). The optical
density was recorded and percentage of inhibition was
calculated using the equation: % of inhibition of DPPH
activity = (A–B) /A × 100; where A is optical density of
the blank and B is optical density of the sample.
HPTLC STUDIES
Air dried (45-55°C) powdered stem and twig of S. persica
(2.0 g) in triplicate were extracted separately with 3 X 20
ml methanol. Extracts were concentrated under vacuum
and re dissolved in methanol, ltered and nally made
up to 100 ml with methanol prior to HPTLC analysis.
Reagents used were from Merck (Germany) and standard
ferulic acid was procured from Sigma-Aldrich (Stein-
heim), (Verma et al 2012, Gupta A et al. 2015).
Antimicrobial effects
Different antimicrobial activity was performed which
can An in vitro study showed that the aqueous extract
of S. persica miswak had an inhibitory effect on the
growth of Candida albicans that may be attributed to
its high sulfate content (al-Bagieh, N. et al. 1995). Some
studies investigated the derivatives of S. persica mis-
wak using three different laboratory methods, and dem-
onstrated strong antimicrobial effects on the growth of
Streptococcus sp. and Staphylococcus aureus. (Al La ,
et al. 1995) In addition, some showed that Enterococcus
faecalis is the most sensitive microorganism affected by
the use of S. persica miswak, and noted no signi cant
difference in the antimicrobial effects of freshly cut and
1-month-old miswak. A comparison of the alcoholic and
aqueous extracts of S. persica miswak revealed that the
alcoholic extract had more potent antimicrobial activity
than did the aqueous extract (Al-Bagieh, et al. 1997).
Aqueous extract of plant inhibited all the microor-
ganisms, showing greater activity on Streptococcus spe-
cies. Methanolic extract was resisted by L. acidophilus
and P. aeruginosa. At highest concentration tested (200
mg/ml), the aqueous extract was more ef cient than the
methanolic extract but were less ef cient than the posi-
tive control streptomycin and amphotericin B. (Al-Bayati
et al. 2008) Miswak extract displayed greater reduction
in both S. mutans and Lactobacillus cariogenic bacte-
ria counts. Reduction of microbial count in females was
more for both microorganisms as compared to males,
(Bhat et al. 2012). Ethanol extract was more effective
than the aqueous extract in inhibiting the S. mutans, L.
acidophilus, E. coli, S. aureus, and P. aeruginosa micro-
organisms. The aqueous extract did not display any
inhibitory effect on P. aeruginosa. (Mohammed, et al.

Sharma, Shah and Dave
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS A REVIEW ON THE PHARMAGNOSTIC EVALUATION OF MESWAK,
SALVADORA PERSICA
741
2013). The miswak extracts showed comparable or
slightly stronger activity compared to some toothpastes.
The aqueous extract exhibit antibacterial activity on M.
bovis (Fallah et al., 2015).
All solvent-extracts inhibited the S. aureus, S.
mutans, S. sanguinis, S. sobrinus, S. salivarius, L. aci-
dophilus microorganisms. Methanol extract was more
effective than the other extracts. (Kumar et al., 2016)
S. persica aqueous extract showed higher activity than
methanol extract against S. aureus while the opposite
effect was observed against E. coli. Compared to C.
mopane and D. cinerea, S. persica was most effective
against S. aureus but was least effective against E. coli,
(Mudzengi et al., 2017).
CONCLUSION
Our literature review concludes that the use of S. per-
sica miswak as an oral hygiene aid is effective. Descrip-
tive and experimental studies have provided consider-
able evidence that the S. persica plant and its extracts
exert bene cial effects on the oral tissues and help to
maintain good oral hygiene. It is encouraging to note
the large number of studies and clinical trials that have
examined the effects of S. persica miswak and the value
that people have attached to it since ancient times. The
use of S. persica miswak alone or in combination with
conventional toothbrushes, when performed judiciously,
will result in superior oral health and hygiene. It is to
be noted that there had been earlier attempts to summa-
ries the medicinal potential of S. persica, even though
with a different or a less broad ethnopharmacological
focus. However, this review can be considered as the
rst attempt to broaden and critically assess scienti c
evidence on the ethnopharmacology of S. persica. It is
obvious from this review that S. persica can be regarded
as an important traditionally used medicinal plant har-
boring a panoply of bioactive compounds, pharmaco-
logical properties, and modern applications in emerg-
ing elds of interest. It is anticipated that this review
article will open new avenues for research and stimulate
further studies that will ll research gaps highlighted
above.
REFERENCES
Abdel-Motaal Foudaa, Amany Ragab Youssef (2017) Anti-
osteoporotic activity ofSalvadora persicasticks extract in an
estrogen de cient model of osteoporosis. Osteoporosis and
Sarcopenia, Volume-3, 132-137
Almas K. (2001) The effect of extract of chewing sticks (Sal-
vadora persica) on healthy and periodontal involved human
dentin A SEM study. Indian J Dent Res 12:127-132.
Al-Bayati, F.A., Sulaiman, K.D. (2008) In vitro antimicrobial
activity of Salvadora persica L. extracts against some isolated
oral pathogens in Iraq. Turk. J. Biol. 32 (1), 57–62.
AI-Bagieh NH, Weinberg ED. (1988) Benzylisothiocyanate:
a possible agent for controlling dental caries. Microbios Lett
39:143–51.
Al-Bagieh, N., Almas, K. (1997) In vitro antibacterial effects of
aqueous and alcohol extracts of miswak (chewing sticks). Cairo
Dent. J. 13, 221–224
Al-Bagieh, N., Idowu, A., Salako, N.O. (1994). Effect of aqueous
extract of miswak on the in vitro growth of Candida albicans.
Microbios 80 (323), 107–113.
Al-sieni AI. (2013) The antibacterial activity of traditionally
used Salvadora persica L. (miswak) and Commiphora gileaden-
sis (palsam) in Saudi Arabia. Afr J Tradit Complement Altern
Med. 11(1): 23-7.
Almas K, AI-Bagieh NH (1999) The antimicrobial effects of
bark and pulp extracts of meswak Salvadora persica Biomed
Lett 60:71–5.
Almas K, Al-La TR. (1995) The natural toothbrush. World
Health Forum 16:206-210.
Al La , T., Ababneh, H. (1995) The effect of the extract of the
miswak (chewing sticks) used in Jordan and the Middle East on
oral bacteria. Int. Dent. J. 45, 218–222.
Anonymous (2007) Ayurvedic Pharmacopeoeia of India. Part
I, Vol I. Department of Health, Ministry of Health and Fam-
ily Welfare, Government of India, New Delhi; 2004. p. 152-
165.
Anonymous (2007a) Indian pharmacopoeia, Government of
India, Ministry of Health and Family Welfare, Controller of
Publications, New Delhi; 2007. p. 191.
Anonymous (1984) Of cial methods of Analysis (AOAC), 4th
edn. Association of Of cial Chemists, Inc. U.S.A; 1984. p. 187-
8.
Anonymous. (1972) The wealth of India - Raw materials (New
Delhi, CSIR), 1972; 9: 193-195.
Aumeeruddya, M.Z.; Zenginb, G.; Mahomoodally, M.F. A
review of the traditional and modern uses of Salvadora persica
L. (Miswak): Toothbrush tree of Prophet Muhammad. J. Eth-
nopharm. 2017, 213, 409–444.
Bhat, P.K., Kumar, A., Sarkar, S., (2012) Assessment of immedi-
ate antimicrobial effect of miswak extract and toothbrush on
cariogenic bacteria-A clinical study. J. Adv. Oral. Res. 3 (1)
13–18.
Bhatia B, Sharma HL (2000) Fuelwood production and waste-
land reclamation, Botanica 2000; 14: 84-93.
Dahiya P, Kamal R, Luthra RP, Mishra R, Saini G. (2012) Mis-
wak: a periodontist’s perspective. J Ayurveda Integr Med
3(4):184–7.
Darout IA, Christy AA, Nils Skaug, Egeberg PK. (2000) Identi-
cation and quanti cation of some potentially antimicrobial
anionic components in miswak extract. Indian J Pharmacol.
32: 11-14.

Sharma, Shah and Dave
742 A REVIEW ON THE PHARMAGNOSTIC EVALUATION OF MESWAK,
SALVADORA PERSICA
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Delazar A, Nahar L, Hamedeyazdan S, Sarker SD. (2012) Micro-
wave-assisted extraction in natural products isolation. Meth-
ods Mol Biol. 864:89-115
Doughari, J.H.; Human, I.S, Bennade, S. & Ndakidemi, P.A.
(2009). Phytochemicals as chemotherapeutic agents and anti-
oxidants: Possible solution to the control of antibiotic resistant
verocytotoxin producing bacteria. Journal of Medicinal Plants
Research. 3(11): 839-848.
E.Noumi, M.Snoussi, H.Hajlaoui, E.Valentin, A.Bakhrouf.,
(2009). Antifungal properties ofSalvadora persicaandJuglans
regiaL. extracts against oralCandidastrains. European Jour-
nal of Clinical Microbiology & Infectious Diseases,
E. Riggs, C. van Gemert, M. Gussy, E. Waters, N. Kilpatrick
(2012) Re ections on cultural diversity of oral health pro-
motion and prevention, Global Health Promot. 19 (1) 60–63,
http://dx.doi.org/10.1177/1757975911429872.
Fallah, M., Fallah, F., Kamalinejad, M., Malekan, M.A.,
Akhlaghi, Z., Esmaeili, M., (2015). The antimicrobial effect of
aquatic extract of Salvadora persica on Mycobacterium bovis
in vitro. Int. J. Mycobacteriology 4, 167–168.
Goldman R. (1962) Ultrasonic Technology. Van Nostrand Rein-
hold, New York. 1962.
Gordy WWV, Smith RF Trambarulo. (1953) Microwave Spec-
troscopy. Wiley, New York. 1953.
Gupta A, Verma A, Kushwaha P, Srivastava S, Rawat AKS.
(2015) Phytochemical and Antioxidant Studies of Salva-
dora persica L. Stem & Twig. Indian Journal of Pharmaceu-
tical Education and Research | Vol 49 | Issue 1 | Jan-Mar,
2015
Gururaja GR, Nayak AK, Chinchmalatpure AR, Nath A, Babu
VP. (2004) Growth and Yield of Salvadora persica, A facul-
tative halophyte grown on saline black soil (Vertic Haplus-
tept), Arid Land Research and Management 18(1): 165-
168.
Harborne JB. (1973) Phytochemical methods. Chapman and
Hall Ltd., London. 49-88
H. Ahmad, K. Rajagopal, Biological activities of Salvadora
persica L. (Meswak), Med. Aromat. Plants 2 (4) (2013), http://
dx.doi.org/10.4172/21670412.1000129.
H.S. Halawany (2012) A review on Miswak (Salvadora persica)
and its effect on various aspects of oral health, Saudi Dent. J.
24 (2012) 63–69.
Hyson JM. (2003) History of the toothbrush. J Hist Dent
51(2):73–80.
Jindal SK, Bhansali R, Satyavir R. (1996) Salvadora tree - A
potential source of non-edible oil, In: Proc of All India seminar
on rabi oil seed crop (Jodhpur, CAZRI); 1996
Kumari, A., Parida, A.K., Rangani, J., Panda, A., (2017). Antiox-
idant activities, metabolic pro ling, proximate analysis, min-
eral nutrient composition of Salvadora persica fruit unravel
a potential functional food and a natural source of pharma-
ceuticals. Front. Pharmacol. 8 (61). http://dx.doi.org/10.3389/
fphar.2017.00061.
Malik S, Ahmed SS, Haider SI, Muzaffer A. (1987) Salvadori-
cine - a new indole alkaloid from the leaves of Salvadora per-
sica, Tetrahedron Lett 28: 163-164.
Mohammad Abhary , Abdul-Aziz Al-Hazmi. (2016) Antibacte-
rial activity of Miswak (Salvadora persica L.) extracts on oral
hygiene. Journal of Taibah University for Science 10 513–520
Mohammed, S.G., (2013). Comparative study of in vitro anti-
bacterial activity of miswak extracts and different toothpastes.
Am. J. Agric. Biol. Sci. 8 (1), 82–88.
Mohamed Farag, Wael M. Abdel-Mageed, Omer Basudan and
Ali El-Gamal, (2018), Persicaline, A New Antioxidant Sulphur-
Containing Imidazoline Alkaloid from Salvadora persica Roots,
Molecules 23, 483; doi:10.3390/molecules2302048
Mudzengi, C.P., Murwira, A., Tivapasi, M., Murungweni, C.,
Burumu, J.V., Halimani, T., (2017). Antibacterial activity of
aqueous and methanol extracts of selected species used in
livestock health management. Pharm. Biol. 55 (1), 1054–1060.
Noumi E, Snoussi M, Hajlaoui H, Valentin E, Bakhrouf A (2010)
Antifungal properties of Salvadora persica and Juglans regia
L. extracts against oral Candida strains. Eur J Clin Microbiol
Infect Dis 29: 81-88.
Patil PS, Shettigar R.(2010) An advancement of analytical
techniques in herbal research J Adv Sci Res. 1(1):08-14.
Peach K,Tracy MV.(1955) Modern Methods of Plant Analysis.
Vol III and IV Springer, Heidelberg p. 258-61.
Ramadan, K.S., Alshamrani, S.A., (2016.) Phytochemical anal-
ysis and antioxidant activity of Salvadora persica extracts. J.
Basic Appl. Res.2, 390–395
Rasouli GAA, Rezaei A, Hosein MSS, Yaghoobee S, Khorsand
A, Kadkhoda Z, (2014) et al. Inhibitory activity of Salvadora
persica extracts against oral bacterial strains associated with
periodontitis: An in-vitro study. Journal of Oral Biology and
cranio facial research 4: 19-23.
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha
L.(2011) Extraction, isolation and characterization of bioactive
compounds from plants’ extracts. Afr J Tradit Complement
Altern Med. 2011; 8(1):1-10.
S. Dutta, A. Shaikh (2012) The active chemical constituent and
biological activity of Salvadora persica (Miswak), Int. J. Curr.
Pharmaceut. Rev. Res. 3 (1) ISSN: 0976-822X.
Verma S, Gupta A, Kushwaha P, Khare V, Srivastava S, Rawat
AKS. (2012)Phytochemical Evaluation and Antioxidant Study
of Jatropha curcas Seeds. Pharmcog J. 4(29): 5-54.
WHO (1987) Preventive methods and programmes for oral dis-
eases. World Health Organization. Technical Report Series 713,
Geneva; 1987.
Wolf W. (1966) A history of personal hygiene – customs, meth-
ods and instruments – yesterday, today, tomorrow. Bull Hist
Dent 14(4):54–66.
Wu, C., Darout, I., Skaug, N. (2001). Chewing sticks: timeless
natural toothbrushes for oral cleansing. J. Periodontal. Res. 36
(5), 275–284.