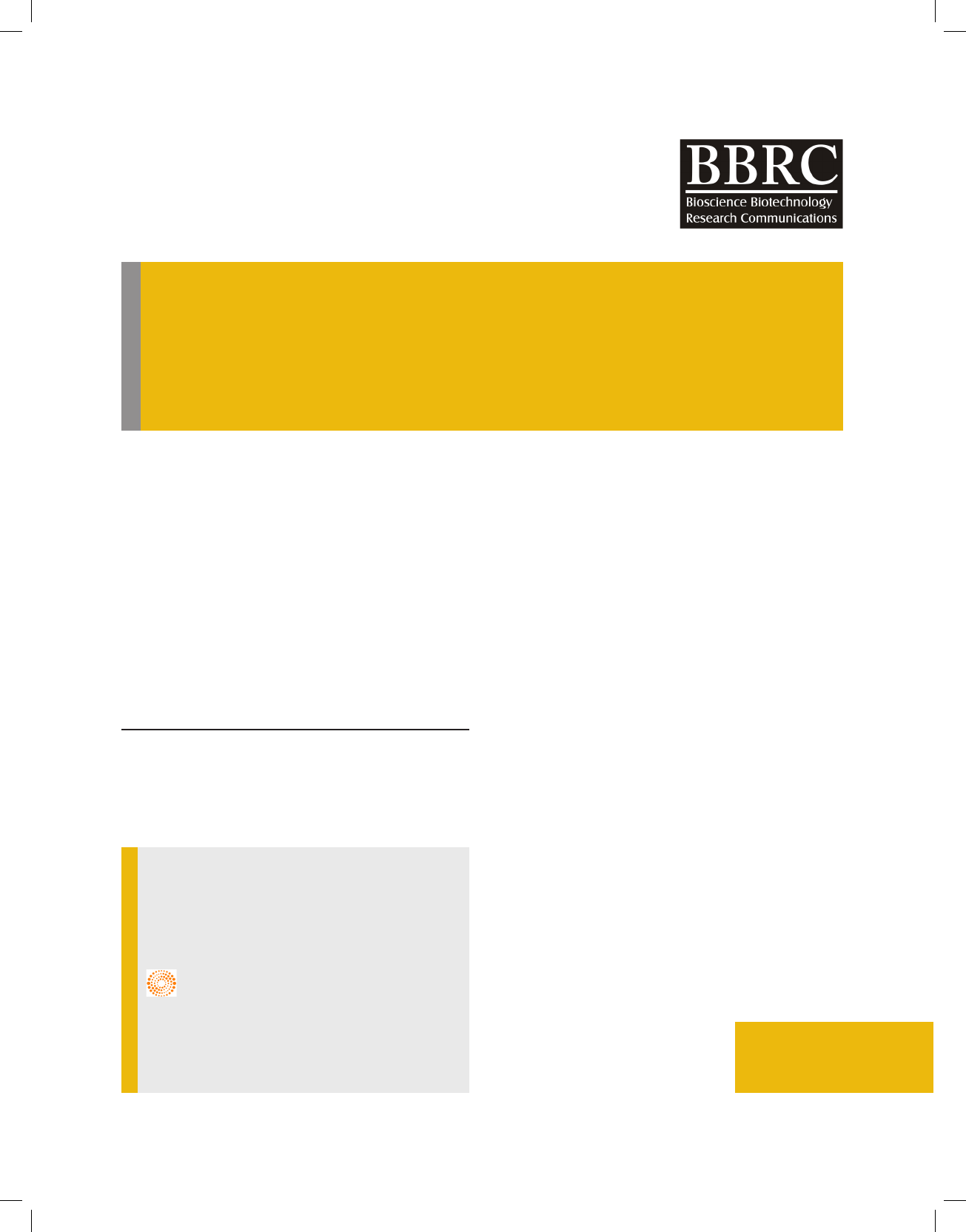
Extraction of bacitracin from
Bacillus subtilis
BSG and
its optimization using response surface methodology
Diptikanta Acharya, Sagarika Satapathy and Manoja Das*
Department of Biotechnology, Gandhi Institute of Engineering and Technology (Autonomous),
Gunupur-765022, India
ABSTRACT
Bacillus sp. was isolated from the soil sample of Gunupur, Odisha, India and screened to produce bacitracin at the
laboratory. The bacitracin from the bacterium was extracted using butanol-ether solution as a solvent and its anti-
bacterial properties against ve bacteria namely Staphylococcus aureus (MTCC 98), Escherichia coli (MTCC 739),
Micrococcus luteus (MTCC 106), Salmonella typhimurium (MTCC 1254), and Pseudomonas aeruginosa (MTCC 2453)
have been studied in vitro by agar well diffusion method. Simultaneously, the individual, square and combined effect
of incubation time, pH and temperature on the bacitracin production was studied using Response Surface Method-
ology (RSP). Analysis of variance (ANOVA) has shown a coef cient of correlation value of 0.9611) and a quadratic
correlation for bacitracin production was derived with 95% con dence level. Incubation time of 26.5 h; pH of 5.67;
and temperature of 30.65°C have been found to be as optimum operating conditions for a maximum bacitracin pro-
duction from Bacillus sp. BSG of 8.72μg/100 ml.
KEY WORDS: SOIL MICROORGANISM; BACITRACIN; ANTIMICROBIAL ACTIVITY; ANOVA; RESPONSE SURFACE METHODOLOGY
710
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(4): 710-718 (2018)
INTRODUCTION
The use of antibiotics in clinical biology is a common
phenomenon and their uses are rapidly increasing day
by day. Most of the antibiotics have been obtained from
microorganisms. Antibiotics which have been used
today are either natural or semi synthetic or synthetic
by nature (Trookman et al., 2011). Semi synthetic anti-
biotics are prepared by modifying the natural antibi-
otic, either chemically or enzymatically with an aim to
enhance the ef ciency of original antibiotics. Antibiot-
ics used in medical practices have been obtained from
few groups of microorganisms only. The two genera
of bacteria Streptomyces and Bacillus are the maxi-
ARTICLE INFORMATION:
Corresponding Authors: manoja2003@rediffmail.com
Received 28
th
Sep, 2018
Accepted after revision 19
th
Dec, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
Mono of Clarivate Analytics and Crossref Indexed
Journal Mono of CR
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.4/23
Acharya et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EXTRACTION OF BACITRACIN FROM
BACILLUS SUBTILIS
BSG 711
mum contributor of antibiotics (Pavithra et al., 2009).
The antibiotics produced by Bacillus species are being
recommended for infection caused by gram positive
bacteria. Bacillus species produces about 167 antibiot-
ics which are polypeptide by nature (Arias et al., 1999).
The two species of Bacillus such as B. subtilis and B.
brevis produce 66 and23 polypeptide respectively. The
most important peptide antibiotics produced by Bacil-
lus species is Bacitracin. It is commonly used in medi-
cal preparations, either alone or with other antibiotics,
against all kinds of gram positive bacteria and to some
extent gram negative bacteria. The antibiotic interferes
with protein synthesis and disrupts the cell of parasitic
bacteria. The bacitracin production by Bacillus licheni-
formis can be enhanced by acetoin reductase activity
and the transcription factors Spo0A and AbrB regulate
the bacitracin synthesis, (Kasetty et al., 2015 Lagzian
et al., 2018).
Many investigations have been conducted on baci-
tracin, but the optimization o ts production remains
unexplored. The present work aims at the isolation of
bacitracin producing the soil inhabitant Bacillus bac-
terium obtained from the local soil sample with the
purpose of establishing its antibacterial activity against
ve bacteria such as Staphylococcus aureus (MTCC 98),
Escherichia coli (MTCC 739), Micrococcus luteus (MTCC
106), Salmonella typhimurium (MTCC 1254), and Pseu-
domonas aeruginosa (MTCC 2453). Simultaneously, cen-
tral composite design was used to investigate the effect
of incubation time, pH and temperature on the produc-
tion of bacitracin. The production parameters are opti-
mized in order to maximize the production of bacitracin.
MATERIALS ANDMETHODS
EXPERIMENTAL PROCEDURE
Isolation of Bacillus species
Soil samples were collected by sterilized plastic bags
from different parts of Gunupur area. The soil inhabitant
microorganism was isolated by following serial dilution
(10
-4
folds) using nutrient agar plate (Coppuccino et al.,
1996) and CFU of the soil microbes’ were recorded.
Sub culture and characterization of Bacterium
The isolated bacterium was sub cultured in the labo-
ratory using the same nutrient by following agar slant
technique, and preserved at the low temperature (4°C)
for further use. The characterization of the bacterium
was made at the laboratory by morphological observa-
tion and biochemical tests. The suspected Bacillus spe-
cies were identi ed by molecular techniques. The growth
curve of the bacterium was determined by spectrophoto-
metric techniques (Goodfellow et al., 1980).
Extraction, puri cation and identi cation of Bacitracin
The batch culture technique was used for the extrac-
tion of bacitracin from isolated bacterium. The bacte-
rium was inoculated in the nutrient broth at 37° C at
slow agitation condition for 72 hrs and at log phase of
growth the extraction was obtained. The puri cation of
the bacitracin in the culture medium was done using
butanol-ether solution (pH=4) as a solvent (Murphy
et al., 2007). At acidic condition, the bacitracin remained
in lower aqueous layer was collected carefully followed
by neutralizing with sodium bicarbonate and was lyo-
philized to obtain the puri ed bacitracin. Thin Layer
Chromatography (TLC) and GC-MS had been used to
identify the obtained bacitracin . The silica phase acts
as the solid phase of the TLC and the mobile phase was
prepared with chloroform and methanol in the ratio of
9:1. The Rf value is considered for identi cation of baci-
tracin (Phillips, 1999). Con rmation of bacitracin was
made by GC-MS (MSGC- 11) instrument with the capil-
lary column of HP-3 (50 mm × 0.521mm, lm thick-
ness 0.25μm). 1μL of extract was carefully injected into
GC-MS for analysis. The chemical compositions were
identi ed by comparing their retention indices (RI) and
mass fragmentation pattern.
Standardization of Production of Bacitracin
The production of bacitracin was carried out with solid
state fermentation. The fermentation was made in an
Erlenmeyer ask (capacity 1 liter) by using wheat bran
as substrate. The substrate (100 gm) was added to 100
ml of phosphate buffer. Then the ask along with the
substrate had been sterilized in autoclave at 15 psi for
20 minutes. Isolated microorganisms were inoculated
carefully into the ask and incubated at 37° C for 24
hrs. The antibiotic was extracted by following standard
procedure with little modi cations (Rajan et al., 2014),
followed by ltration of aliquot and then centrifuging at
10,000 rpm at 4°C for 20 minutes.
Study of Antibacterial activity
Agar well diffusion method was used to investigate the
antimicrobial properties of the bacitracin obtained from
solid state fermentation. The Muller-Hinton agar (MHA)
plates were prepared by using 20ml of the medium and
left overnight at room temperature to check for any con-
tamination to the plates. The test bacteria was grown in
a nutrient broth and diluted (OD 620 nm = 0.1) to obtain
a bacterial suspension of 1×10
8
CFU/ml before apply-
ing onto the agar plate. Agar wells of 5mm diameter
were prepared using sterilized steel gel puncher and each
well received 10μL of the extracts. The agar plates were
incubated in a BOD chamber at 37
0
C for 48 h. The anti-
bacterial activity of the isolated compound against each
Acharya et al.
712 EXTRACTION OF BACITRACIN FROM
BACILLUS SUBTILIS
BSG BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
test organisms was quanti ed by measuring the zone of
inhibition. The average diameter of three replicates for
each organism was determined and expressed in mil-
limeter (mm).
STATISTICAL ANALYSIS
The CCD comprises of a full fractional factorial design
with center points ampli ed by a group of ‘star points’.
Statistical methodologies such as RSM based on CCD
can also be used to maximize the production of a sub-
stance by optimization of operational factors. In com-
pare to conventional methods, apart from the individual
contribution of process variables, the contributions of
interaction among process variables can be investigated
using RSM (Dora et al., 2013). Thus, Response Surface
Methodology (RSM) based on Central Composite Design
(CCD) was used to explore the effect of the different pro-
duction parameters viz. temperature, pH and time on the
production of bacitracin.
RESULTS ANDDISCUSSION
CHARACTERIZATION OF BACILLUS SUBTILIS
The isolated soil microorganisms obtained from different
soil samples of the Gunupur area was characterized mor-
phologically and biochemically (Table 1). The cultured
bacteria showed irregular colonies in culture media and
shape of the bacteria was rod shaped. The gram staining
test had shown gram positive. Similarly, glucose fermen-
tation test and VP tests of the isolated bacterium were
positive whereas catalase and lactose fermentation test
found to be negative. The isolated microorganism was
identi ed by following Bergay’s manual of systematic
bacteriology (Sharma et al., 2010) and found as Bacil-
lus subtilis as shown in g 1. The batch culture of the
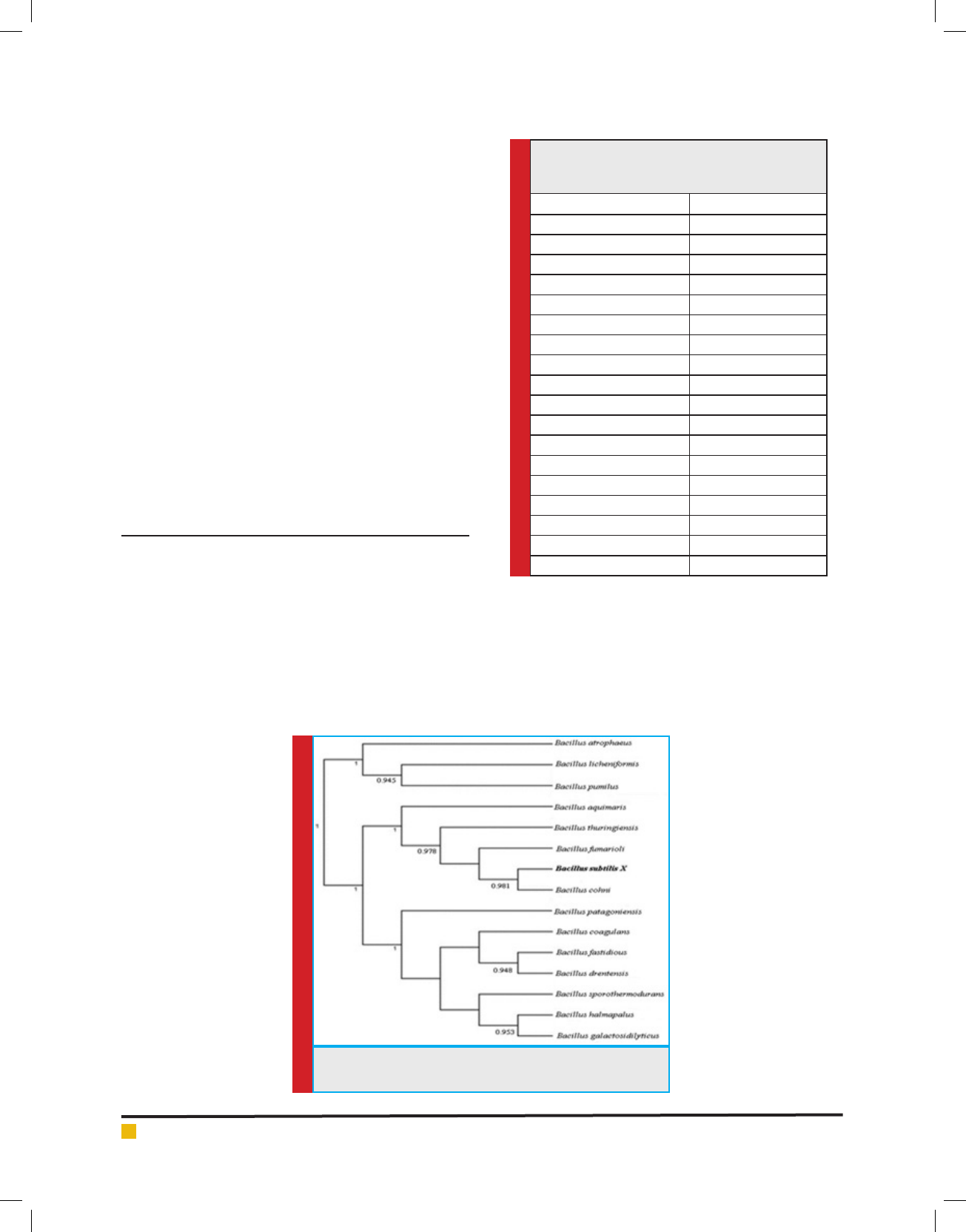
isolated bacterium retained lag phase up to 4 hrs fol-
Table 1. Morphological and biochemical
characterstics of microorganism isolated from soil
sample of Gunupur area, Odisha
Characters Observation
Shape Irregular
Margin Lobate
Color of the Colony White
Structure Filamentous
Size Rod Shaped
Gram Strian +ve
Carbohydrate fermentation -ve
Mannitol Fermentation -ve
Glucose Fermentation +ve
Catalase fermentation -ve
Nitrate reduction -ve
Methyl -ve
Indole -ve
Litmus -ve
Oxidose -ve
Urase -ve
Lactose Fermentation -ve
Voges proskauer +ve
FIGURE 1. Molecular characterization and phylogenic analy-
sis of the isolated Bacillus subtilis
Acharya et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EXTRACTION OF BACITRACIN FROM
BACILLUS SUBTILIS
BSG 713
Table 2. Effect of different carbon sources
for production of bacitraction by the isolated
microorganism
Carbon Sources Production of bacitracin
(μ/100ml of culture)
Glucose 6.0
Fructose 3.0
Sucrose 6.5
Mannitol 8.6
Sorbitol 4.8
Starch 4.5
FIGURE 2. Growth curve of the isolated Bacillus sub-
tilis in batch culture with nutrient medium
Table 3. Effect of different nitrogen sources
for production of bacitracin by the isolated
microorganism
Nitrogen Sources Production of bacitracin
(μ/100ml of culture)
Asparagine 7.0
Tyrosine 7.5
Methionine 8.0
NH4Cl 3.0
NaNO3 4.0
Urea 4.5
of Bacillus species (
Kasetty et al., 2015)
. In the study,
least production was observed when NH
4
Cl is used as the
nitrogen
source.
Individual effect of incubation time, pH and
temperature on bacitracin production
The production of bacitracin by the isolated bacte-
rium was high at log phase of growth. The initiation
of production of bacitracin was observed after 8 hours
of inoculation and maximum amount, after 30 hours of
inoculation. The activity continued up to 60 hours of
inoculation and then found to decline as depicted in
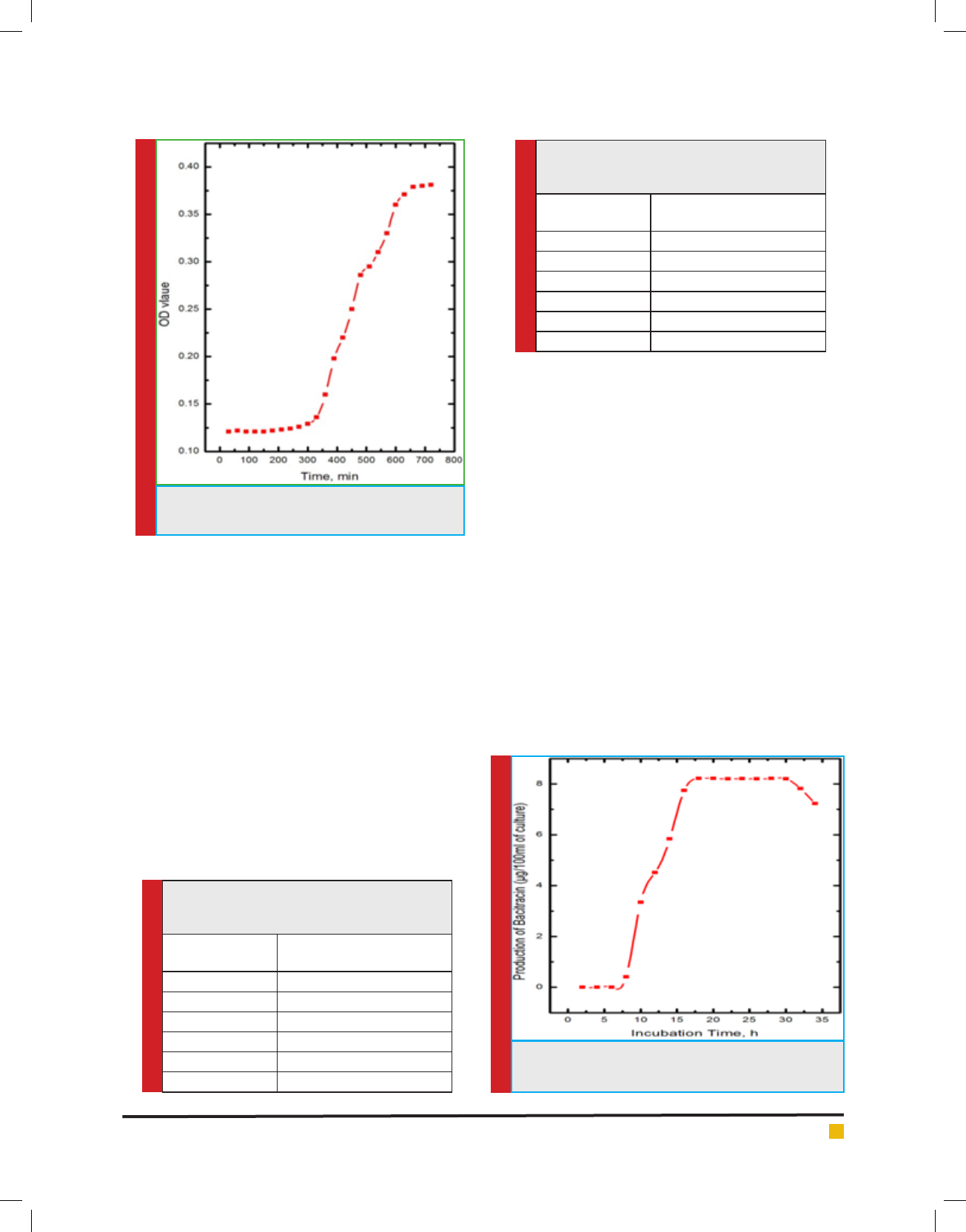
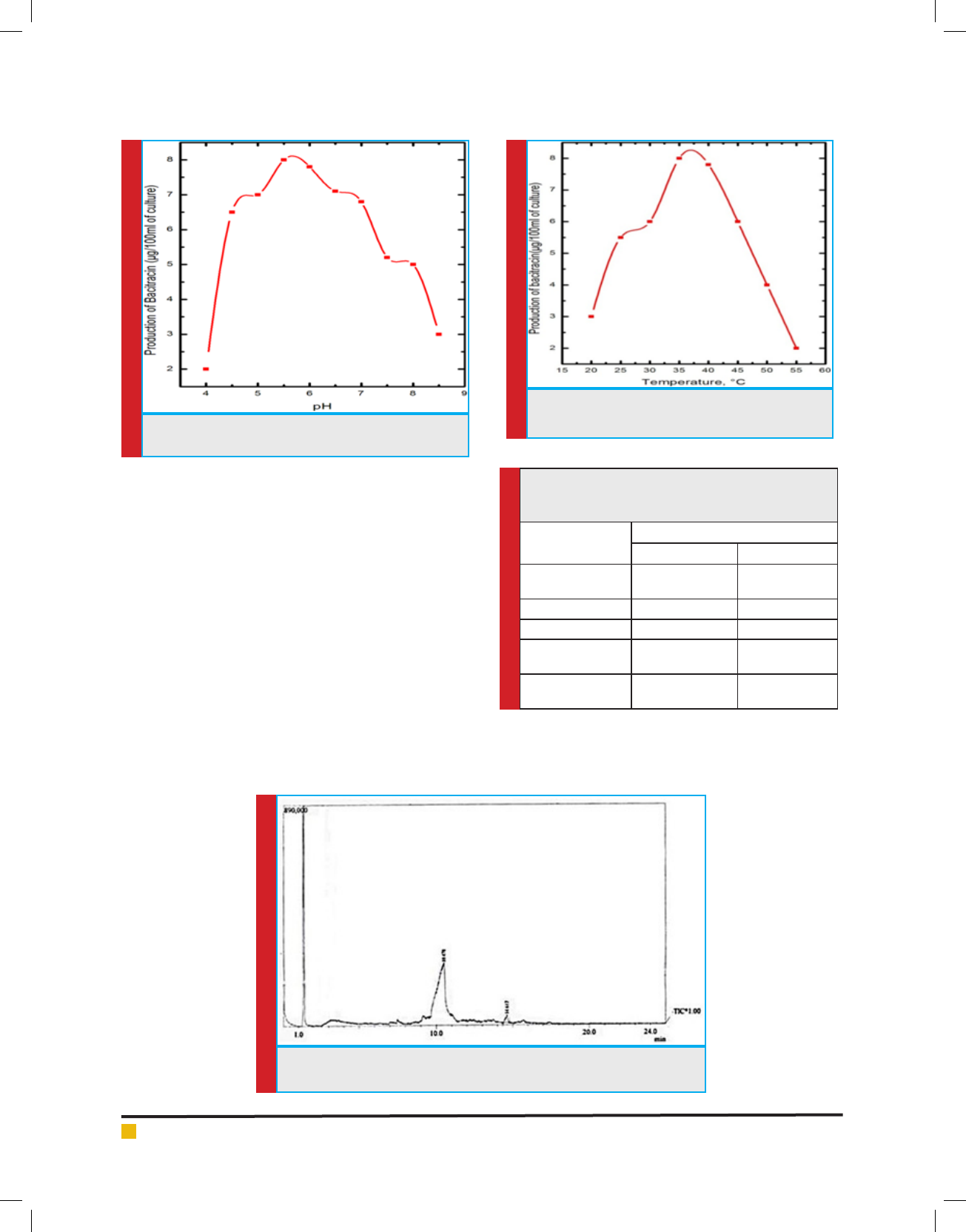
g. 3. The effect of pH on bacitracin production is
shown in g. 4. The maximum production of bacitracin
was obtained in acidic condition (pH 5.5). The environ-
mental conditions play an important role for the growth
and production of metabolites in Bacteria (Kang et al.,
2017). There was no production of antibiotic at 20° C by
the isolated microorganism. The maximum production
of bacitracin was obtained at 35°C. Beyond 350 C, the
lowed by log phase (Fig. 2). The morphological features
of isolated microorganisms presenting the gram + ve,
rod shaped, and having endospore con rms the presence
of Bacillus subtilis (Das et al., 2012).
Effect of carbon and nitrogen sources on the production
of bacitracin
The effect of carbon sources on production of bacitra-
cin is represented in table 2. The extreme production of
bacitracin was obtained when maltose is used as the car-
bon source. Further, minimum production was obtained
when fructose is used as the carbon source. To standard-
ize the effect of nitrogen sources on bacitracin produc-
tion, it was observed that methionine found to be the
best nitrogen source among the studied nitrogen com-
pounds
(table 3). Earlier report shows that methionine
can be used as suitable source of nitrogen for growth
FIGURE 3. Production of bacitracin by isolated Bacillus
subtilis in batch culture with respect to incubation time
Acharya et al.
714 EXTRACTION OF BACITRACIN FROM
BACILLUS SUBTILIS
BSG BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 5. Effect of temperature on production of
bacitracin by isolated Bacillus subtilis
FIGURE 6. Chromatogram of GC-MS analysis of Bacitracin by the isolated
Bacillus subtilis
production of antibiotic decreases as observed in g. 5.
Because Bacillus subtilis is a mesophillic bacterium, the
growth shall be optimum at 250C - 350C. Therefore, the
production of bacitracin is optimum at this temperature
(Deslouches, et al., 2015).
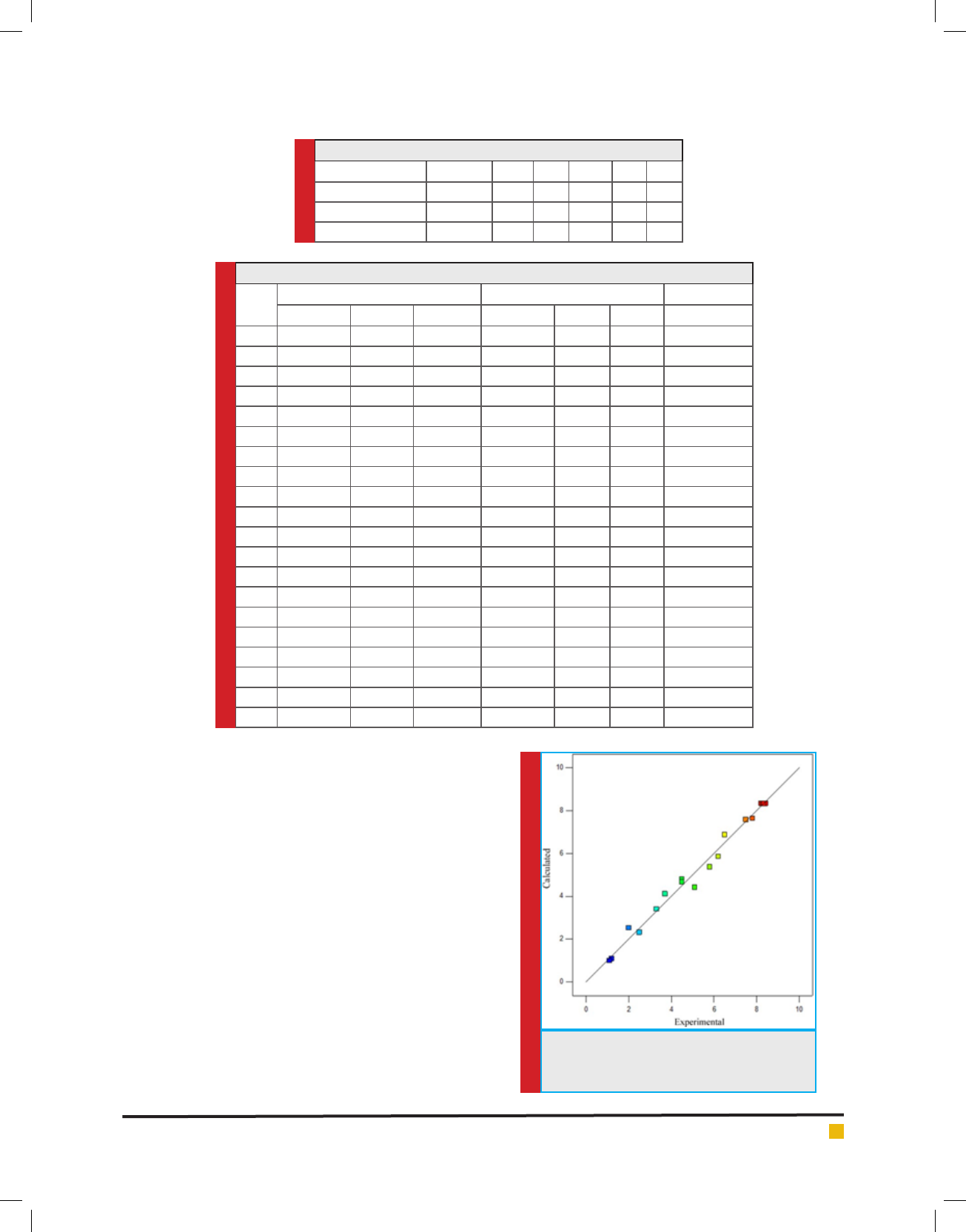
The chromatogram of GC-MS of extract is represented
as g. 6. It is observed that, there are two major peaks
having the retention time of 10.478 and 14.617 and the
area occupied are 69.27% and 2.51% respectively. The
molecular weight of the bacitracin was measured as 1.6
k Da. The l yophilized antibiotic is faint yellow in color,
amorphous and water soluble. The zone of inhibition
of isolated bacitracin against ve pathogens namely
Staphylococcus aureus, Escherichia coli, Micrococ-
cus luteus, Salmonella typhimurium and Pseudomonas
aeruginosa is represented in the table 4. The maximum
FIGURE 4. Effect of pH on production of bacitracin by
isolated Bacillus subtilis
Table 4. Zone of Inhibition (in mm) against ve
pathogens by the bacitracin extracted from the
isolated microorganism.
Test Organism
Zone of Inhibition (in mm)
After 24 hours After 48 hours
Staphylococcus
aureus
16.6
Escherichia coli 12.4
Micrococcus luteus 14.5
Salmonella
typhimureum
8.3 12.0
Pseudomonas
aureginosa
11.4
zone of inhibition was observed against Staphylococcus
aureus (23 mm) and the minimum zone of inhibition
was observed against Salmonella typhimurium (12 mm).
Acharya et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EXTRACTION OF BACITRACIN FROM
BACILLUS SUBTILIS
BSG 715
Table 5. Level of independent variables.
Variables Symbol - -1 0 +1 +
Incubation Time, h A 2 8 18 28 34
pH B 4 5 6.5 7.5 8.5
Temperature, °C C 20 28 37.5 48 55
Table 6. Experimental design matrix and response
Run
Coded variables Actual variables Response
ABC ABC (Y)
1 -1 -1 -1 8 5 28 1.2
2 +1 -1 -1 28 5 28 7.5
3 -1 +1 -1 8 7.5 28 2
4 +1 +1 -1 28 7.5 28 6.5
5 -1 -1 +1 8 5 48 2.5
6 +1 -1 +1 28 5 48 6.2
7 -1 +1 +1 8 7.5 48 3.3
8 +1 +1 +1 28 7.5 48 4.5
9- 0 0 2 6.5 37.5 1.1
10 + 0 0 34 6.5 37.5 7.8
11 0 - 0 18 4 37.5 3.7
12 0 + 0 18 8.5 37.5 5.1
13 0 0 - 18 6.5 20 5.8
14 0 0 + 18 6.5 55 4.5
15 0 0 0 18 6.5 37.5 8.22
16 0 0 0 18 6.5 37.5 8.36
17 0 0 0 18 6.5 37.5 8.3
18 0 0 0 18 6.5 37.5 8.42
19 0 0 0 18 6.5 37.5 8.33
20 0 0 0 18 6.5 37.5 8.34
Statistical analysis
From the analysis of experimental data, it was found
that the production of bacitracin is in uenced by three
process parameters viz. incubation time, pH and tem-
perature. Thus the RSM based on CCD is employed in
order to estimate the combined effect of the operating
parameters on the bacitracin production. The range of
the process parameters and the complete design of the
matrix along with the experimental data are presented as
tables 5 and 6 respectively. Table 6 is planned to obtain
a quadratic correlation for the production of bacitracin
consisting 2
3
numbers of axial runs, 6 numbers of star
con gurations and 5 numbers of center points. The runs
15-19 around the center points in table 6 are repeated
in order to determine experimental errors. The correla-
tion obtained for the production of bacitracin in terms
of coded factor is presented as eq. 1.
Y
=
8
.
33
+
1
.
97
A
+
0
.
092
B
−
0
.
211
C
−
0
.
53
AB
−
0
.
73
AC
−
0
.
087
BC
−
1
.
42
A
2
−
1
.
43
B
2
−
1
.
172
C
2
(
1
)
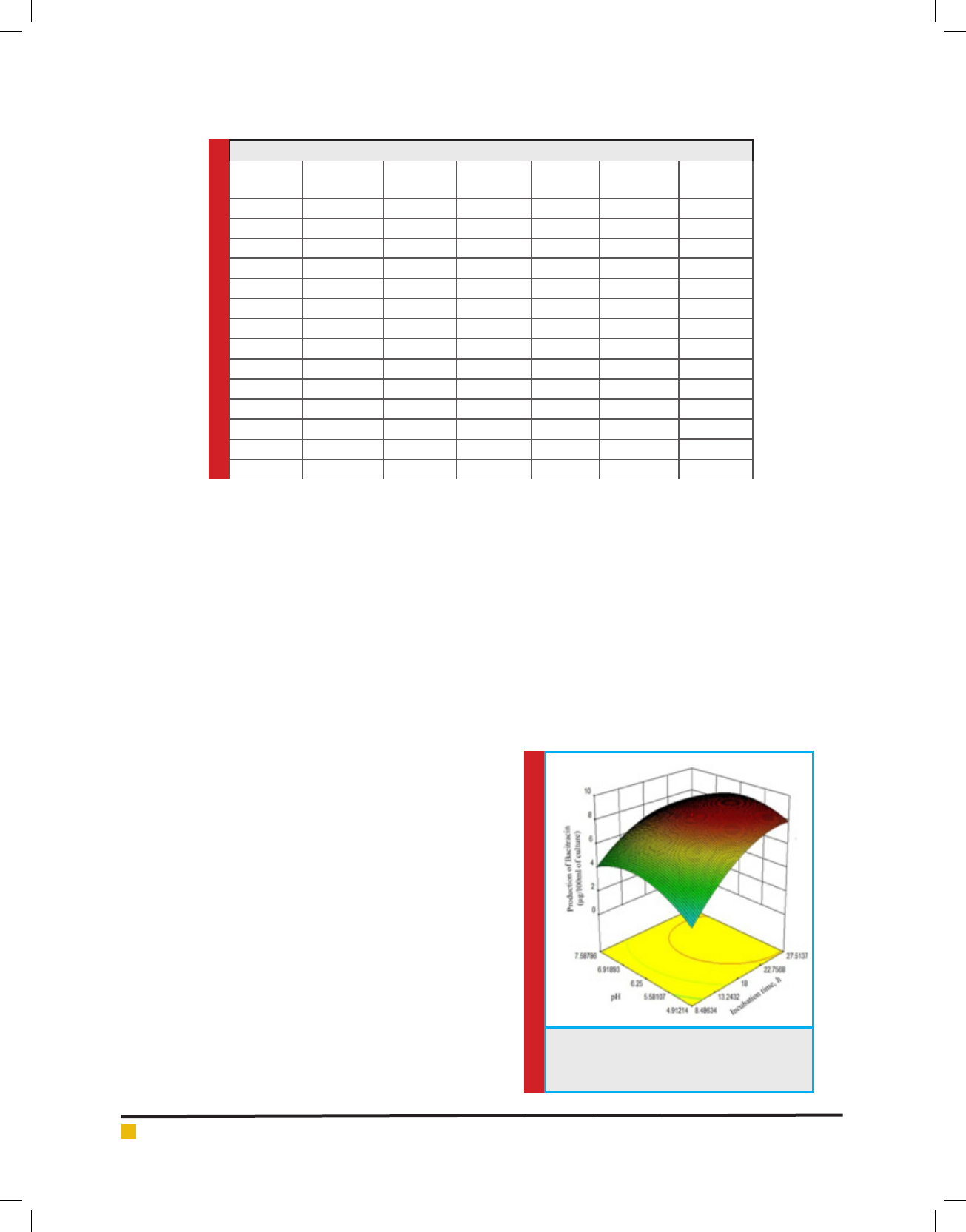
FIGURE 7. Comparison of calculated values
of bacitracin production from eq. 1 with the
experimental ones
Acharya et al.
716 EXTRACTION OF BACITRACIN FROM
BACILLUS SUBTILIS
BSG BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
The positive and negative signs against each factor in
eq. 1 show the synergistic and antagonistic effect of
those respective terms on bacitracin production. The cal-
culated values of bacitracin production from eq. (1) are
compared with the experimental ones and represented
as g. 7. The values of coef cient of correlation (R
2
),
adjusted R
2
(R
adj
2
), predicted R
2
(R
pred
2
) are found to be
0.986, 0.973 and 0.901 respectively, which shows that
the calculated values are analogous with the experimen-
tal ones. The adequate precision, which measures the
signal to noise ratio, is found to be 24.21, indicates that
the correlation developed can be used to navigate the
design space. The Analysis of variance (ANOVA) for the
production of bacitracin is represented as table 7. The
F-value” of 75.47 indicates that the model is signi cant
and there is only 0.01% chance of failure of this corre-
lation. The signi cance of the model terms can be esti-
mated through the “P-value”. The terms A, B, C, AB, AC,
A
2
, B
2
and C
2
are signi cant, because the “P-value” for
these terms are less than 0.05.
Combined effect of process parameters on the
bacitracin production
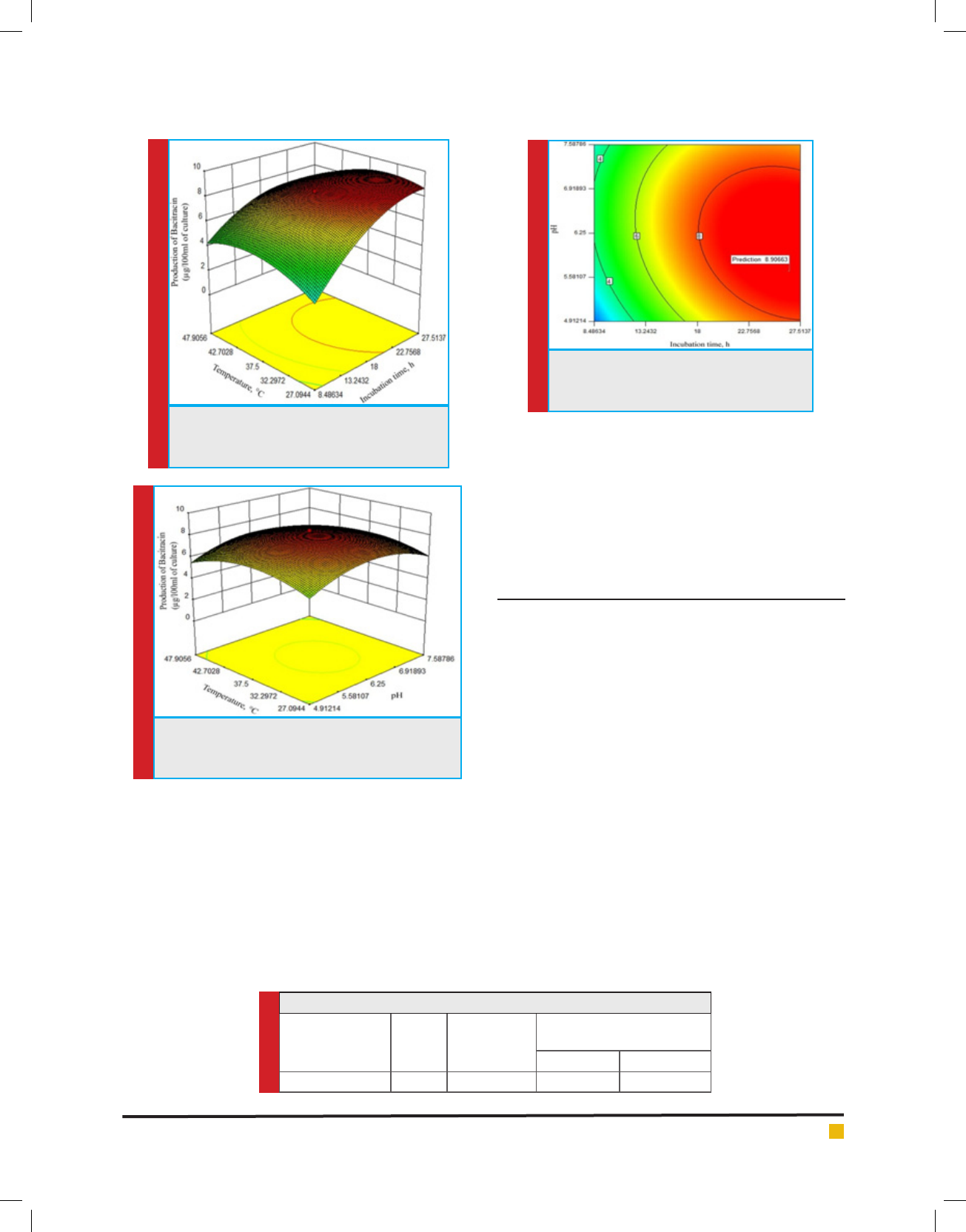
Fig. 8 shows the combined effect of pH and incubation
time on the bacitracin production at a temperature of
37.5°C. Bacitracin production increases with the increase
in pH up to 6.5, beyond which it decreases, which may
be due to the effect on the rate of nutrient transport
and consumption from the medium to bacterial body.
Again, the bacitracin production increases exponentially
with the increase in incubation time up to 22.75 h, then
start decreasing. This may be due to decrease in essential
nutrients in the medium. A maximum bacitracin pro-
duction of 8.3 μg/100ml is found as depicted in g.7.
Similarly, the combined effect of temperature and incu-
bation time on the bacitracin production at pH of 6.25
is presented as g. 9. It was observed that the bacitracin
production increases up to a temperature of 38°C and
after that the production decreases. The increase may be
due to proper functions of enzymes involved in the bac-
itracin synthesis pathway of microorganisms. Beyond
38°C what happens so that it decreases, it may be due to
the inhibitory effect of enzymes involved in bacitracin
synthesis pathway also write down. Fig. 10 shows the
combined effect of temperature and pH on the bacitracin
Table 7. ANOVA for bacitracin production
Source
Sum of
Squares
Degree of
freedom
Mean
Square
F Value p-value
Prob > F
Remarks
Model 118.5209 9 13.16898 75.4718 2.13265E-07 significant
A 53.25338 1 53.25338 305.1967 2.98275E-08 significant
B 0.115238 1 0.11523 0.6604 0.037374471 significant
C 0.610016 1 0.61001 3.4960 0.034333927 significant
AB 2.31125 1 2.31125 13.2458 0.005405229 significant
AC 4.35125 1 4.35125 24.9371 0.000745341 significant
BC 0.06125 1 0.06125 0.3510 0.568118214
A
2
27.5197 1 27.51969 157.7162 5.22146E-07 significant
B
2
1 28.20920 161.6678 4.69653E-07 significant
C
2
1 18.76232 107.5274 2.64328E-06 significant
Residual 1.570399
0.17448
Lack of t 1.548479
0.30969 56.5138 0.000840525
Pure Error 0.02192 4
Cor Total 120.0913 18
FIGURE 8. Combined effect of pH and incu-
bation time on the production of bacitracin at
a temperature of 37.5°C
Acharya et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EXTRACTION OF BACITRACIN FROM
BACILLUS SUBTILIS
BSG 717
FIGURE 9. Combined effect of temperature and
incubation time on the bacitracin production at
a pH of 6.25
FIGURE 10. Combined effect of temperature and pH
on the bacitracin production at 18h of incubation
time.
FIGURE 11. Optimization region of pH and
incubation time for maximum production of
bacitracin.
Table 8. Optimized conditions for bacitracin production
Incubation time,
h (A)
pH (B) Temperature,
°C (C)
Production of bacitracin
(μg/100ml of culture) (Y)
observed predicted
26.5 5.67 30.65 8.72 8.90663
production is 18h of incubation time. A maximum baci-
tracin of production of 7.2 μg/100mlis found ( g. 11).
Optimization of the production of bacitracin
The focal objective of this study is to optimize the pro-
cess parameters for the production of bacitracin using
the developed correlation (eq 1). The quadratic corre-
lations are optimized using quadratic programing to
maximize the production of bacitracin (Bodroth et al.,
2012). The optimum region of pH and time for the maxi-
mum production of bacitracin is presented as g. 11.The
optimized conditions are found to be incubation time
of 26.5 h; pH of 5.67 and temperature of 30.65°C for a
maximum production of bacitracin of 8.9 μg/100ml as
shown in table 8.
CONCLUSION
The Bacillus subtilis BSG isolated from Gunupur soil is a
gram +ve, rod shaped bacteria has the capabilities to pro-
duce bacitracin, a peptide antibiotic. The bacitracin pro-
duction was optimized in different parameter and found
the suitable production point. The bacitracin production
will be affected by imbalanced carbon and nitrogen
sources. The present work will give a milestone for the
production of antibiotics in pilot plant scale. Bacitracin
also has potential antimicrobial properties with maxi-
mum inhibition for pathogenic bacteria. Simultaneously,
RSM based CCD was used to estimate the individual,
combined and the square effect of all incubation time,
pH and temperature on the bacitracin production. Using
ANOVA results a quadratic correlation was developed
with a coef cient of correlation of 0.986. Again, the
process parameters are optimized so as to maximize the
bacitracin production. The optimized conditions is found
of incubation time of 26.5h; pH of 5.67 and temperature
of 30.65°C for a maximum production of bacitracin of
8.9 μg/100ml. Therefore, the correlation developed and

Acharya et al.
718 EXTRACTION OF BACITRACIN FROM
BACILLUS SUBTILIS
BSG BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
the data reported may provide convenient information
for the economic production of bacitracin within ranges
of the operating parameters investigated.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the management of
GIET for encouragement and providing laboratory facil-
ity to carry out the investigation.
REFERENCES
Arias, R.S., Sagardoy, M.A. and Vanvuurde, J.W.L. (1999). Spa-
tio-temporal distribution of naturally occurring Bacillus spp.
and other bacteria on the phylloplane of soybean under eld
conditions. J Basic Microbiol. 39, 283–292.
Bodroth, R.P. and Das, M. (2012). Phytochemical Screening
and antimicrobial activity of ethanol and chloroform extract
of Zizyphus nummularis Wt. & Arm. African J Biotechn. 11,
4929-4933.
Coppuccino, J.G. and Sherman. N, (1996). A Laboratory Man-
ual in General Microbiology, fourth ed. Benjamin Commius
Publication Company, California.
Das, M. and Kumar, S. (2012). Characterization of lactamase
producing bacterium from soil of Mahendragiri region, Orissa.
Asian Jr. Microbiol. Biotech. Env. Sc. 14 (2), 229- 232.
Deslouches, B., Steckbeck, J. D., Craigo, J. K., Doi, Y., Burns,
J. L., and Montelaro, R. C. (2015). Engineered cationic antimi-
crobial peptides to overcome multidrug resistance by ESKAPE
pathogens. Antimicrob. Agents Chemother. 59, 1329–1333.
doi: 10.1128/AAC.03937-14.
Dora, D.T.K., Mohanty, Y.K. and Roy, G.K. (2013). Hydrody-
namics of three-phase uidization of a homogeneous ternary
mixture of regular particles - Experimental and statistical
analysis. Pow Techn. 237, 594 – 601.
Goodfellow, M. and Board, R.G. (1980). Microbiological Clas-
si cation and Identi cation, Aca Press New York 152
Kang, H. K., Kim, C., Seo, C. H., and Park, Y. (2017). The thera-
peutic applications of antimicrobial peptides (AMPs): a patent
review. J. Microbiol. 55, 1–12. doi: 10.1007/s12275-0176452-1
Kasetty, G., Kalle, M., Morgelin, M., Brune, J. C., and
Schmidtchen, A. (2015). Anti-endotoxic and antibacterial
effects of a dermal substitute coated with host defense peptides.
Biomat. 53, 415–425. doi: 10.1016/j.biomaterials.2015.02.111
Lagzian, M., Shahraki, A., Besharatian, M. and Asoodeh, A.
(2018). A thermostable alkaliphilic protein-disul de isomerase
from Bacillus subtilis DR8806: cloning, expression, biochemi-
cal characterization and molecular dynamics simulation. Inter-
nat J Biomacromol. 107, 703-712.
Murphy, T., Roy, I., Harrop, A., Dixon K. and Keshavarz, T.
(2007). Effect of oligosaccharide elicitors on bacitracin a pro-
duction and evidence of transcriptional level control. J bio-
techn 131, 397–403.
Pavithra, P.S., Sreevidya, N. and Verma, R.S. (2009). Antibacte-
rial activity and chemical composition of essential oil of Pam-
burusmissionis. J. Ethnopharmaco. 124, 151-153.
Phillips, I. (1999). The use of bacitracin as a growth promoter
in animals produces no risk to human health. J Antimicrob
Chemother. 44(6),.725−728.
Rajan, B.M. and Kannabiran, K. (2014). Extraction and iden-
ti cation of antibacterial secondary metabolites from marine
Streptomyces sp. VITBRK2. Int J Mol Cell Med. 3,130–137.
Sharma, D. and Das, M. (2010). Ef cacy of antimicrobial
metabolites of Pseudomonas uroscenes PFG against phy-
topathogenic fungi. Pro Nat Aca. 1, 68-71.
Trookman, N.S., Rizer, R.L. and Weber, T. (2011). Treatment of
minor wounds from dermatologic procedures: A comparison
of three topical wound care ointments using a laser wound
model. J. Am. Acad. Dermatol. 64, 08–15.