
Studies on NOx removal using
Dunaliella salina
algae
in photobioreactors
Kethineni Chandrika
1
, S. F. Choragudi
2
, Krishna Kireeti Kakarla
3
, Kolluru Sumanth
4
and
Ch Devika
5
1
Associate Professor, Department of Biotechnology, Koneru Lakshmaiah Education Foundation,
Vaddeswaram, Guntur, AP, India–522502
2,3,4,5
Department of Biotechnology, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Guntur, AP,
India–522502
ABSTRACT
The capability of an algal species to remove NO2 and NO in the simulated ue gas was established using Dunaliella
salina in Photobioreactors under two variants of NOx sources. The concentrations studies were in the range between
25ppm to 150ppm. The diffusion of NOx and subsequent reaction with water resulted in NO3- and NO2- in the growth
medium. Algal growth by absorption of NO3- and NO2- created a nitrate gradient in the bulk medium resulting in
NOx uptake rates from the gas phase of up to 96%, leaving the unconsumed nitrogen of up to 7 mg-N/L in the growth
medium. Algal species having an initial cell density of 2.8x105 cells/mL grew to the cell density of 1.73x107 cells/mL
and dry weight of 262 mg/L. The Nitrogen content of cells varied from 3-6%. The treatment of NOx in Photobioreactors
was investigated with reference to the gas removal ef ciency, cell growth and total nitrogen content in the biomass
KEY WORDS:
DUNALIELLA SALINA
, PHOTOBIOREACTORS, ALGAL GROWTH
674
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(4): 674-680 (2018)
INTRODUCTION
Disproportionate usage of fossil fuels has been consid-
ered as the source for manmade toxic emissions com-
prising carbon dioxide, sulfur dioxide, nitrogen oxides,
volatile organic compounds and heavy metals (Mulhol-
land, 2008; Attilo et al., 2009). The by-products of fossil
fuels have been identi ed as one of the major anthro-
pogenic sources of this gas, contributing to global warm-
ing by the greenhouse effect. Therefore, it has become
obligatory to reduce these toxic emissions before they
are disposed into the environment. Nitric oxide (NO) and
ARTICLE INFORMATION:
Corresponding Authors: kkchandrika@kluniversity.in
Received 19
th
Oct, 2018
Accepted after revision 24
th
Dec, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
Mono of Clarivate Analytics and Crossref Indexed
Journal Mono of CR
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at:
http//www.bbrc.in/
DOI: 10.21786/bbrc/11.4/18
Kethineni Chandrika et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS NOX REMOVAL USING DUNALIELLA SALINA ALGAE IN PHOTOBIOREACTORS 675
Nitrogen dioxide (NO2) are the two main components
that make up NOx. These components are toxic and have
various environmental hazards as per Environmental
Protection Agency (Ronda, 2014). The nitrogen removal
is 94.9 % in the ammonium form nitrogen group using
bio lm (Yuxuan Zhu 2018).
There are several methods for treatment of NOx.
Selective catalytic reduction (SCR) is used, however it
is more expensive when applied for large-scale power
plants (Miller et al., 2010). Another way to treat NOx
from stationary sources is to use scrubbers to trans-
fer the risk into an aqueous solution, which still must
be treated or disposed of (Raja et al., 2007). Hence, to
develop an economical and practical process to handle
NOx exists. Cultivation of an algae to take up dissolved
NOx from a scrubber as a nitrogen source, and oxygen
only released as a by-product. This concept has been
worked to estimate that, algal strains and conditions,
algae can take up nitrogen from dissolved NOx (Nagase
et al., 1997). Toxic compounds present in the ue gas
inhibit the growth rate. NO2 has high solubility in water
and therefore, reacts with water to form aqueous nitrates
(NO3) and nitrites (NO2), some of the unaccounted-for
nitrogen was lost due to volatization of gaseous nitro-
gen species, (Kaitlyan 2018).
Aqueous nitrate and nitrites are used by the algae as
a source of nitrogen for cell synthesis, (Mulholland &
Lomas, 2008). The dissolved NO2 and NO react to form
dissolved nitrogen compounds which are available to
ingest, biological conditions for the uptake of nitrate
or nitrite by the algae, (Lee & Schwartz 1981). The
nitrate or nitrite uptake by the algae reduce the soluble
NO2, thus increases the concentration gradient of NO2
between liquid and the air. Thus, apparent solubility
of NO2 is proportional to the NO2 gradient in the bulk
medium, (Skalska, et al 2010). Nitrogen mono-oxide and
sulfur dioxide can be removed by simultaneous absorp-
tion into aqueous mixed solutions of sul te and [Fe II
(edta)]H2O)]2−, ferrous ion coordinated to an anion of
ethylene-diaminetetraacetic acid, (Tomasz et al 2016). A
sequential process for the recovery and puri cation of
multiple products was used on a mixture of algal bio-
mass comprised of Spirulina platensis and Dunaliella
salina (Kethineni 2017).
Dunaliella salina is a green algae known to with
stand high salinity and accumulate carotenes. The
nitrate requirement for algae is more for accumulation
of biomass than for accumulation of carotene. Nitrates
in the range of 1- 10mM is suitable for algal growth
(Tafreshi et al., 2009). Harter et al, (2012) performed a
mass balance for nitrogen from NOx for Dunaliella cul-
tures in a column reactor. In a lab scale trail under simu-
lated ue gas the results indicate that with an in ow of
150 μg N/L day NOX along with CO2 the alga could able
to maintain a net in ux of 0.52.73 μg N L-1 d-1 which
amounts to 35% NOx removal. Nagase et al, (1997) stud-
ied the removal rate of nitric oxide by Dunaliella tertio-
lacta supplied in the range between 25-500ppm. At an
inlet concentration of 500ppm NO in addition to CO2, it
was shown to remove 110 μmoles per hour at a ow rate
of 150mL/min. Also, it was shown that within a range of
100 to 400mL/min gas ow rate, a maximum of 60% of
the NO was removed. These results suggest that D. salina
is a potential algal species for NOx removal.
the ability
of thegreen algae, Chlorellato acclimate to high level
of NOx and the potential usage of Chlorella strains in
biological NOx removal (DeNOx) from industrial ue
gases,
(Tianpei and Gang Xu 2016).
To understand the
NOX removal process and to increase its range of appli-
cability. The use of microalgae for simultaneous removal
of CO
2
, SO
x
and NO
x
from ue gas is an environmen-
tally benign process, (Hong‐Wei Yen et al, 2016), 75%
decrease of the nitrogen concentration in the medium,
with respect to the optimal values for growth, increased
the lipid fractions of algal species, ( Attilio Converti et
al 2009).
It is very important to undertake biological NO
X
xa-
tion. Therefore, in this work, two individual experiments
were conducted to productively remove the NOx from
simulated ue gas with varying NOx loading rates by
estimating the optimal growth parameters. Different
NOx concentrations were supplied to each photobiore-
actor inoculated with Dunaliella salina. NOx removal
ef ciency and algal growth were determined in each
experiment.
MATERIAL AND METHODS
DUNALIELLA CULTURE
All three reactors were inoculated with 600 mL of pure
Dunaliella salina (SAG:42.88) grown in Dunaliella
medium (=Dun) at 250C and a pH of 7.0. The inoculum
was grown to a 1 x107 cells/mL, with an initial cell den-
sity of 2.8x105 cells/mL
GROWTH MEDIUM
The modi ed Dunaliella growth medium was used for
inoculum and algae growth experimentation. All the
nitrogen uptake by the algal cells was provided through
inlet simulated gas. Dunaliella salina was grown in
modi ed Dunaliella medium. A nitrogen free stock solu-
tion was prepared with K2HPO4, 0.1 g/ 100 mL. 20 mL
of this nutrient was mixed with 30 mL of the soil extract
and 930 mL arti cial seawater to make a liter solution.
The growth medium was given in Table 1.
Kethineni Chandrika et al.
676 NOX REMOVAL USING DUNALIELLA SALINA ALGAE IN PHOTOBIOREACTORS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Operating conditions of the reactor
Case1: pure NO2 feed source:
NO2 gas diluted with ambient air was used as the simu-
lated ue gas for the rst run. As the boiling point of
NO
2
is approximately 20
o
C at atmospheric pressure; NO
2
was initially released as a liquid in the tubing. NO2 was
blended with 3Lpm of air to get NOx concentrations of
100ppm, 200ppm,350 ppm in photobioreactors 1a, 1b,
and 1c respectively. Experimental conditions were given
in Table 2.
ing 5000 ppm and 9000 ppm NO2 concentration. Thus,
in this case, the need for the liquid NO2 trap was ignored
as the gas mixture was already in a vapor state. Thus,
pumping calibration gas achieved steady inlet concen-
trations. Experimental conditions were given in table3.
The calibrated NO2 is blended using air for the required
NOX concentrations. The calibrated gases were supplied
to the reactor at 3 Lpm, having NO2 concentrations of
25 ppm, 50 ppm, 120 ppm respectively. The concentra-
tion range was chosen to resemble real-time power plant
NOx concentrations. Two aquarium stones of 12cm were
used to diffuse the gas into the reactor. CO2 was sup-
plied in the system, at a concentration of 400ppm until
the pH remains 7 However when the pH is below 7, the
pH 7 was maintained using 1.0 g/L solution of sodium
bicarbonate (NaHCO3). The system was run for six days
after loading of NOx. In uent and ef uent concentra-
tions of NO and NO2 in gas samples were measured
using an analyser, (Testo 350-S/-XL, USA).
RESULTS AND DISCUSSION
NOX REMOVAL EFFICIENCY
Case 1: pure NO2 feed source
In this case, the reactors were fed with pure NO2 which
is delivered as a liquid at room temperature and then the
collected vapors were diluted with air. Using pure NO2
gas, actual average inlet concentrations for reactors 1a,
1b, and 1c were 108 ppm, 35ppm, 70 ppm respectively
(Table 4). Fig. 1 shows the Dunaliella growth curve in
reactors 1a, 1b, and 1c during 90-hour run.
Table1. Modi ed Dunaliella growth medium
Component
Stock sol.
(g/100mL)
Nutrient concentrate
ion (mL)
KOH 0.1 20
Soil extract 30 -
Arti cial seawater 930 -
Table 2. Experimental conditions for case 1
Setting Reactor 1a Reactor 1b Reactor 1c
Inlet NO
x
(ppm) 100 200 350
Inlet Gas Flow
Rate (Lpm)
333
pH 7-8 7-8 7-8
Temperature (
0
C) 20 20 20
The simulated gas entered each photobioreactor
through a sparger, pH was maintained in the range
of 7.0 to 8.0 using CO2. The carbon dioxide feed was
monitored by separate valves to each reactor which was
controlled based on the pH in the reactor. NOx removal
rates were monitored for four days. The reactors were
illuminated with three 1 m long uorescent white lights
emitting, a total of 2700 Klux.
Case 2: Gas feed source calibration:
For the case 2, the reactors were inoculated before to
the start of NOx loading and left for two days during
which, only ambient air was supplied to the system. NOx
was given from NO2 calibration gas cylinders compris-
Table 3. Experimental conditions for case 2
Setting
Reactor
2a
Reactor
2b
Reactor
2c
Inlet NO
x
(ppm) 25 50 120
Inlet Gas Flow Rate (Lpm) 3 3 3
In uent CO
2
(g) (ppm) 400 400 400
pH 7-8 7-8 7-8
Temperature (
0
C) 20 20 20
Table 4. In uent and ef uent NO
x
concentrations in reactors
Reactor
Ratio
(NO:NO2)
Inlet NOx (g)
Conc (ppm)
Outlet NO
x
(g)
Conc (ppm)
Average removal
Conc (%)
1a 0.47 108±55 47±33 49
1b 0.15 35±16 15±12 51
1c 0.35 70±32 8±7 81
2a 0.038 27±6 11±4 59
2b 0.035 57±9 2±8 96
2c 0.043 126±12 7±11 95
Kethineni Chandrika et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS NOX REMOVAL USING DUNALIELLA SALINA ALGAE IN PHOTOBIOREACTORS 677
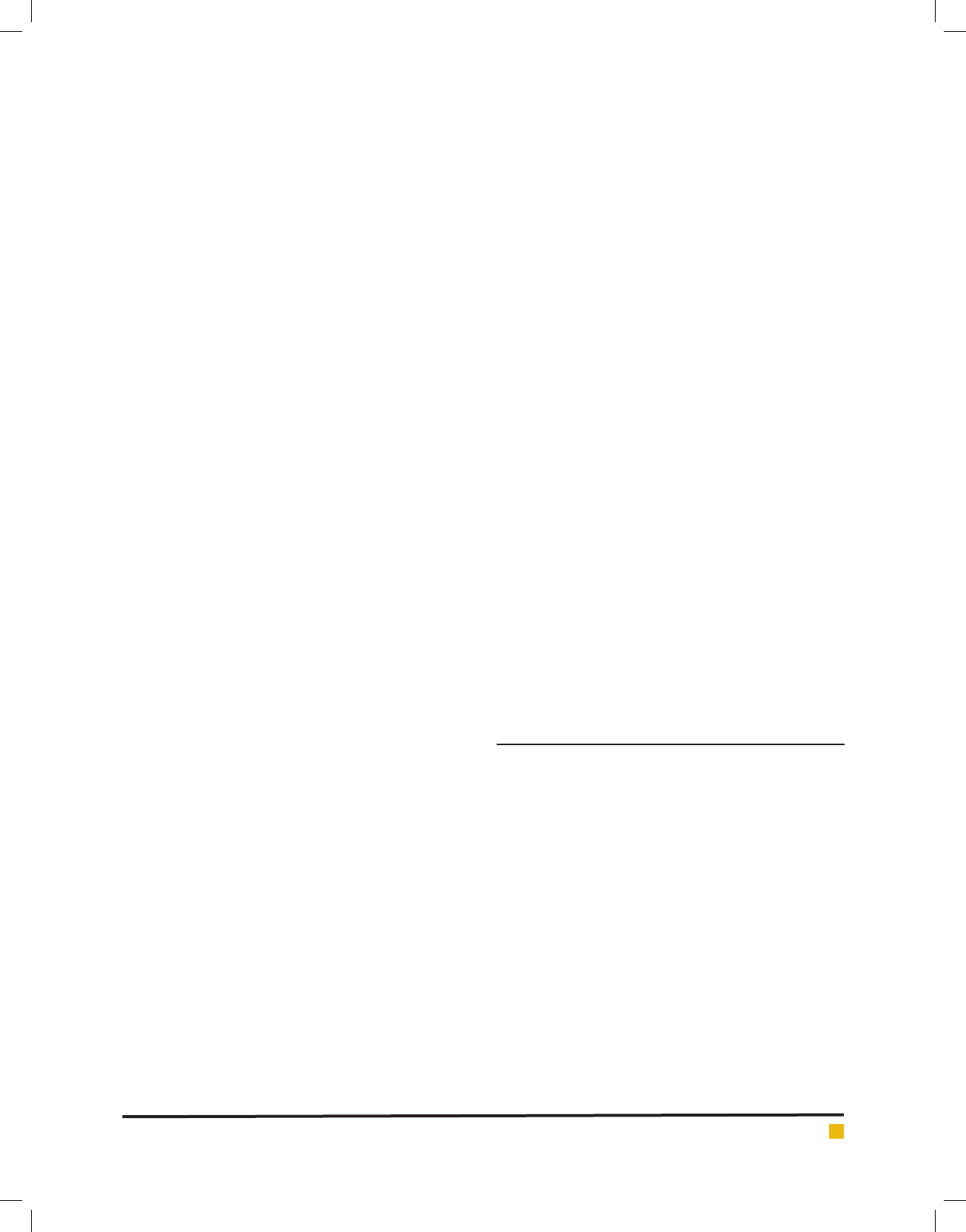
FIGURE 1. Growth curves in Photobioreactors for case 1
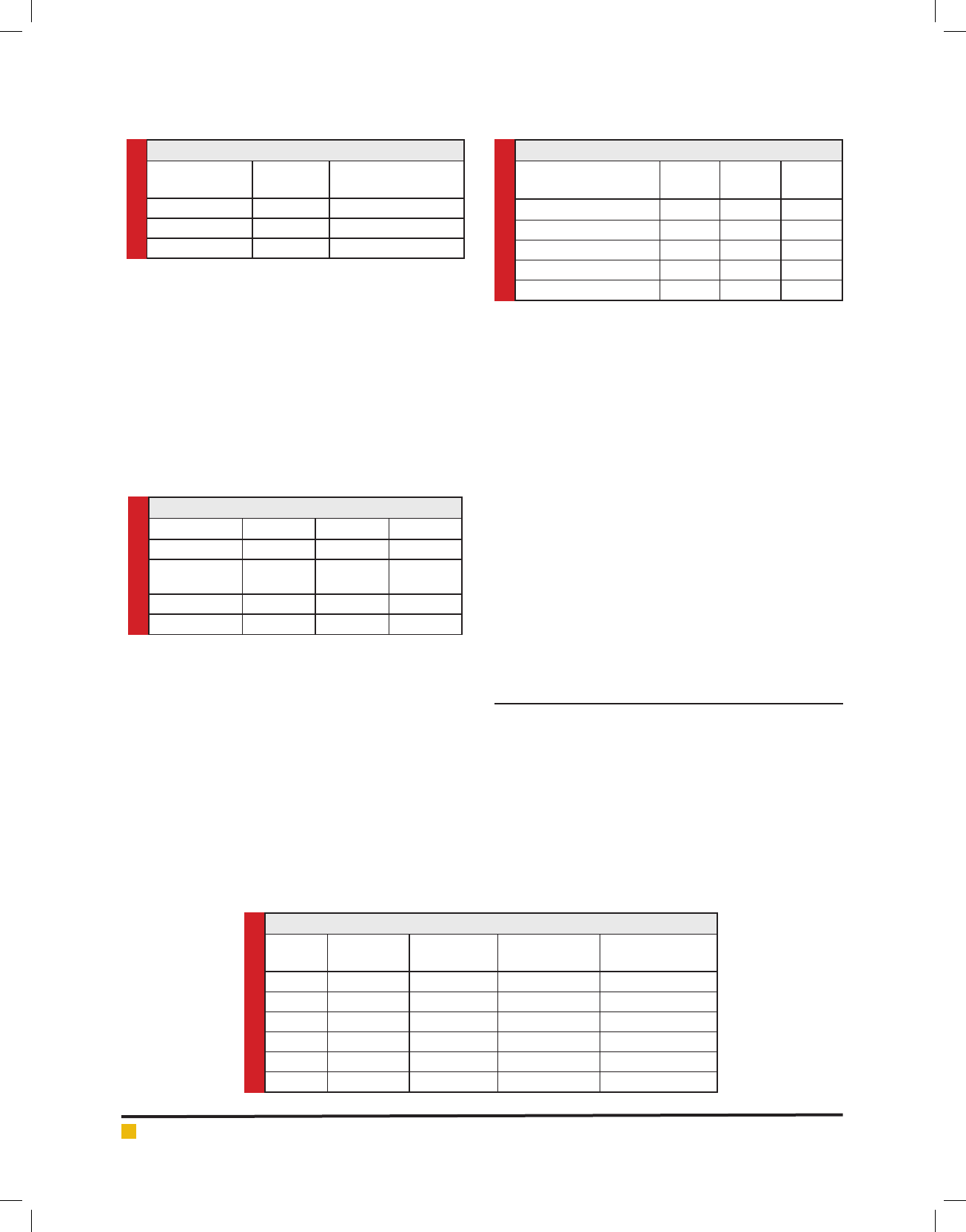
FIGURE 2. Growth curves in Photobioreactors for case 2
All three reactors began with 2.8x10
5
cells/ml. As
shown in Fig. 1, the culture in reactor 1a began exhaus-
tive during the rst 24 hours, therefore, the cells never
attained a density greater than the initial. The maximum
cell densities, for 1b, 1c were 1.46x106 cells/mL and
1.48x106 cells/mL respectively. Table 3 presents the NOx
removal data for the reactors under case 1. Inlet and out-
let NOx in Table 3 is the summation of measured NO and
NO2 concentrations in the inlet and outlet streams. The
ef ciency of NOx removal by the algal system is based
on total nitrogen through the reactor system and not
on any particular NOx component. Therefore, removal
percent of total NOx is only considered in the analysis.
Reactor 1a obtained an average NOx removal of 49%,
Kethineni Chandrika et al.
678 NOX REMOVAL USING DUNALIELLA SALINA ALGAE IN PHOTOBIOREACTORS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Table 5. Total suspended solids of Photobioreactors in
Case 2
Photo-bioreactor TSS Initial (mg/L) TSS Final (mg/L)
2a 6.0 210
2b 5.7 243
2c 6.3 222
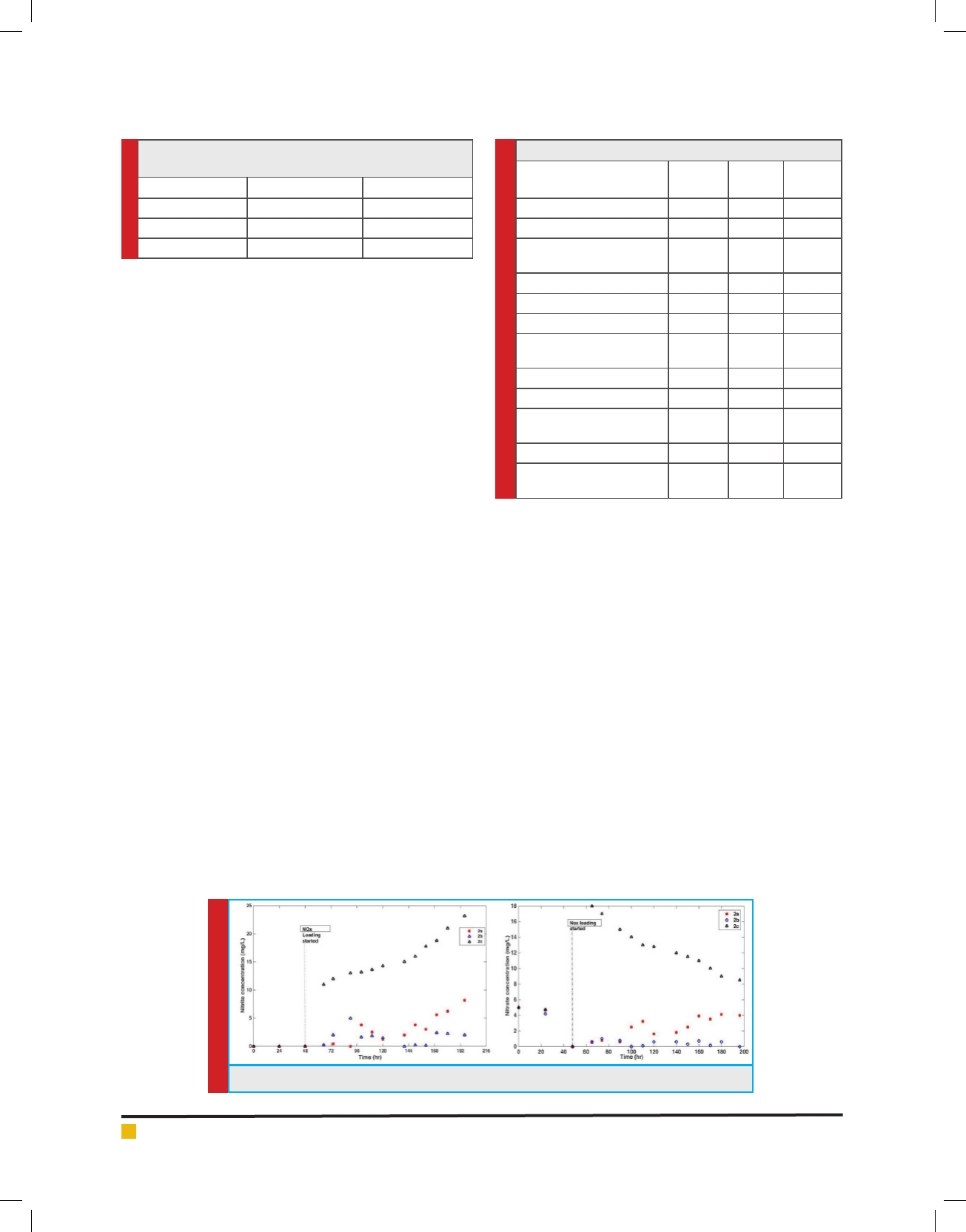
FIGURE 3. I) & II) Concentrations of NO3 & NO2 in Reactors 2a, b, c
Table 6. Nitrogen mass balance data
Reactor
2a
Reactor
2b
Reactor
2c
NO
x
Input 890 1740 3450
NO
x
Output 450 60 90
N Consumed from NO
x
gas
phase
440 1680 3360
Initial N in growth medium 23 22 23
Final NO
3
-271448
Final NO
2
- 74 13 163
Net Accumulation of NO
3
-
+NO
2
-
78 5 188
Initial Organic N 0 0 0
Final Organic N 310 750 1140
N Accumulated in Algal
Cells
310 750 1140
Total N Accumulated 388 755 1228
Mass Balance (% NO
x
uptake accounted for)
88% 45% 36%
Reactor 1b achieved an average 51% NOx removal, and
Reactor 1c has removed an average of 81% of inlet NOx.
The average NOx removal ef ciency for the rst 60 h
was 39%, but the average removal for the last 25 hours
was 52%. As the algal culture was declining, removal
of NOx could be associated with the dissolution of NO2
into the medium.
Reactors 1b and 1c presented moderate growth fol-
lowing an extended lag phase, as shown in the gure1.
Algae in reactors 1b, 1c has not truly experienced log
phase, and the run was ended after 90 hours.
Case 2: Calibration gas as a feed source
As shown in Fig. 2 all three reactors began with a cell
concentration of 2.8x105 cells/mL for three reactors 2a,
2b, and 2c .Maximum cell densities were 1.24x107 cells/
mL, 1.46x107 cells/mL and 1.72x107 cells/mL respec-
tively. To overcome some of the problems that appeared
from using pure NO2 gas in case 1, NO2 calibration
gases of 5000ppm and 10000 ppm NO2 were used to
supply NOx for case 2. This allowed for accurate NOx
loading concentrations. Actual NOx Loading rates for
case 2 were 27 ppm, 57 ppm and 126 ppm for reactors
2a, 2b, and 2c respectively.
The data in Table 4 presents the removal of NOx for
the reactors (case 2). Inlet and outlet NOx in Table 4 is
the summation of measured NO and NO2 concentrations
in the inlet and outlet streams. Reactor 2a got an aver-
age NOx removal of 59% reactor 2b got an average 96%,
and reactor 2c was able to remove an average of 95%
of inlet NOx. The lag phase was reduced to less than 24
hours due to delay in NOx loading, and log phase was
attained between 24 and 48 hours. NOx loading started
at 48 hours, and three reactors showed continued growth,
but with a signi cant decline in growth rate (Fig. 2).
Total suspended solids (TSS) for initial and nal sam-
ples were taken to quantify algal growth and to esti-
mate the nitrogen content of the cells. The results were
summarized in the Table 5. Total suspended solid results
show that 37 fold average mass growth was accom-
plished over the 190-hour run. For nitrate and nitrite
concentrations, liquid samples from case 2 were ana-
lyzed, and the results were shown in Fig.3. Nitrate was
completely drained in all three reactors before NOx was
loaded into the system at 49 hours. Nitrogen source
available for algal growth only after that point was from
dissolved NO2. Initial and nal total organic nitrogen
content of the algal cultures was used to estimate the
uptake of nitrogen by algae and to determine the nitro-
gen content of the cells. These analyses summarized in
the table: the cells in reactors 2a, 2b, and 2c were found
to contain 6.2%, 4.1%, and 7.9% nitrogen respectively.

Kethineni Chandrika et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS NOX REMOVAL USING DUNALIELLA SALINA ALGAE IN PHOTOBIOREACTORS 679
A mass balance of nitrogen was conducted to assess the
effect of NOx through the system. In reactor 2a, the mass
balance alleged for 88% of the observed NOx removal,
as slightly more nitrogen was found in the cells and
growth medium than entering the system.
For reactors 2b and 2c, only 45% and 36% respec-
tively of the nitrogen that entered the system was found
in the cells and growth medium. NOx feed stream: Load-
ing NOx at a particular concentration tested to be very
dif cult for the rst case, as can see by more standard
deviations in the table 6:. In the rst case, NOx was
given from a pure NO2 cylinder, and vapors from this
liquid were pumped using peristaltic pumps into the
reactors. To overcome large deviations in concentration,
case 2 was operated using calibration gases. Data analy-
sis considered only in case 2 because of the NOx feed
was not consistent during case1.Nitrogen mass balance
data summarized in Table 6. The only difference in the
three reactors in case 2 was the loading rates of NOx.
NOx removal: In case 2, NOx removal rates are 59%,
95%, 96% for reactors 2a, 2b, and 2c respectively. Only
59% NOx removal was achieved in reactor 2a, which
had the lowest NOx loading rate. NOx loading rates were
different in three reactors. Aqueous nitrate and nitrite
concentrations in the reactors are shown in Figure3.
Cell growth: The average speci c growth rate for the
rst 2 days in reactors 2a, 2b, and 2c was 0.03, 0.06,
0.12 respectively, the average speci c growth rates for
the same period in reactors 1a, 1b were both negative,
the growth curves of case 2 presented in Fig. 3, after
loading NOx, growth rates started to decline in all three
reactors and never achieved the value as before NOx
loading, and this declining growth is proportional to the
NOx concentration entering the system. Approximately
48 hours of NOx loading, the inlet concentrations of
NOx do not affect algal growth. Algae took nearly 48
hours to adopt new nitrogen source.
Nitrate /Nitrite: The algae was able to take nitrate in
solution before the loading of into the reactors. After 48
hours Nitrogen source is available only from NOx. The
steady fall in NO3- concentrations and NO2- accumula-
tion in reactor 2c suggests that algae preferred NO3- as
its nitrogen source over NO2-.
CONCLUSION
The primary purpose of this study was to test the
hypothesis that Dunaliella can grow on nitrogen from
dissolved NO2 as its only nitrogen source for cell syn-
thesis. Dunaliella grew used only nitrate /nitrite gener-
ated by the NOx dissolution for cell synthesis, reaching
a maximum cell density of 1.75x107cell/ml. A reactor
with lower NO2 loading concentrations resulted in lower
NOx removal rates, for this reactor, nitrate was not accu-
mulated as ef ciently as a similar with grated NOx load-
ing. In case 2: cell growth of mass between 1850mg/L
to 198mg/L. Nitrogen was removed from gaseous NOx
at a rate of 0.06-0.45 mg N/mg cell growth. Assuming
a 700 MW natural gas red power plant can produce up
to 1,70,000m3/h of ue gas with approx. 50ppm NOx
concentrations, growth of a minimun110 kg algal cell/h
would be required to treat this stream.
REFERENCES
Attilo Converti et al (2009) Effect of temperature and nitrogen
concentration on the growth and lipid content of Nanochloro-
psi lataandChlorella vulgarisfor biodiesel production Chemi-
cal Engineering and Processing Vol.48 1146-1151
Harter H J (2013) Carbon and nitrogen mass balance during
ue gas treatment with Dunaliella salina cultures, Journal of
Applied Phycology, Volume 2 : 359-368
Hong‐Wei Yen (2015) CO2, NOx and SOx removal from ue
gas via microalgae cultivation, Biotechnology Journal Vol. 10:
890-899.
Kaitlyan D (2018) The fate of nitrogen through algal treatment
of land ll leachate, Algal Research, Volume 30: 50-58
Kethineni, C. (2017) Development of Sequential Processes for
Multiple Product Recovery from Microalgae, Industrial Bio-
technology Vol 14 No 2:94-99
Lee, Y. N., & Schwartz, S. E. (1981) Reaction kinetics of nitro-
gen dioxide with liquid water at low partial pressure. The Jour-
nal of Physical Chemistry.Vol 85 No7: 840-848
Miller, J. S., & Ledakowicz, S. (2010) Trends in NOx abate-
ment: A review. Science of the Total Environment, Vol 408 No
19:3976-3989.
Mulholland, M. R., & Lomas, M. W. (2008) Nitrogen Uptake and
Assimilation. In D. G. Capone, D. A. Brown, M. R. Mulholland,
& E. J. Carpenter (Eds.), Nitrogen in the marine environment
Amsterdam: Elsevier Academic Press. (2nd Ed.: 303-384).
Nagase, H. (1997) Characteristics of biological NOx removal
from ue gas in a Dunaliella tertiolecta culture system. Journal
of Fermentation and Bioengineering,Vol. 83 No 5: 461-465
Raja R S. Hemaiswarya, R. Rengasamy (2007) Exploitation of
Dunaliella for -carotene production. Applied Microbiology &
Biotechnology.Vol 74 No 3: 517–523
Ronda JI (2014) A growth inhibitory model with SO
x
in u-
enced effective growth rate for estimation of algal biomass
concentration under ue gas atmosphere, Bioresource Tech-
nology, Volume 152: 283-291
Skalsa K (2010), Trends in NOx abatement. Implementation of
stringent regulations of NOxemission requires the development
of new technologies for NOxremoval from exhaust gases.Sci-
ence of The Total Environment Vol. 408: 3976-3989

Kethineni Chandrika et al.
680 NOX REMOVAL USING DUNALIELLA SALINA ALGAE IN PHOTOBIOREACTORS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Tianpei Li and Gang Xu (2016). The acclimation ofChlorellato
high-level nitrite for potential application in biological NOx
removal from industrial ue gases. Journal of Plant Physiol-
ogy Vol. 195:73-79.
Tomasz T & Hidehiro Kumazawa (2016) Reactivity of nano-
size zinc powder in the aqueous solution of (FeIII(Edta)
(H2O) Science of The Total Environment Vol. 628–629: 870-
881
Yuxuan Zhu (2018), Biological activities and nitrogen and
phosphorus removal during the Anabaena os-aquae bio lm
growth using different nutrient form, Bioresource Technology,
Volume 251: 7-12